Abstract
Rho-associated kinase (ROCK) is a serine/threonine kinase and one of the major downstream effectors of the small GTPase RhoA. The Rho/ROCK pathway is closely related to the pathogenesis of several central nervous system (CNS) disorders, and involved in many aspects of neuronal functions including neurite outgrowth and retraction. In the adult CNS, the damaged neuron regeneration is very difficult due to the presence of myelin-associated axon growth inhibitors such as Nogo, myelin-associated glycoprotein (MAG) and oligodendrocyte-myelin glycoprotein (Omgp), etc. The effects of these axon growth inhibitors are reversed by blocking the Rho/ROCK pathway in vitro, and the inhibition of Rho/ROCK pathway can promote axon regeneration and functional recovery in the injured CNS in vivo. In addition, the therapeutic effects of the Rho/ROCK inhibitors have also been demonstrated in some animal models and the Rho/ROCK pathway becomes an attractive target for the development of drugs for treating CNS disorders. In this review, we summarized on the effect of the Rho and the downstream factor ROCK in neural regeneration, and the potential therapeutic effect of Rho/ROCK inhibitors in the survival and axonal regeneration of retinal ganglion cells was also discussed.
Keywords: Rho/ROCK pathway, neural regeneration, potential therapeutic effect, optic nerve damage
INTRODUCTION
An important reason that axon regeneration is very difficult, following the grown-up mammal central nervous system (CNS) damaged, is due to the existence of some growth suppression molecules in the damaged environment. Until now had it mainly been discovered three kinds of molecules derived from myelin which can suppress axon growth: Nogo-A, myelin-associated glycoprotein (MAG) and oligodendrocyte-myelin glycoprotein (Omgp)[1]. The axon growth derived from the growth cone, and the growth cone can decide the direction of axon growth, as well as extend length by feeling information from the external environment[2]. After the growth cone contacts these suppression molecules in myelin, the cell skeleton structure changes, subsequently causes the growth cone to collapse, retract, and stop the axon growth[3]. At present, the functional mechanism of myelin-derived inhibitors is not completely clear. Rho is a member of small GTP enzyme of Rho family. Since the first time in 1985 Rho genes had been found[4], the pathophysiology of Rho and Rho-associated kinase (ROCK) has been conducted extensive research. Rho is a member of Rho subfamily of small molecular GTPases superfamily, and the mammalian Rho gene is homologous with the Ras superfamily. Previous studies have confirmed that Rho and their associated signaling molecules participate in and mediate the biological processes of axon regeneration, extension and fiber projection[5]-[8]. Rho regulates cell actin cytoskeleton by it's downstream effective factor ROCK, which extensively involve in the biological processes of cell migration, movement, apoptosis, gene transcription, nerve regeneration[9]. Previous researches indicate that Nogo-A, MAG, Omgp may activate Rho by common or different way, subsequently causes the growth cone collapse[10],[11]. This review summaries on the effect of the Rho and the downstream factor ROCK in neural regeneration.
Rho Profile
Rho is a member of Rho subfamily of small molecular GTPases superfamily related to Ras. Rho-GTP kinase, which includes three members: Rho, Rac and Cdc42, is closely related to the development of nerve[12]-[14]. They are considered as a molecular switch on the signal pathway of neuronal cell membrane surface receptor to actin skeleton, which has played a vital role in adjusting neuron development and axon construction[3]. Rho plays a key role in mediating the process of axon regeneration inhibitors which cause the growth cone collapse[15]-[17]. Rho has three kind of isomers: RhoA, RhoB and RhoC, mainly RhoA in neuron[18]. Rho normally exists in two forms: one is the non-activated form combined with GDP (Rho-GDP), and the other is the activated form combined with GTP (Rho-GTP)[19]. Rho realizes its molecular switch function by constantly transformation between two forms. At the same time, the combination state of Rho (Rho-GDP or Rho-GTP) is also regulated by many of regulatory proteins, in which guanine nucleotide exchange factors (GEFs)[20]-[22] GTPase-activating proteins (GAPs)[23]-[25] and GDP dissociation inhibitors (GDIs)[26],[27] play a key role. GEFs can urge Rho to release GDP and to unify GTP[20]-[22]. GAPs can stimulate the Rho activity of their own GTP molecules, which causes GTP to hydrolyze into GDP[23]-[25]. GDIs may suppress the transformation between Rho-GDP and Rho-GTP[26],[27]. The three types of protein interact with each other and regulate mutually the conversion of Rho between the two forms. Rho-associated kinase (ROCK) belongs to one of serine/threonine protein activating enzyme family members[28]. The Rho kinase has 2 kinds of isomers: ROCK-I and ROCK-II, 65% of amino acid sequence is the same, the similarity of activating enzyme area reaches as high as 92%[29]. Although their structure is very similar, the distribution of them in organization is relatively different, and their functions have also some differences. ROCK-I mRNA is ubiquitously expressed except in the brain and muscle, whereas ROCK-II mRNA is expressed abundantly in the brain, muscle, heart, lung and placenta[29]. The main biological effect of ROCK is to inactivate myosin light chain phosphatase (myosin light chain phosphatase, MLCP)[30]. MLCP causes phosphorylated myosin light chain to dephosphorylate, which is the main mechanism of contracted-MLC diastole. In the physiological situation, ROCK exists in the non-active form in the cytoplasm, and it may be activated by Rho and arachidonic acid[9],[31]. ROCK-I and ROCK-II are, at present, the most distinctive Rho downstream effect members that have researched ever[32]-[35]. After activated, Rho kinase leads to the phosphorylation and inactivation of myosin phosphatase, makes the extent of myosin phosphorylation increase, thus effects actin-myosin system which led to axon growth cone collapse and axon growth inhibition[32]-[37].
Inhibition Effect of Rho in Neuronal Regeneration
After central nervous system damaged, due to the lack of stimulating factor and a variety of inhibitory molecules in the environment, the axon regeneration process was blocked[38]-[41]. Some studies have also shown that many of extracellular information-oriented elements, including Nogo.A, MAG, Omgp can inhibit the growth of axons by activating the Rho-mediated signal transduction pathway[42]-[44]. Lingor et al[35] evaluated three inhibitors of ROCK, including Y-27632, fasudil (HA-1077), and dimethylfasudil (H-1152), in the models of neurite outgrowth in vitro. All three ROCK inhibitors partially restored neurite outgrowth of Ntera-2 neurons on the inhibitory chondroitin sulphate proteoglycan substrate. In the rat optic nerve crush model, Y-27632 dose-dependently increased regeneration of retinal ganglion cell axons in vivo. Application of dimethylfasudil showed a trend toward increasing axon regeneration in an intermediate concentration[35]. Recently, several studies suggested that oxygen-dependent gene expression was of crucial importance in governing the essential steps of neuronal regeneration, such as cell proliferation, survival and differentiation[45]-[47]. Pacary et al[45] analyzed the effect of the hypoxia inducible factor-1 (HIF-1) activation-mimicking agent CoCl2 on mesenchymal stem cells (MSC). CoCl2 treatment increased the expression of the anti-proliferative gene BTG2/PC3 and decreased cyclin D1 expression. Meanwhile, the expressions of HIF-1 alpha and its target genes erythropoietin (EPO), vascular endothelial growth factor (VEGF) and p21 were also upregulated. These changes were followed by inhibiting cell proliferation and morphological changes. Additionally, by using Y-27632, it demonstrated that Rho/ROCK inhibition potentiated CoCl2-induced MSC differentiation, in particular, into dopaminergic neuron-like cells as attested by its effect on tyrosine hydroxylase expression[45],[47]. Lingor et al[48] evaluated the role of pharmacological inhibition of Rho/ROCK and ciliary neurotrophic factor (CNTF), a potent neurotrophic factor for retinal ganglion cells, in the models of retinal ganglion cell apoptosis and neurite outgrowth regeneration in vitro and in vivo. It showed for the first time that the ROCK inhibitor Y-27632 significantly enhanced the survival of retinal ganglion cells in vitro and in vivo. In vitro, the co-application of CNTF and Y-27632 potentiated the effect of either substance alone. ROCK inhibition resulted in the activation of the intrinsic mitogen-activated protein kinases (MAPK) pathway[31],[48]-[50], and the combination of CNTF and Y-27632 resulted in even more pronounced MAPK activation[48]. In vivo, ROCK activity was also decreased in an additive manner by both substances. Both CNTF and Y-27632 enhanced the regeneration of RGC into the non-permissive optic nerve crush model and additive effects were observed after combination treatment[48]. Subsequent studies found that the promoting effect of Y-27632 and fasudil to axon regeneration had the time and dose-dependent[51],[52]. Histological analysis showed that, following the spinal cord injury for 4 weeks, local application of fasudil was invalid to axon regeneration and the restoration of motor function, which indicated that the activation of Rho/ROCK pathway mediated the irreversible inhibition effect of axon regeneration in the chronic phase of the central nervous system damaged[53]. These studies have shown that Rho/ROCK pathway plays an important role in mediating inhibitory signals to block the inhibition process of axon regeneration in neuron.
The Role of Rho in Actin Cytoskeleton and Neuronal Regeneration Inhibition
Rho has regulated many activities of cell functions which the actin involved in[54]. The studies in the neural cells have discovered that Rho may cause the growth cone to collapse by affecting the actin skeleton system[15],[55]-[57]. The growth cone is an active vehicle and locates in the expansion part of the terminal of neurite, its surface has the filiform pseudopod and the laminated pseudopod. The filiform pseudopod is the finger-liked protuberance stretched out from the growth cone, which can feel the information from the environment to guide forward the movement of the growth cone[58]. The actin micro silk is the main constitution part of filiform pseudopod, and it is also the main driving force for impelling its advance[58]. Prior to the significant morphogenesis changes of the apical cone, the cell skeleton system, including it's internal actin structures, has changed constantly through the gathering and the depolymerization of actin as well as the unceasing myosin participation, the retraction movements of the filiform pseudopod and the laminated pseudopod allow the apical cone to extend along the specific direction, by which Rho precisely affects the axon growth[58],[59]. Alabed et al[60] have identified the cytosolic phosphoprotein in collapsin-response mediator protein 4b (CRMP4b) as a protein that physically and functionally interacts with RhoA to mediate neurite outgrowth inhibition. Disruption of CRMP4b-RhoA binding with a competitive inhibitor attenuates neurite outgrowth inhibition on myelin and aggrecan substrate[60]. Stimulation of neuronal growth cones with Nogo leads to colocalization of CRMP4b and RhoA at discrete regions within the actin-rich central and peripheral domains of the growth cone, indicative of a potential function in cytoskeletal rearrangements during neurite outgrowth inhibition[60].
Sarasa-Renedo et al[61] found that the actin contractility controlled by RhoA/ROCK has a mechanosensory function in fibroblasts that correlates directly with tenascin-C gene expression. Previous RhoA/ROCK activation, either by chemical or mechanical signals, might render fibroblasts more sensitive to external tensile stress, on the contrary, RhoA/ROCK inhibitors can inhibit tenascin-C gene expression[61],[62].
Upstream and Downstream Signal Relation of Rho
As a molecular switch that connections molecular surface recceptor and the actin cell skeleton, Rho is playing the vital role in the adjustment of cell skeleton dynamics and the cell movement. Some studies have discovered that the activation of Rho plays an essential function in the mechanism of axon regeneration suppression members that cause the growth cone to collapse[43],[44], simultaneously blocking Rho or its downstream signal pathway can reverse the suppression effect of these suppression members against the axon regeneration[35], which shows that these suppression molecules possibly cause the apical cone collapse through Rho-mediated signal pathway[63]. To a great extent, this discoveries have promoted the research for the action mechanism of axon regeneration suppression molecules. Eickholt et al[64] have demonstrated that inactivation of p110delta in mice does not affect gross neuronal development, but leads to an increased vulnerability of dorsal root ganglia neurons to exhibit growth cone collapse and results in axon extension decrease. Loss of p110delta activity also dampened axon regeneration following peripheral nerve injury in adult mice and impaired functional recovery of locomotion. The inactivation of p110delta resulted in reducing neuronal signaling by the Akt protein kinase, and increasing the activity of the small GTPase RhoA[64],[65]. Pharmacological inhibition of ROCK, a downstream effector of RhoA, can restore axon extension defects in neurons with the inactive of p110delta, suggesting a key role of RhoA in p110delta signaling in neurons[64]. Kubo et al[66] have shown that blocking of the Rho/ROCK pathway can reverse the inhibitory effects of these inhibitors in vitro, and promote axon regeneration in vivo. Therefore, the inhibition of Rho/ROCK has a therapeutic potential against injuries to the human CNS, such as spinal cord injuries[66]. Fu et al[67] have reported that the non-steroidal anti-inflammatory drugs (NSAIDs) ibuprofen and indomethacin, can surmount axon growth restrictions from myelin and proteoglycans by potently inhibiting their downstream pathway ROCK. Systemic administration of ibuprofen to spinal cord lesion rodents reverses the activity of RhoA around injury area measured via Rho-GTP binding assay. Subcutaneous injections of ibuprofen via minipumps to rats with a thoracic spinal cord transection or contusion injury result in substantial corticospinal and serotonergic axon sprouting in the caudal spinal cord and promote locomotor functional recovery, even delaying the treatment 1 week after trauma. In contrast, the non-RhoA-inhibiting NSAID naproxen does not have the axon growth promoting effects on the cultured or lesioned neurons.[67] Gopalakrishnan et al[68] have found that in an in vitro model of the nerve growth factor-differentiated PC12 cell, the chondroitin sulfate proteoglycans (CSPG) can increase the phosphorylation of myosin phosphatase, which is a substrate immediately downstream of ROCK activation. Fasudil, dimethylfasudil and Y27632 can inhibit the phosphorylation of myosin phosphatase induced by CSGP. In addition, ROCK inhibitors can also reverse cofilin phosphorylation induced by CSPG. These data demonstrate that the interaction between CSPG and the ROCK pathway involves in the downstream effectors of ROCK, such as myosin phosphatase and cofilin[68]. Alabed et al[69] have provided the direct evidence that ROCK-II is activated in response to the myelin-associated inhibitor Nogo. Nogo enhances ROCK-II translocation to the cellular membrane of the PC12 cells and enhances ROCK-II kinase activity in vitro. In addition, Nogo also enhances the phosphorylation of myosin light chain II, a known ROCK substrate. Furthermore, the primary dorsal root ganglia neurons can be rendered insensitive to the inhibitory effects of myelin via infection with dominant negative ROCK[69].
Recently, Sagawa et al[70] investigated the effect of a novel ROCK inhibitor, Y-39983, on neurite regeneration in vitro and axon regeneration in the crushed cat optic nerve in vivo. Y-39983 was injected into the vitreous and the crushed site. An injection of 10 microM Y-39983 induced the crushed axons to regenerate and pass over the crush site. In contrast, very few axons passed beyond the crush site in the optic nerve with phosphate-buffered saline injection. The second injection of 10 microM Y-39983 on day 7 doubled the number of regenerated axons[70].
CONCLUSION AND PROSPECT
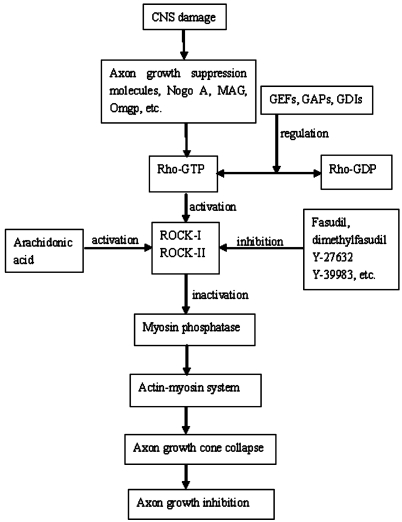
To sum up, some axon growth suppression molecules in the damaged environment, including Nogo.A, MAG, Omgp can inhibit the growth of axons by activating the Rho-mediated signal transduction pathway (Figure 1). Many of these processes demonstrate that the dynamic reorganization of actin cytoskeleton of which Rho signaling has now emerged as a major molecular switch. The involvement of dynamic changes of Rho GTPases in the axon regeneration underscores the need to produce effective inhibitors for their therapeutic applications[71]. Fasudil, Y-27632 and Y-39983, with many newer additions, are three classes of widely used chemical compounds that inhibit ROCK, an important downstream effector of RhoA subfamily GTPases. These inhibitors have been successful in some animal models, indicating the potential benefit of clinical Rho pathway inhibition. Because of a rapidly growing number of studies deciphering the role of the Rho proteins in CNS diseases, specific and potent pharmaceutical modulators of various steps of Rho GTPase signaling pathway are critically needed to target for therapeutic intervention in CNS disease.
Figure 1. Proposed Rho/ROCK pathway leading to axon growth inhibition in CNS damage.
Footnotes
Foundation items: Supported by National Nature Science Foundation of China (No.81070728); Shanghai “Science and Technology Innovation Action Plan” Basic Research Key Project, China (No.11JC1407700 and 11JC1407701); Shanghai Nature Science Foundation, China (No.08ZR1413900); Shanghai Leading Academic Discipline Project, China (No.S30205)
REFERENCES
- 1.Woolf CJ, Bloechlinger S. Neuroscience. It takes more than two to Nogo. Science. 2002;297(5584):1132–1134. doi: 10.1126/science.1076247. [DOI] [PubMed] [Google Scholar]
- 2.Laishram J, Avossa D, Shahapure R, Torre V. Mechanical computation in neurons. Dev Neurobiol. 2009;69(11):731–751. doi: 10.1002/dneu.20733. [DOI] [PubMed] [Google Scholar]
- 3.Hall A, Nobes CD. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos Trans R Soc Lond B Biol Sci. 2000;355(1399):965–970. doi: 10.1098/rstb.2000.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madaule P, Axel R. A novel ras-related gene family. Cell. 1985;41(1):31–40. doi: 10.1016/0092-8674(85)90058-3. [DOI] [PubMed] [Google Scholar]
- 5.Sivasankaran R, Pei J, Wang KC, Zhang YP, Shields CB, Xu XM, He Z. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat Neurosci. 2004;7(3):261–268. doi: 10.1038/nn1193. [DOI] [PubMed] [Google Scholar]
- 6.Zhou FQ, Walzer M, Wu YH, Zhou J, Dedhar S, Snider WD. Neurotrophins support regenerative axon assembly over CSPGs by an ECM-integrin-independent mechanism. J Cell Sci. 2006;119(Pt 13):2787–2796. doi: 10.1242/jcs.03016. [DOI] [PubMed] [Google Scholar]
- 7.Douglas MR, Morrison KC, Jacques SJ, Leadbeater WE, Gonzalez AM, Berry M, Logan A, Ahmed Z. Off-target effects of epidermal growth factor receptor antagonists mediate retinal ganglion cell disinhibited axon growth. Brain. 2009;132(Pt 11):3102–3121. doi: 10.1093/brain/awp240. [DOI] [PubMed] [Google Scholar]
- 8.Duffy P, Schmandke A, Schmandke A, Sigworth J, Narumiya S, Cafferty WB, Strittmatter SM. Rho-associated kinase II (ROCKII) limits axonal growth after trauma within the adult mouse spinal cord. J Neurosci. 2009;29(48):15266–15276. doi: 10.1523/JNEUROSCI.4650-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhadriraju K, Yang M, Alom Ruiz S, Pirone D, Tan J, Chen CS. Activation of ROCK by RhoA is regulated by cell adhesion, shape, and cytoskeletal tension. Exp Cell Res. 2007;313(16):3616–3623. doi: 10.1016/j.yexcr.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schimchowitsch S, Cassel JC. Polyamine and aminoguanidine treatments to promote structural and functional recovery in the adult mammalian brain after injury: a brief literature review and preliminary data about their combined administration. J Physiol Paris. 2006;99(2-3):221–231. doi: 10.1016/j.jphysparis.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Schweigreiter R, Walmsley AR, Niederöst B, Zimmermann DR, Oertle T, Casademunt E, Frentzel S, Dechant G, Mir A, Bandtlow CE. Versican V2 and the central inhibitory domain of Nogo-A inhibit neurite growth via p75NTR/NgR-independent pathways that converge at RhoA. Mol Cell Neurosci. 2004;27(2):163–174. doi: 10.1016/j.mcn.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Negishi M, Katoh H. Rho family GTPases and dendrite plasticity. Neuroscientist. 2005;11(3):187–191. doi: 10.1177/1073858404268768. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed I, Calle Y, Iwashita S, Nur-E-Kamal A. Role of Cdc42 in neurite outgrowth of PC12 cells and cerebellar granule neurons. Mol Cell Biochem. 2006;281(1-2):17–25. doi: 10.1007/s11010-006-0165-9. [DOI] [PubMed] [Google Scholar]
- 14.Causeret F, Hidalgo-Sanchez M, Fort P, Backer S, Popoff MR, Gauthier-Rouvière C, Bloch-Gallego E. Distinct roles of Rac1/Cdc42 and Rho/Rock for axon outgrowth and nucleokinesis of precerebellar neurons toward netrin 1. Development. 2004;131(12):2841–2852. doi: 10.1242/dev.01162. [DOI] [PubMed] [Google Scholar]
- 15.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279(5350):509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 16.Jiang P, Enomoto A, Takahashi M. Cell biology of the movement of breast cancer cells: intracellular signalling and the actin cytoskeleton. Cancer Lett. 2009;284(2):122–130. doi: 10.1016/j.canlet.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 17.Arnsdorf EJ, Tummala P, Kwon RY, Jacobs CR. Mechanically induced osteogenic differentiation--the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci. 2009;122(Pt 4):546–553. doi: 10.1242/jcs.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erschbamer MK, Hofstetter CP, Olson L. RhoA, RhoB, RhoC, Rac1, Cdc42, and Tc10 mRNA levels in spinal cord, sensory ganglia, and corticospinal tract neurons and long-lasting specific changes following spinal cord injury. J Comp Neurol. 2005;484(2):224–233. doi: 10.1002/cne.20471. [DOI] [PubMed] [Google Scholar]
- 19.Fauré J, Dagher MC. Interactions between Rho GTPases and Rho GDP dissociation inhibitor (Rho-GDI) Biochimie. 2001;83(5):409–414. doi: 10.1016/s0300-9084(01)01263-9. [DOI] [PubMed] [Google Scholar]
- 20.Estrach S, Schmidt S, Diriong S, Penna A, Blangy A, Fort P, Debant A. The Human Rho-GEF trio and its target GTPase RhoG are involved in the NGF pathway, leading to neurite outgrowth. Curr Biol. 2002;12(4):307–312. doi: 10.1016/s0960-9822(02)00658-9. [DOI] [PubMed] [Google Scholar]
- 21.Overbeck AF, Brtva TR, Cox AD, Graham SM, Huff SY, Khosravi-Far R, Quilliam LA, Solski PA, Der CJ. Guanine nucleotide exchange factors: activators of Ras superfamily proteins. Mol Reprod Dev. 1995;42(4):468–476. doi: 10.1002/mrd.1080420415. [DOI] [PubMed] [Google Scholar]
- 22.Blomquist A, Schwörer G, Schablowski H, Psoma A, Lehnen M, Jakobs KH, Rümenapp U. Identification and characterization of a novel Rho-specific guanine nucleotide exchange factor. Biochem J. 2000;352 Pt 2:319–325. [PMC free article] [PubMed] [Google Scholar]
- 23.Moon SY, Zheng Y. Rho GTPase-activating proteins in cell regulation. Trends Cell Biol. 2003 Jan;13(1):13–22. doi: 10.1016/s0962-8924(02)00004-1. [DOI] [PubMed] [Google Scholar]
- 24.Brinkmann T, Daumke O, Herbrand U, Kühlmann D, Stege P, Ahmadian MR, Wittinghofer A. Rap-specific GTPase activating protein follows an alternative mechanism. J Biol Chem. 2002;277(15):12525–12531. doi: 10.1074/jbc.M109176200. [DOI] [PubMed] [Google Scholar]
- 25.Kozma R, Ahmed S, Best A, Lim L. The GTPase-activating protein n-chimaerin cooperates with Rac1 and Cdc42Hs to induce the formation of lamellipodia and filopodia. Mol Cell Biol. 1996;16(9):5069–5080. doi: 10.1128/mcb.16.9.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiao J, Holian O, Lee BS, Huang F, Zhang J, Lum H. Phosphorylation of GTP dissociation inhibitor by PKA negatively regulates RhoA. Am J Physiol Cell Physiol. 2008;295(5):C1161–1168. doi: 10.1152/ajpcell.00139.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15(7):356–363. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Riento K, Totty N, Villalonga P, Garg R, Guasch R, Ridley AJ. RhoE function is regulated by ROCK I-mediated phosphorylation. EMBO J. 2005;24(6):1170–1180. doi: 10.1038/sj.emboj.7600612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392(2):189–193. doi: 10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Zheng XR, Riddick N, Bryden M, Baur W, Zhang X, Surks HK. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ Res. 2009;104(4):531–540. doi: 10.1161/CIRCRESAHA.108.188524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia MC, Ray DM, Lackford B, Rubino M, Olden K, Roberts JD. Arachidonic acid stimulates cell adhesion through a novel p38 MAPK-RhoA signaling pathway that involves heat shock protein 27. J Biol Chem. 2009;284(31):20936–20945. doi: 10.1074/jbc.M109.020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bito H, Furuyashiki T, Ishihara H, Shibasaki Y, Ohashi K, Mizuno K, Maekawa M, Ishizaki T, Narumiya S. A critical role for a Rho-associated kinase, p160ROCK, in determining axon outgrowth in mammalian CNS neurons. Neuron. 2000;26(2):431–441. doi: 10.1016/s0896-6273(00)81175-7. [DOI] [PubMed] [Google Scholar]
- 33.Nikolic M. The role of Rho GTPases and associated kinases in regulating neurite outgrowth. Int J Biochem Cell Biol. 2002;34(7):731–745. doi: 10.1016/s1357-2725(01)00167-4. [DOI] [PubMed] [Google Scholar]
- 34.Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003;23(4):1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lingor P, Teusch N, Schwarz K, Mueller R, Mack H, Bähr M, Mueller BK. Inhibition of Rho kinase (ROCK) increases neurite outgrowth on chondroitin sulphate proteoglycan in vitro and axonal regeneration in the adult optic nerve in vivo. J Neurochem. 2007;103(1):181–189. doi: 10.1111/j.1471-4159.2007.04756.x. [DOI] [PubMed] [Google Scholar]
- 36.Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci. 2002;22(15):6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wettschureck N, Offermanns S. Rho/Rho-kinase mediated signaling in physiology and pathophysiology. J Mol Med. 2002;80(10):629–638. doi: 10.1007/s00109-002-0370-2. [DOI] [PubMed] [Google Scholar]
- 38.Jones LL, Sajed D, Tuszynski MH. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J Neurosci. 2003;23(28):9276–9288. doi: 10.1523/JNEUROSCI.23-28-09276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7(8):617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Filbin MT. Recapitulate development to promote axonal regeneration: good or bad approach? Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1565–1574. doi: 10.1098/rstb.2006.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen ZJ, Ughrin Y, Levine JM. Inhibition of axon growth by oligodendrocyte precursor cells. Mol Cell Neurosci. 2002;20(1):125–139. doi: 10.1006/mcne.2002.1102. [DOI] [PubMed] [Google Scholar]
- 42.Bouquier N, Vignal E, Charrasse S, Weill M, Schmidt S, Léonetti JP, Blangy A, Fort P. A cell active chemical GEF inhibitor selectively targets the Trio/RhoG/Rac1 signaling pathway. Chem Biol. 2009;16(6):657–666. doi: 10.1016/j.chembiol.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Kang MJ, Seo JS, Park WY. Caveolin-1 inhibits neurite growth by blocking Rac1/Cdc42 and p21-activated kinase 1 interactions. Neuroreport. 2006;17(8):823–827. doi: 10.1097/01.wnr.0000220139.83671.60. [DOI] [PubMed] [Google Scholar]
- 44.Niederöst B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci. 2002;22(23):10368–10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pacary E, Legros H, Valable S, Duchatelle P, Lecocq M, Petit E, Nicole O, Bernaudin M. Synergistic effects of CoCl2 and ROCK inhibition on mesenchymal stem cell differentiation into neuron-like cells. J Cell Sci. 2006;119(Pt 13):2667–2678. doi: 10.1242/jcs.03004. [DOI] [PubMed] [Google Scholar]
- 46.Pacary E, Tixier E, Coulet F, Roussel S, Petit E, Bernaudin M. Crosstalk between HIF-1 and ROCK pathways in neuronal differentiation of mesenchymal stem cells, neurospheres and in PC12 neurite outgrowth. Mol Cell Neurosci. 2007;35(3):409–423. doi: 10.1016/j.mcn.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Pacary E, Petit E, Bernaudin M. Concomitant inhibition of prolyl hydroxylases and ROCK initiates differentiation of mesenchymal stem cells and PC12 towards the neuronal lineage. Biochem Biophys Res Commun. 2008;377(2):400–406. doi: 10.1016/j.bbrc.2008.09.145. [DOI] [PubMed] [Google Scholar]
- 48.Lingor P, Tönges L, Pieper N, Bermel C, Barski E, Planchamp V, Bähr M. ROCK inhibition and CNTF interact on intrinsic signalling pathways and differentially regulate survival and regeneration in retinal ganglion cells. Brain. 2008;131(Pt 1):250–263. doi: 10.1093/brain/awm284. [DOI] [PubMed] [Google Scholar]
- 49.Khatiwala CB, Kim PD, Peyton SR, Putnam AJ. ECM compliance regulates osteogenesis by influencing MAPK signaling downstream of RhoA and ROCK. J Bone Miner Res. 2009;24(5):886–898. doi: 10.1359/JBMR.081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodrigues-Díez R, Carvajal-González G, Sánchez-López E, Rodríguez-Vita J, Rodrigues Díez R, Selgas R, Ortiz A, Egido J, Mezzano S, Ruiz-Ortega M. Pharmacological modulation of epithelial mesenchymal transition caused by angiotensin II. Role of ROCK and MAPK pathways. Pharm Res. 2008;25(10):2447–2461. doi: 10.1007/s11095-008-9636-x. [DOI] [PubMed] [Google Scholar]
- 51.Chan CC, Khodarahmi K, Liu J, Sutherland D, Oschipok LW, Steeves JD, Tetzlaff W. Dose-dependent beneficial and detrimental effects of ROCK inhibitor Y27632 on axonal sprouting and functional recovery after rat spinal cord injury. Exp Neurol. 2005;196(2):352–364. doi: 10.1016/j.expneurol.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Z, Ottens AK, Larner SF, Kobeissy FH, Williams ML, Hayes RL, Wang KK. Direct Rho-associated kinase inhibition [correction of inhibiton] induces cofilin dephosphorylation and neurite outgrowth in PC-12 cells. Cell Mol Biol Lett. 2006;11(1):12–29. doi: 10.2478/s11658-006-0002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishio Y, Koda M, Kitajo K, Seto M, Hata K, Taniguchi J, Moriya H, Fujitani M, Kubo T, Yamashita T. Delayed treatment with Rho-kinase inhibitor does not enhance axonal regeneration or functional recovery after spinal cord injury in rats. Exp Neurol. 2006;200(2):392–397. doi: 10.1016/j.expneurol.2006.02.123. [DOI] [PubMed] [Google Scholar]
- 54.Santarius M, Lee CH, Anderson RA. Supervised membrane swimming: small G-protein lifeguards regulate PIPK signalling and monitor intracellular PtdIns(4,5)P2 pools. Biochem J. 2006;398(1):1–13. doi: 10.1042/BJ20060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kubo T, Endo M, Hata K, Taniguchi J, Kitajo K, Tomura S, Yamaguchi A, Mueller BK, Yamashita T. Myosin IIA is required for neurite outgrowth inhibition produced by repulsive guidance molecule. J Neurochem. 2008;105(1):113–126. doi: 10.1111/j.1471-4159.2007.05125.x. [DOI] [PubMed] [Google Scholar]
- 56.Shi L, Fu WY, Hung KW, Porchetta C, Hall C, Fu AK, Ip NY. Alpha2-chimaerin interacts with EphA4 and regulates EphA4-dependent growth cone collapse. Proc Natl Acad Sci U S A. 2007;104(41):16347163–52. doi: 10.1073/pnas.0706626104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gallo G, Letourneau PC. Regulation of growth cone actin filaments by guidance cues. J Neurobiol. 2004;58(1):92–102. doi: 10.1002/neu.10282. [DOI] [PubMed] [Google Scholar]
- 58.Withers GS, James CD, Kingman CE, Craighead HG, Banker GA. Effects of substrate geometry on growth cone behavior and axon branching. J Neurobiol. 2006;66(11):1183–1194. doi: 10.1002/neu.20298. [DOI] [PubMed] [Google Scholar]
- 59.Thies E, Davenport RW. Independent roles of Rho-GTPases in growth cone and axonal behavior. J Neurobiol. 2003;54(2):358–369. doi: 10.1002/neu.10135. [DOI] [PubMed] [Google Scholar]
- 60.Alabed YZ, Pool M, Ong Tone S, Fournier AE. Identification of CRMP4 as a convergent regulator of axon outgrowth inhibition. J Neurosci. 2007;27(7):1702–1711. doi: 10.1523/JNEUROSCI.5055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sarasa-Renedo A, Tunç-Civelek V, Chiquet M. Role of RhoA/ROCK-dependent actin contractility in the induction of tenascin-C by cyclic tensile strain. Exp Cell Res. 2006;312(8):1361–1370. doi: 10.1016/j.yexcr.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 62.Maier S, Lutz R, Gelman L, Sarasa-Renedo A, Schenk S, Grashoff C, Chiquet M. Tenascin-C induction by cyclic strain requires integrin-linked kinase. Biochim Biophys Acta. 2008;1783(6):1150–1162. doi: 10.1016/j.bbamcr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 63.Borisoff JF, Chan CC, Hiebert GW, Oschipok L, Robertson GS, Zamboni R, Steeves JD, Tetzlaff W. Suppression of Rho-kinase activity promotes axonal growth on inhibitory CNS substrates. Mol Cell Neurosci. 2003;22(3):405–416. doi: 10.1016/s1044-7431(02)00032-5. [DOI] [PubMed] [Google Scholar]
- 64.Eickholt BJ, Ahmed AI, Davies M, Papakonstanti EA, Pearce W, Starkey ML, Bilancio A, Need AC, Smith AJ, Hall SM, Hamers FP, Giese KP, Bradbury EJ, Vanhaesebroeck B. Control of axonal growth and regeneration of sensory neurons by the p110delta PI 3-kinase. PLoS One. 2007;2(9):e869. doi: 10.1371/journal.pone.0000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Papakonstanti EA, Ridley AJ, Vanhaesebroeck B. The p110delta isoform of PI 3-kinase negatively controls RhoA and PTEN. EMBO J. 2007;26(13):3050–3061. doi: 10.1038/sj.emboj.7601763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kubo T, Hata K, Yamaguchi A, Yamashita T. Rho-ROCK inhibitors as emerging strategies to promote nerve regeneration. Curr Pharm Des. 2007;13(24):2493–2499. doi: 10.2174/138161207781368657. [DOI] [PubMed] [Google Scholar]
- 67.Fu Q, Hue J, Li S. Nonsteroidal anti-inflammatory drugs promote axon regeneration via RhoA inhibition. J Neurosci. 2007;27(15):4154–4164. doi: 10.1523/JNEUROSCI.4353-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gopalakrishnan SM, Teusch N, Imhof C, Bakker MH, Schurdak M, Burns DJ, Warrior U. Role of Rho kinase pathway in chondroitin sulfate proteoglycan-mediated inhibition of neurite outgrowth in PC12 cells. J Neurosci Res. 2008;86(10):2214–2226. doi: 10.1002/jnr.21671. [DOI] [PubMed] [Google Scholar]
- 69.Alabed YZ, Grados-Munro E, Ferraro GB, Hsieh SH, Fournier AE. Neuronal responses to myelin are mediated by rho kinase. J Neurochem. 2006;96(6):1616–1625. doi: 10.1111/j.1471-4159.2006.03670.x. [DOI] [PubMed] [Google Scholar]
- 70.Sagawa H, Terasaki H, Nakamura M, Ichikawa M, Yata T, Tokita Y, Watanabe M. A novel ROCK inhibitor, Y-39983, promotes regeneration of crushed axons of retinal ganglion cells into the optic nerve of adult cats. Exp Neurol. 2007;205(1):230–240. doi: 10.1016/j.expneurol.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 71.Lu Q, Longo FM, Zhou H, Massa SM, Chen YH. Signaling through Rho GTPase pathway as viable drug target. Curr Med Chem. 2009;16(11):1355–1365. doi: 10.2174/092986709787846569. [DOI] [PMC free article] [PubMed] [Google Scholar]