Abstract
AIM
To compare the corneal endothelial cell density (ECD) of clear grafts after penetrating keratoplasty (PK) and deep anterior lamellar keratoplasty (DALK).
METHODS
The study included 44 and 54 patients treated with PK and DALK, respectively, between March 2006 and April 2010. Corneal ECD was examined using specular microscopy at postoperative 1, 3, 6, 12, and 18 months, and the values were compared.
RESULTS
Corneal ECD reduction in the PK group was 7.4%, 15.2%, 23.5%, and 28.9% at 3, 6, 12 and 18 months respectively after surgery, compared with 4.2 % in the first month (P<0.01). These figures were 3.0%, 6.7%, 7.2%, and 7.7% at 3, 6, 12 and 18 months respectively, compared with 2.2 % in the first month in the DALK group (P>0.05).
CONCLUSION
Compared with DALK, PK significantly reduced ECD of the clear grafts. These results suggest that survival of endothelial cells in grafts is better after DALK than after PK.
Keywords: lamellar keratoplasty, penetrating keratoplasty, endothelial cell density, specular microscopy
INTRODUCTION
The fundamental aim of a successful keratoplasty surgery is to obtain a clear corneal graft and maintain its survival. Previous studies have demonstrated the importance of endothelial cell density (ECD) for maintaining graft transparency and survival after keratoplasty[1]. The mean annual rate of endothelial cell loss during the first 3 to 5 years after penetrating keratoplasty (PK) (7.8%/year) is higher than the physiological endothelial cell loss(0.52%/year)[2],[3]. The cumulative endothelial cell loss 10 years after PK rises to above 50% and the resulting decrease in cell density constitutes the most important reason for late graft failure[4].
In order to obtain long-term protection of corneal graft transparency, deep anterior lamellar keratoplasty (DALK) has become the preferred procedure in recent years. In this approach, the corneal endothelium of the recipient is kept intact and the anterior layers of donor cornea, without endothelium and Descemet's membrane are transplanted. Today, sophisticated DALK surgery techniques enable sufficient stromal dissection and preparation of a smooth recipient bed without interface problems. With DALK, loss of endothelial cells appears to be similar to the physiological loss; and additionally, the impairment in the corneal graft transparency due to tissue mismatch can be prevented[5]. This study was undertaken to investigate the changes in endothelial cell count following PK and DALK.
MATERIALS AND METHODS
Materials
In this retrospective study, we reviewed the clinical charts of 98 patients who underwent corneal transplantation in the Cornea Clinic of the Ophthalmology Department at Haydarpasa Numune Education and Research Hospital, Istanbul, Turkey, between 2006 and 2010. Of these 98 patients, 44 had undergone PK and 54 had undergone DALK. Patients with hydrops or altered Descemet's membrane due to corneal pathology had undergone PK. Patients with keratoconus without hydrops, corneal scars, and dystrophies with a healthy Descemet's membrane, had undergone DALK. Patients who had participated to regular follow-up visits, maintaining graft transparency until the last follow-up visit (18 months) were included. Exclusion criteria included the presence of ocular pathologies other than corneal pathology and diabetes mellitus, increased intraocular pressure that could influence graft transparency, graft rejection, graft ulcer, graft detachment, development of cataract during follow up period, need for additional surgery, or combined surgery during keratoplasty.
Age and gender of the patients, indications for primary keratoplasty, and graft transparency were recorded. Then, we evaluated central corneal ECD by specular microscopy at 1, 3, 6, 12 and 18 months. In addition, the patients were examined for diabetes mellitus as this disease has a negative impact on corneal endothelium. Before surgery, all participants had undergone visual acuity test using a snellen chart, biomicroscopic assessment, intraocular pressure measurement by Goldmann applanation tonometry, and fundus examination.
The study was carried out in accordance with the ethical standards of the 1964 Helsinki Declaration, and informed consent was obtained from every patient.
Donor corneas were taken from cadavers and stored in Optisol-GS (Bauch&Lomb, Inc., New York, USA) cornea storage solution, containing 100µg/mL gentamicin and 200µg/mL streptomycin sulfate as antimicrobials. The corneas were used for keratoplasty within a maximum of four days.
Methods
Pre-operative preparation
Pilocarpine hydrochloride 2% was administered to the eyes of all patients to obtain miosis before surgery. Mannitol (300mL) was given intravenously to reduce intraocular pressure. To achieve akinesia and anesthesia, retrobulbar injections of 2mL lidocaine hydrochloride 2% and 2mL bupivacaine were performed. The periocular area was cleaned using povidone-iodine 10% as the antiseptic agent.
Surgical techniques
a) Penetrating keratoplasty: After choosing the appropriate trephine size for the recipient cornea, the donor cornea was cut with a 0.25mm larger (with respect to the recipient cornea) punch trephine and was kept in cefuroxime solution (10mg/mL). The recipient cornea was dissected by a vacuum trephine and the excision was completed using scissors. The corneal graft was placed on the recipient bed and sutured with 10/0 monofilament nylon by four sutures for fixation, followed by continuous sutures. Sutures were tightened and the wound site was closed without leakage. The anterior chamber was filled with Balanced Salt Solution. Operation was completed by intracameral cefuroxime administration (10mg/mL); b) Deep anterior lamellar keratoplasty: Recipient cornea was trephinized using a vacuum trephine at 60%-80% depth. A big bubble was then formed in the stroma by injecting air using a 30-gauge injector. Superficial keratotomy was performed. A lateral port was opened at the 3 o'clock position and the anterior chamber was filled entirely with air from the port. A puncture was made on Descemet's membrane 45° from the center of the cornea. Descemet's membrane and the remaining stromal tissue were detached using a spatula inserted between Descemet's membrane and the posterior stromal tissue. The remaining stromal bed was cut with cornea scissors. The donor cornea was cut with a 0.25mm larger (with respect to recipient cornea) punch trephine. Trypan blue (0.06%) was used to stain the endothelial surface of the donor cornea, and the Descemet's membrane was removed with a dry cellulose sponge. The donor cornea was placed on the recipient bed and sutured with 10/0 monofilament nylon by four sutures for fixation. Then, the graft was fixed with a continuous suture. Sutures were tightened and the wound site was closed without leakage. The operation was completed by intracameral cefuroxime administration (10mg/mL).
Follow-up visits were scheduled at post-operative 1, 3, 6, 12 and 18 months, and both biomicroscopic examinations and intraocular pressure measurements using an applanation tonometry were performed. Endothelial cell parameters were obtained using a specular microscope combined with a camera (Tomey Endothelium Specular Microscope EM-2000, Tokyo, Japan). Endothelial photographs of both eyes of each patient were taken twice by the same researcher (B.T.A.). The clearest area in each photograph was magnified and 20 cells were assessed. Endothelial cells were assessed using the analysis function of the specular microscopy device. Central ECD was measured and the mean values were recorded.
Statistical Analysis
Statistical analyses were performed using the SPSS for Windows 10.0 software. Inter-group comparisons were performed by the Student's t-test for the parametric variables and by Mann Whitney U test for the non-parametric variables. Inter-group comparisons were made by analysis of variance for repeated measures and paired samples t-test for distinguishing differences in parameters among the follow-up months. The intragroup changes in the parameters without normal distribution were compared using the Friedman test and the Wilcoxon signed rank test to determine the difference in parameters between the follow-up months. The significance level was adjusted to P<0.05, with a 95% confidence interval.
RESULTS
Penetrating keratoplasty group (Group 1) included 44 eyes and DALK group (group 2) 54 eyes. Table 1 summarizes the baseline characteristics and preoperative data of the patients in the study.
Table 1. Preoperative data of the patients who underwent penetrating keratoplasty and deep anterior lamellar keratoplasty.
| Characteristics | PK | DALK |
| Number of eyes | 44 | 54 |
| Male | 23(52.3%) | 30(55.6%) |
| Female | 21(47.7%) | 24(44.4%) |
| Age (mean±SD) (years) | 30.5±6.2 | 28.4±7.1 |
| Follow-up(mean±SD) (months) | 28.5±4.7 | 22.5±3.0 |
| Preoperative diagnosis | ||
| Keratoconus | 24(54.6%) | 36(66.7%) |
| Corneal scarring | 8(18.2%) | 10(18.5%) |
| Macular dystrophy | 6(13.6%) | 4(7.4%) |
| Granular dystrophy | 6(13.6%) | 3(5.6%) |
| Reis-Buckler's dystrophy | - | 1(1.8%) |
PK: penetrating keratoplasty; DALK: deep anterior lamellar keratoplasty; SD: standard deviation.
The endothelial cell density during the follow up periods in both groups is shown in Table 2.
Table 2. Endothelial cell density over time.
| Characteristics | PK(mean±SD) | DALK(mean±SD) | P |
| 1 month | 2790.36±568.54 | 2452.82±646.52 | 0.012 |
| 3 months | 2598.80±583.66 | 2378.63±622.18 | 0.036 |
| 6 months | 2400.36±593.62 | 2274.67±612.20 | 0.364 |
| 12 months | 2156.44±648.30 | 2265.54±636.42 | 0.145 |
| 18 months | 1994.26±618.42 | 2252.68±634.28 | 0.022 |
PK: penetrating keratoplasty, DALK: deep anterior lamellar keratoplasty, SD: standard deviation.
ECD at 1 and 3 months in Group 2 was significantly lower than that in Group 1 (P<0.05). ECD at 6 and 12 months, was similar in the study groups (P>0.05). There was, however, a significant difference between the two groups in terms of ECD measured at 18 months (P<0.05).
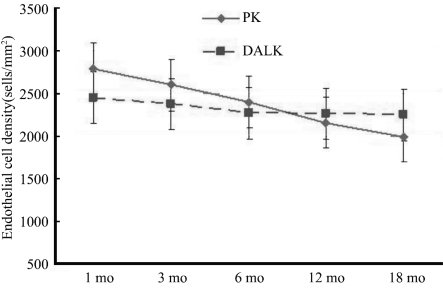
In the PK (Group 1) group, ECD was significantly different among the time points of 1, 3, 6, 12, and 18 months after surgery (P<0.01). In this group, ECD values at 3, 6, 12, and 18 months were significantly different compared with that at the first month (P=0.001; P<0.01). The ECD values at 6, 12, and 18 months were significantly lower than that at 3 months (P=0.001; P<0.01). The ECD values at 12 and 18 months were significantly lower than that at 6 months (P=0.001; P<0.01) and, the ECD at 18 months was significantly lower than the ECD at 12 months(P=0.001; P<0.01, Figure 1).
Figure 1. Mean endothelial cell density after PK and DALK. At 18 months following surgery, endothelial cell density was significantly higher in the DALK group than that in the PK group (P<0.05).
Estimated cell loss, as a percentage of the original central corneal endothelial cell count at 1 month was 4.2%, 7.4% at 3 months, 15.2% at 6 months, 23.5% at 12 months and 28.9% at 18 months (Table 3). In the DALK group, ECD were similar at the 1, 3, 6, 12 and 18 month time points (P>0.05, Figure 1). Estimated cell loss, as a percentage of the original central corneal endothelial cell count at the first month was 2.2%, 3.0% at 3 months, 6.7% at 6 months, 7.2% at 12 months and 7.7% at 18 months (Table 3).
Table 3. Estimated endothelial cell loss after PK and DALK(relative to the first month).
| PK (%) | DALK (%) | |
| 3 months | 7.4 | 3.0 |
| 6 months | 15.2 | 6.7 |
| 12 months | 23.5 | 7.2 |
| 18 months | 28.9 | 7.7 |
PK: penetrating keratoplasty, DALK: deep anterior lamellar keratoplasty
DISCUSSION
Endothelial damage after keratoplasty is compensated for by migration of peripheral cells into the damaged area and by hypertrophy of the surviving cells. Adequate amounts of corneal ECD are required to obtain a long-term functional transparent graft. During the early post-operative period after PK, there are significant decreases both in endothelial cell count and in hexagonal cell percentage, accompanied by increases in the coefficient of variation of cell area and the mean cell area[6]. Progressive decrease in the number of central endothelial cells is considered to result from the migration of these cells towards the peripheral areas of endothelial damage[7]. It has been shown that endothelial cell loss was almost 33% during the post-operative 2 years after PK and continues for 20 years after the operation[2],[8],[9]. It is considered that surgical trauma, redistribution of endothelial cells, and allograft rejection lead to substantial decreases in ECD[2],[10].
Obata et al[11] determined that post-operative endothelial cell loss reached 10.4% at 2 weeks, 16.3% at 1 month, 33.6% at 3 months, 39.4% at 6 months, and 48.2% at 12 months after PK procedure. In the same study, the rate of cell loss in patients with keratoconus was 1.9% at 2 weeks, 1.2% at 1 month, 9.9% at 3 months, 30.6% at 6 months and 33.4% at 12 months, whereas in the bullous keratopathy group, these values were 13.8%, 25.9%, 52.6%, 47.2%, and 66.9%, respectively. Furthermore, the authors stated that cell loss in the post-operative first year was due to primary disease. Ing et al[12] studied 500 keratoplasty patients and reported endothelial cell loss to be 17% at 2 months and 34% at 1 year after surgery.
In our study, the reduction in central corneal cell count in the PK group was 7.44% at 3 months, 15.20% at 6 months, 23.46% at 12 months and 28.88% at 18 months, compared with cell counts at the first month (4.22%). Our values were lower than those observed in the previous studies, and we considered that this might be a consequence of the patient characteristics in the PK group, which consisted of 75% keratoconus patients.
Following DALK surgery, endothelial cell loss due to allograft rejection decreases substantially. Sugita et al[13] reported that endothelial cell loss after DALK was 13% at the end of first year. Van Dooren et al[14] found that ECD showed an 11% decrease during the first six months after DALK, and afterwards the decrease was 1%-2% per year. They also found that the decrease in ECD was similar to that of non-operated healthy corneas[14]. Among the reasons for large decreases in ECD after DALK surgery perioperative air injection into the anterior chamber, and trauma of the recipient endothelium during deep stromal dissection have been proposed[14],[15]. In our study, we found similar results. Reduction in central endothelial cell count after DALK was 3.0% at 3 months, 6.7% at 6 months, 7.2% at 12 months and 7.6% at 18 months, compared with the first-month after surgery (2.2%).
In conclusion, we observed that endothelial cell loss after PK increased over time. Surgical trauma, redistribution of endothelial cells, and allograft rejection are considered to be responsible for endothelial cell loss after PK[2],[10],[16]. In contrast, the rate of cell loss after DALK in the recipient's healthy endothelium under the lamellar graft was similar to the cell loss in natural corneal endothelium[14]. This is especially important for long-term survival of the endothelial cells in young patients. Such patients may have corneas that can tolerate a possible cataract surgery in their older ages. Therefore, DALK is advantageous compared with PK for the treatment of corneal anterior surface disorders such as keratoconus and corneal superficial ulcers.
REFERENCES
- 1.Bourne WM. One-year observation of transplanted human corneal endothelium. Ophthalmology. 1980;87(7):673–679. doi: 10.1016/s0161-6420(80)35188-9. [DOI] [PubMed] [Google Scholar]
- 2.Bourne WM. Cellular changes in transplanted human corneas, Castroviejo lecture. Cornea. 2001;20(6):560–569. doi: 10.1097/00003226-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Bourne WM, Hodge DO, Nelson LR. Corneal endothelium five years after transplantation. Am J Ophthalmol. 1994;118(2):185–196. doi: 10.1016/s0002-9394(14)72898-3. [DOI] [PubMed] [Google Scholar]
- 4.Patel SV, Hodge DO, Bourne WM. Corneal endothelium and postoperative outcomes 15 years after penetrating keratoplasty. Am J Ophthalmol. 2005;139(2):311–319. doi: 10.1016/j.ajo.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 5.Melles GRJ, Remeijer L, Geerards AJM, Beekhuis WH. A quick surgical technique for deep lamellar keratoplasty using visco-dissection. Cornea. 2000;19(4):427–432. doi: 10.1097/00003226-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Traffers WF. Human corneal endothelial wound repair; in vitro and in vivo. Ophthalmology. 1982;89(6):605–613. doi: 10.1016/s0161-6420(82)34757-0. [DOI] [PubMed] [Google Scholar]
- 7.Inoue K, Kimura C, Amano S, Oshika T, Tsuru T. Corneal endothelial cell changes twenty years after penetrating keratoplasty. Jpn J Ophthalmol. 2002;46(2):189–192. doi: 10.1016/s0021-5155(01)00485-3. [DOI] [PubMed] [Google Scholar]
- 8.Panda A, Bageshwar LMS, Ray M, Singh JP, Kumar A. Deep lamellar keratoplasty versus PK for corneal lesions. Cornea. 1999;18(2):172–175. doi: 10.1097/00003226-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Shimazaki J, Shimmura S, Isioka M, Tsubota K. Randomized clinical trial of deep lamellar keratoplasty vs. penetrating keratoplasty. Am J Ophthalmol. 2002;134(2):159–165. doi: 10.1016/s0002-9394(02)01523-4. [DOI] [PubMed] [Google Scholar]
- 10.Reinhard T, Bohringer D, Huschen D, Sundmacher R. Chronic endothelial cell loss of the graft after penetrating keratoplasty: influence of endothelial cell migration from graft to host. Klin Monatsbl Augenheilk. 2002;219(6):410–416. doi: 10.1055/s-2002-32876. [DOI] [PubMed] [Google Scholar]
- 11.Obata H, Ishida K, Murao M, Miyata K, Sawa M. Corneal endothelial cell damage in penetrating keratoplasty. Jpn J Ophthalmol. 1991;35(4):411–416. [PubMed] [Google Scholar]
- 12.Ing JJ, Ing HH, Nelson LR, Hodge DO, Bourne WM. Ten-year postoperative results of penetrating keratoplasty. Ophthalmology. 1998;105(10):1855–1865. doi: 10.1016/S0161-6420(98)91030-2. [DOI] [PubMed] [Google Scholar]
- 13.Sugita J, Kondo J. Deep lamellar keratoplasty with complete removal of pathological stroma for vision improvement. Br J Ophthalmol. 1997;81(3):184–188. doi: 10.1136/bjo.81.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Dooren BTH, Mulder PGH, Nieuwendaal CP, Beekhuis WH, Melles GRJ. Endothelial cell density after deep anterior lamellar keratoplasty: Melles Technique. Am J Ophthalmol. 2004;137(3):397–400. doi: 10.1016/j.ajo.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 15.Eiferman RA, Wilkins EL. The effect of air on human corneal endothelium. Am J Ophthalmol. 1981;92(3):328–331. doi: 10.1016/0002-9394(81)90520-1. [DOI] [PubMed] [Google Scholar]
- 16.Bohringer D, Reinhard T, Godehardt E, Sundmacher R. Regression analysis of chronic endothelial cell loss after penetrating normal risk keratoplasty: a retrospective clinical study. Klin Monatsbl Augenheilk. 2001;218(6):412–417. doi: 10.1055/s-2001-16254. [DOI] [PubMed] [Google Scholar]