Abstract
AIM
To observe effect of adenovirus-mediated brain derived neurotrophic factor in early retinal neuropathy of diabetes in rats.
METHODS
Adult male Wistar rats, 9 weeks of age, were injected intraperitoneally with STZ to induce diabetes. Two weeks after the models were established, Ad.BDNF was administered into the vitreous cavities of rats. Four weeks after the models were set up, the rats were killed and the retina was removed for Western blotting and whole-mount immunohistochemistry for tyrosine hydroxylase (TH) to observe the changes of TH and dopaminergic amacrine cells in retina.
RESULTS
The protein levels of TH and the number of positive staining dopaminergic amacrine cells and the staining gray scale of experimental group without Ad.BDNF were lower statistically. But there was no statistically significant difference between the experimental group with Ad.BDNF and control group.
CONCLUSION
In the early stage of STZ diabetic, the administration of Ad.BDNF into the vitreous cavities can increase TH protein levels and the density of dopaminergic amacrine cells in the STZ rats' retina.
Keywords: diabetic retinopathy, tyrosine hydroxylase, brain derived neurotrophic factor, adenovirus
INTRODUCTION
Diabetes is a disease characterized with glucose metabolic disturbance, of all the complications of it, the earliest incidence and the maximal morbidity is the complications of eyes, in them, the most serious one is diabetic retinopathy (DR). Diabetic retinopathy which is classically defined as microangiopathy, but actually it is a neural degeneration of retina. There were lots of evidences showed that just after the diabetes onset and before the angio-complications, the molecule functions are variation in human being and experimental animals. The neuropathy is earlier than the angio-complications in the retina of diabetes, continue through all the time of diabetes; threaten the acuity of vision significant seriously. Neurotrophic factors (NTFs) produce a significant effect in the procedure of reparation and regeneration at microenvironment after neural degeneration, brain-derived neurotrophic factor (BDNF) is one of them who have been affirmed earlier[1]. Dopaminergic neurons respond multitude stimulus by regulating the synthesis and delivery of dopamine. TH is the rate-limiting enzyme of the dopamine synthesis, so the tyrosine hydroxylase (TH) protein level is one of the identification of dopaminergic amacrine cells. We injected the recombinant adenovirus containing the BDNF gene (Ad.BDNF) into the vitreous cavity of diabetic rats and observed the variation of tyrosine hydroxylase (TH) protein level and the number of dopaminergic amacrine cells in the diabetic rat retina before and after the injection of Ad.BDNF, to aim at providing experimental evidence for the clinical treatment of diabetic retinopathy and the application of BDNF in eyes.
MATERIALS AND METHODS
Materials
Recombinant adenovirus containing the BDNF gene (Ad.BDNF), (Academy of military medical sciences); streptozotocin (STZ) (Sigma), TH immunohistochemistry kit (Boster). Adult male Wistar rats, 9 weeks of age, 180-220g, no retinopathy, were purchased from the Animal Center of China Medical University and housed under standard lighting conditions (12h light/dark cycle) with available ad libitum food and water. The cages are well ventilated and room temperature maintained at between 18-25°C, relative humidity are maintained at between 40%-70%.
Methods
STZ-derived diabetic rats
STZ (in 0.1mmol/L sodium citrate buffer, pH4.5), of which the concentration was 10g/L, was prepared just before use. Wistar rats was fasting during 12 hours; streptozotocin (STZ) (70mg/kg) was administered intraperitoneally under general anesthesia with chloral hydrate. We drew blood from vena caudalis to measure fasting blood glucose at 48 hours, 72 hours, and 1 week after the injection subsequently. Rats with glucose levels>16.7mmol/L were classified as diabetic. Fasting blood glucose concentrations were measured weekly thereafter. Nondiabetic rats were injected with an equal volume of citrate buffer. Wistar rats were anesthetized by intraperitoneal injection of chloral hydrate and killed by dislocation of cervical vertebra 4 weeks after the STZ injection. The eyes were processed for analyses.
Intravitreal injection of Ad.BDNF
Using a glass micropipette, the intraocular administration of Ad.BDNF was beginning 2 weeks after the injection of STZ or placebo, under anesthesia with chloral hydrate and superficial anaesthesia with a drop of 20g/L lidocaine. Recombinant adenovirus containing the BDNF gene 5µL was injected into the vitreous cavity of one of the rats' eye randomly. An equal volume of vehicle (Ad.LacZ) was injected into the vitreous cavity of the other eye, immediately adjacent to the oraserrata and avoiding the lens. The rats with vitreous hemorrhage or atrophia bulbi were excluded.
TH Western blotting
The level of tyrosine hydroxylase (TH) protein was determined by Western blotting. Remove the anterior segment of the eyes, disassociate and get the retinas carefully, put them in the epphendorf tube, add lysate, hypersound on ice, centrifuge at 4°C.The supernatant fluid was processed for further analyses. Rat anti-TH monoclonal antibody (1:100) and rabbit anti-rat IgG antibody (1:100) were used as primary and secondary antibodies. The gray scale was analyzed by BandScan software.
Whole-mount immunohistochemistry
Retinas were mounted and degreased, then hydrated in 1g/L PBS; incubated in anti-TH monoclonal antibody (1:100) and 5g/L Triton X-100 for 72 hours. Afterward it was incubated in rabbit anti-rat IgG antibody (1:100) for 24 hours at 4°C. The sampling procedures used for counts were used to determine the number of dopaminergic amacrine cells.
Statistical Analysis
The numeration data were analyzed by t-test. Mean values are given ±the standard error of the mean.
RESULTS
TH Protein Level
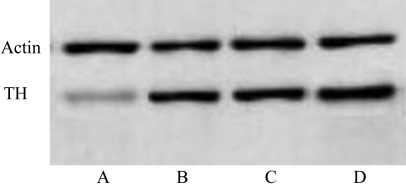
Standardized by the β-actin levels in the same lane, the TH protein in the retinas of the diabetic rats without Ad.BDNF were significantly lower than that in the fellow eyes and the nondiabetic rats (P<0.01). There was no significant difference among the retinas of diabetic rats with Ad.BDNF, nondiabetic rats with and without Ad.BDNF (Figure 1).
Figure 1. TH protein levels in rat retinas (Western blotting staining gray scale).
A: Exp-Ad.BDNF (20.7±4.3); B: Exp+Ad.BDNF (40.4±4.5); C: Control-Ad.BDNF(54.2±4.8); D: Control+Ad.BDNF(57.5±2.6)
TH Immunoreactivity
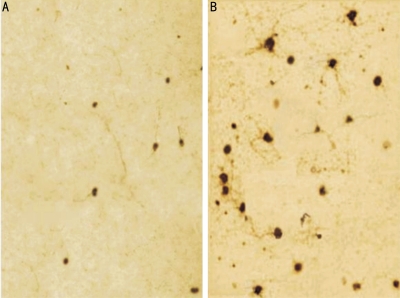
The calculated number (mean±SEM) of cells with TH immunoreactivity was significantly lower than that in the fellow eyes and the nondiabetic rats (P <0.01). There was no statistically significant difference among the retinas of diabetic rats with Ad.BDNF, nondiabetic rats with and without Ad.BDNF (Figure 2, Table 1). The staining gray scale (mean ±EM) of cells with TH immunoreactivity was significantly lower than that in the fellow eyes and the nondiabetic rats (P <0.01). There was no statistically significant difference among the retinas of diabetic rats with Ad.BDNF, nondiabetic rats with and without Ad.BDNF (Table 1).
Figure 2. Immnohistochemisty of TH in experimental rat retinas.
A: Exp-Ad.BDNF; B: Exp+Ad.BDNF
Table 1. TH expression in rat retinas.
| Ad.BDNF | Positive cell counting/mm2 |
Positive cell gray scale |
||
| Exp | Control | Exp | Control | |
| No | 14.3±3.5b | 37.5±6.2 | 37.8±4.4b | 80.2±1.7 |
| Yes | 35.2±3.4 | 36.2±6.8 | 75.3±2.2 | 82.3±3.9 |
bP<0.01
(mean±SEM)
DISCUSSION
In the rat retina, photoreceptors, inner nuclear layer, retinal ganglion cells (RGCs) and nerve fiber layer express BDNF. BDNF is excreted by retina itself mainly, which is important for the survival of retinal ganglion cells. Amacrine cells may be the major source of it[2]. It was reported that after the optic nerve injury, the expression of BDNF gene was increased[3]. BDNF acted an important role to the recovery and regeneration of retina and optic nerve damage, retinal ischemia and chemical damage, photoreceptor cells injury. At present, it was considered as an anti-apoptotic agent, calcium channel blockers and antioxidants.
Dopamine is associated with many of the basic functions of the human body. In the retina dopamine is released by the amacrine cells; the dopamine receptors located in the retina can be activated to produce a marked effect. It plays an important and complex role in the retina functions. The current study suggests[4],[5] that the interaction is a two-way channel between the neural retina and the dopaminergic system, and dopamine may be the mutual feedback messenger among neural retinal cells. There are two receptors of Dopamine, D1 and D2. The periphery of dopaminergic amacrine cells and horizontal cells form synapses, release dopamine to the D1 receptors at the horizontal cells, reduce the response of the latter to light. D2 receptors of dopamine belong to autoreceptor of dopaminergic neurons with presynaptic negative feedback function which can regulate the dopamine release of dopaminergic neurons. The interaction of dopamine receptors D1 and D2 complete the physiological function of dopamine, which can interact with lots of neural active compound. Dopaminergic neurons release dopamine to the dopamine receptors at photoreceptors and retinal pigment epithelial cells, combine with receptors D1 and induce changes in retinal photoreceptor cells. Dopamine is the transmission media from retina to retinal pigment epithelial cells. In addition, dopamine inhibits the activity of adenylate cyclase (AC), decreases the levels of cAMP, and inhibits the release of dopamine by inhibition of feedback through receptors D2. Thus, dopamine acts as nerve-hormones role in the retina. The key of this procedure is TH, the rate-limiting enzyme of dopamine synthesis, like dopamine. It distributes throughout the cell, including the most sophisticated tubercle and peripheral. Therefore, in our study we apply the detection of TH protein levels to reflect the function of dopaminergic amacrine cell in retina, and TH-positive cells to reflect the number of dopaminergic amacrine cells in retina.
The possible mechanism of dopaminergic amacrine cell degeneration in diabetes is as follows: First, the severe insulin deprivation, seen in STZ-induced diabetes. Politi et al[6] studies suggest that insulin is an important factor of the survival of amacrine cells in vitro, and the excess sugar destroy the survival of insulin-stimulated cell and the signaling of insulin receptor in retina in vitro. Second, hyperglycemia. Nakamura et al[7] showed that the excess sugar destroy the survival of insulin-stimulated cell and the signaling of insulin receptor in retina in vitro. Third, the dysfunction of Müller's cell in retina. Li et al[8] and Carrasco et al[9] considered that in diabetes, the transport function of glutamate-aspartate was reduced; the synthesis of aspartic acid was destructed in Müller's cells. These dysfunctions result in the increasing of glutamate levels in retina which is toxic to the amacrine cells. In this study, we detected the level of TH and the number of dopaminergic amacrine cells were significant depression in STZ rats' retina.
The degeneration of dopaminergic amacrine cell may lead to the depression of BDNF levels[10]. A null mutation of bdnf gene in mice can result in the atrophy of dopaminergic amacrine cells. It was reported[11]-[13] that BDNF prevented the death of dopaminergic neurons in substantia nigra and retinal amacrine cells in vivo and in vitro. Agudo et al[14] and Weber et al[15] found that in adult retina, more than 90% of ganglion cells died within 2 weeks after the interception of axis cylinder, if we inject BDNF into vitreous cavity immediately after the cutting off of the optic nerve, retinal ganglion cells will survive. But this approach is temporary and can only delay the death of retinal ganglion cells for about 3 days.
BDNF cannot transport through the blood-brain barrier. Therefore, local application of neurotrophic factors is better than systemic application. It can maintain a high concentration in the micro-environment directly or through local blood circulation and avoid systemic side effects. However, such peptides generally have a relatively short half-life in vivo and their therapeutic effects are only temporary. Repeated injections may prolong neuroprotection but will also cause greater ocular damage. The gene therapy approaches provide high concentrations of protein continuously by the introduction of foreign genes in a period of time which have great advantages in the therapy of central nervous system diseases. In this study, we applied adenovirus-mediated BDNF intravitreal injection to increase the BDNF expression and to enhance the level of TH retina and the number of dopaminergic amacrine cells in rat retina.
In summary, adenovirus-mediated transfection of exogenous BDNF gene can get an efficient and long-lasting expression of BDNF protein, which will provide the prerequisite for the repair of optic nerve damage and the recovery of visual function. It will be an experiment foundation for clinical application of BDNF.
REFERENCES
- 1.Takano M, Horie H, Iijima Y, Dezawa M, Sawada H, Ishikawa Y. Brain-derived neurotrophic factor enhances neurite regeneration from retinal ganglion cells in aged human retina in vitro. Exp Eye Res. 2002;74(2):319–323. doi: 10.1006/exer.2001.1118. [DOI] [PubMed] [Google Scholar]
- 2.Isenmann S, Kretz A, Cellerno A. Molecular determinants of retinal ganglion cell development survival and regeneration. Prog Retin Eye Res. 2003;22(4):483–543. doi: 10.1016/s1350-9462(03)00027-2. [DOI] [PubMed] [Google Scholar]
- 3.Cui ZL, Kang J, Wang L, Wang J, Hui YN, Hu D. Effect of neurotrophic factors on the expression of retinal growth associated protein-43mRNA in retina after optic nerve injuries. Int J Ophthalmol(Guoji Yanke Zazhi) 2005;5(5):902–906. [Google Scholar]
- 4.Brandies R, Yehuda S. The possible role of retinal dopaminergic system in visual performance. Neurosci Biobehav Rev. 2008;32(4):611–656. doi: 10.1016/j.neubiorev.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Dacey DM. The dopaminergic amacrine cell. J Comp Neurol. 1990;301(3):461–489. doi: 10.1002/cne.903010310. [DOI] [PubMed] [Google Scholar]
- 6.Politi LE, Rotstein NP, Salvador G, Giusto NM, Insua MF. Insulin-like growth factor-I is a potential trophic factor for amacrine cells. J Neurochem. 2001;76(4):1199–1211. doi: 10.1046/j.1471-4159.2001.00128.x. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura M, Barber AJ, Antonetti DA, LaNoue KF, Robinson KA, Buse MG, Gardner TW. Excessive hexosamines block the neuroprotective effect of insulin and induce apoptosis in retinal neurons. J Biol Chem. 2001;276(47):43748–43755. doi: 10.1074/jbc.M108594200. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Puro DG. Diabetes-induced dysfunction of the glutamate transporter in retinal Müller cells. Invest Ophthalmol Vis Sci. 2002;43(9):3109–3116. [PubMed] [Google Scholar]
- 9.Carrasco E, Hernàndez C, de Torres I, Farrés J, Simó R. Lowered cortistatin expression is an early event in the human diabetic retina and is associated with apoptosis and glial activation. Mol Vis. 2008;14:1496–1502. [PMC free article] [PubMed] [Google Scholar]
- 10.Loeliger MM, Briscoe T, Rees SM. BDNF increases survival of retinal dopaminergic neurons after prenatal compromise. Invest Ophthalmol Vis Sci. 2008;49(3):1282–1289. doi: 10.1167/iovs.07-0521. [DOI] [PubMed] [Google Scholar]
- 11.Erickson JT, Brosenitsch TA, Katz DM. Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor are required simultaneously for survival of dopaminergic primary sensory neurons in vivo. J Neurosci. 2001;21(2):581–589. doi: 10.1523/JNEUROSCI.21-02-00581.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cusato K, Bosco A, Linden R, Reese BE. Cell death in the inner nuclear layer of the retina is modulated by BDNF. Brain Res Dev Brain Res. 2002;139(2):325–330. doi: 10.1016/s0165-3806(02)00570-9. [DOI] [PubMed] [Google Scholar]
- 13.Kido N, Tanihara H, Honjo M, Inatani M, Tatsuno T, Nakayama C, Honda Y. Neuroprotective effects of brain-derived neurotrophic factor in eyes with NMDA-induced neuronal death. Brain Res 2000;884(1- 2000;884(1-2):59–67. doi: 10.1016/s0006-8993(00)02887-0. [DOI] [PubMed] [Google Scholar]
- 14.Agudo M, Pérez-Marín MC, Lönngren U, Sobrado P, Conesa A, Cànovas I, Salinas-Navarro M, Miralles-Imperial J, Hallböök F, Vidal-Sanz M. Time course profiling of the retinal transcriptome after optic nerve transection and optic nerve crush. Mol Vis. 2008;14(6):1050–1063. [PMC free article] [PubMed] [Google Scholar]
- 15.Weber AJ, Harman CD, Viswanathan S. Effects of optic nerve injury, glaucoma, and neuroprotection on the survival, structure, and function of ganglion cells in the mammalian retina. J Physiol. 2008;586(9):4393–4400. doi: 10.1113/jphysiol.2008.156729. [DOI] [PMC free article] [PubMed] [Google Scholar]