Abstract
AIM
To investigate the antifibrotic effect of freeze-dried bilayered fibrin-binding amniotic membrane on trabeculectomy in a rabbit model.
METHODS
Twenty-four Japanese white rabbits were randomized into three groups: the experimental group (ocular trabeculectomy in combination with freeze-dried bilayered fibrin-binding amniotic membrane transplantation), the control group (ocular trabeculectomy in combination with natural bilayered fibrin-binding amniotic membrane) and the blank group (single trabeculectomy). Clinical observation, hematoxylin-eosin staining, Massion staining, real-time PCR and immunohistochemistry for α-SMA were performed on days 7, 14, 21 and 30 following surgery.
RESULTS
Statistical differences were noted in survival analysis and intraocular pressure (IOP) among groups on days 7, 14, 21 and 30 following surgery. Histology, immunohistochemistry and real-time PCR further demonstrated that trabeculectomy in combination with freeze-dried bilayered fibrin-binding amniotic membrane resulted in good wound healing and no scar formation.
CONCLUSION
Self-made freeze-dried bilayered fibrin-binding amniotic membrane may inhibit the formation of scarring in glaucoma after trabeculectomy.
Keywords: freeze-dried, fibrin-binding, amniotic membrane, trabeculectomy
INTRODUCTION
The amniotic membrane, as a biological material for transplantation, has demonstrated anti-inflammatory and anti-fibrotic effects. Recently, it has been used widely in the treatment of ocular disease. The fibrin glue is a biological agent, generally applied in general, cerebral and gynecological and obstetric surgeries for hemostasis and blockage of local tissues. Application in ophthalmology has also been reported in recent years. This natural high-molecular material originates from mammal (cattle) and human serum. The amniotic membrane blocks the passage of air and fluid by imitating the ultimate stage of the hemagglutination cascade reaction and by forming a stable blood clot. It is easily absorbable, non-toxic and biocompatible. Glaucoma irreversibly induces blindness; surgery is the optimal treatment. However, surgery-induced scar formation in the filtration passageway may result in treatment failure and refractory glaucoma. Although antimetabolites, such as 5-Fu and mitomycin, increase the rate of surgical success[1], these drugs may affect the integrity of conjunctival flaps, thereby resulting in a series of complications, including low intraocular pressure (IOP), filtration blebs in the thin wall, wound leakage, infection of late-filtration blebs and endophthalmitis[2],[3]. In glaucoma surgeries, the amniotic membrane reduces scar formation in the filtration passageway and maintains the integrity of conjunctival flaps. The most commonly used amniotic membrane is currently the unilayered amniotic membrane, which is generally preserved in purified glycerol. This limits use of the membrane by physicians, due to the short storage period, inconvenience of delivery and rapid nature of degradation. Recent clinical studies have sought to decrease scars in the filtration passageway, maintain the patency of the filtration passageway and the integrity of conjunctival flaps and reduce the long-term complications of antimetabolites. Freeze-drying technology allows for preservation at room temperature. We developed a subconjunctival flap freeze-dried fibrin-binding bilayered amniotic membrane by binding the fibrin glue and amniotic membrane. In this article, ocular trabeculectomy followed by freeze-dried fibrin-binding amniotic membrane transplantation was compared with trabeculectomy in combination with subconjunctival flap natural bilayered amniotic membrane and single trabeculectomy.
MATERIALS AND METHODS
Materials
A total of 24 adult male or female Japanese rabbits weighing 2-3kg were provided by the Animal Experiment Center, Huazhong University of Science and Technology, Tongji Medical College. The experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology Statement for the use of animals in ophthalmic and vision research. The rabbits were randomly divided into three groups: the experimental group (16 eyes and 8 animals, ocular trabeculectomy in combination with subconjunctival flap freeze-dried bilayered fibrin-binding amniotic membrane transplantation), the control group (16 eyes and 8 animals, ocular trabeculectomy in combination with subconjunctival flap natural bilayered fibrin-binding amniotic membrane) and the blank control group (16 eyes and 8 animals, single trabeculectomy).
Methods
Experimental animals were anesthetized with an intramuscular injection of 50mg/kg ketamine and 15mg/kg xylazine and a subconjunctival injection of 1% lidocaine. The conjunctival flap was created with the fornix as the base, and the scleral flap was created with the corneoscleral limbus as the base to be 3mm×3mm in size. In the experimental group, rehydrated bilayered freeze-dried fibrin-binding amniotic membrane was placed above the scleral flap and below the conjunctival flap, then sutured four times with 10-0 silk sutures. In the control group, rehydrated bilayered natural fibrin-binding amniotic membrane was placed above the scleral flap and below the conjunctival flap, and then sutured four times with 10-0 silk sutures. In the blank group, only ocular trabeculectomy was performed.
Origin and preparation of amniotic membrane
The study was performed with proper informed consent in accordance with the tenets of the Declaration of Helsinki for research involving human subjects and approval by the Institutional Review Board of Tongji Medical College, Huazhong University of Science and Technology. Fresh placentas were collected from pregnant women with no infectious diseases (e.g., viral hepatitis, acquired immune deficiency syndrome, syphilis) or systemic diseases who underwent caesarean section. Blood on the surface of the amniotic membrane was rinsed with normal saline under sterile conditions. Then, the amniotic membrane was soaked in aseptic normal saline containing antibiotics (50µg/mL penicillin, 50µg/streptomycin, 100µg/neomycin and 2.5µg/amphotericin B) for 20 minutes and was bluntly dissected from the chorion. The amniotic membrane was laid over acetate fiber paper with the epithelium surface up; both were then placed in a container containing disinfectant purified glycerine. After 24 hours, the contents were transferred to another purified glycerine bottle. The bottle was then sealed and stored at 4°C.
Preparation of bilayered freeze-dried fibrin-binding amniotic membrane
Two amniotic membranes were aseptically removed from the glycerine bottle and, after rehydration, were placed in 0.9% normal saline for 20 minutes. Then, the membrane was cut into small pieces of 3.0×3.0cm. The amniotic membrane was positioned with the basement membrane downward; the epithelium was covered evenly with fibrin glue, which was then placed in full contact with the basement membrane of another amniotic membrane. Next, samples were placed in the refrigerator at -80°C for 12 hours, transferred to a freeze-dryer for 12 hours and vacuum-packed at room temperature. Eventually, samples were sterilized with a Cobalt 60 ray.
Preparation of bilayered natural fibrin-binding amniotic membrane
Two amniotic membranes were aseptically removed from the glycerine bottle and rehydrated sufficiently in 0.9% normal saline for 20 minutes. Then, the membrane was cut into small pieces of 3.0×3.0cm. The amniotic membrane was laid on the table with the basement membrane downward, and the epithelium was covered evenly with fibrin glue and then placed in full contact with the basement membrane of another amniotic membrane. Next, samples were placed in the thermostate at 37°C and then vacuum-packed at room temperature (Figure 1). Finally, samples were sterilized with a cobalt 60 ray.
Figure 1. Freeze-dried bilayered fibrin-binding amniotic membrane.
Survival Analysis
Survival analysis was observed on postoperative 7, 14, 21 and 30 days; Bivariate analysis was applied. The survival analysis was illustrated using a Kaplan Meier plot.
IOP measurement
IOP was measured before and on 7, 14, 21 and 30 days after filtering surgery using an applanation tonometer (Tono-Pen XL, Mentor, USA), with rabbits under local anesthesia.
Histology analysis
Eight rabbits were taken from each group on postoperative 7, 14, 21 and 30 days and were sacrificed by injecting the lethal dose of pentobarbital sodium (100mg/kg i.v.). Eyeballs were removed. Filtration blebs of 10mm×10mm were collected and then fixed with 4% paraformaldehyde overnight. Samples were taken along the vertical plane of the filtration region and embedded with paraffin wax followed by serial section, HE staining and Massion staining, as well as immunohistochemistry for α-SMA. HE staining: deparaffinization, staining, dehydration and mounting. Massion's trichrome staining: 1) Deparaffinize and hydrate to distilled water; 2) Stain with hematoxylin for 5-10 minutes; 3) Place in hydrochloric acid-alcohol; 4) Stain with ponceau-acid fuchsin for 5-8 minutes; 5) Rinse in distilled water; 6) Stain in phosphomolybdic acid for 1-3 minutes; 7) Immerse in aniline blue for 5 minutes; 8) Immerse in 1% glacial acetic acid for 1 minute; 9) Dehydrate with alcohol and xylene, and mount with neutral gum. Immunohistochemical staining: 1) Deparaffinize; 2) Incubate in 3% H2O2 at room temperature for 10 minutes; 3) Soak in TBS for 5 minutes; 4) Rinse in distilled water for 5 minutes; 5) Retrieve antigens; 6) Immerse in normal goat serum for 15 minutes at room temperature; 7) Place in 0.01mol/L citrate buffer fluid (pH 6.0) and expose to heat; 8) Cool at room temperature and rinse for 5 minutes three times; 9) Add 40µL of the biotin-labeled secondary antibody and incubate at 37°C for 15 minutes; then add 40µL of horseradish peroxidase-labeled streptavidin and follow the same procedure; 10) Colorate with DAB for 2-5 minutes, counterstain with hematoxylin over 2-5 minutes and ablate with hydrochloric acid-alcohol; 11) Dehydrate, clear, mount and examine under a microscope.
Real-time quantitative reverse transcription-polymerase chain reaction
Rabbits were sacrificed at the end of the follow-up period by intracardiac injection of a lethal dose of pentobarbital under deep general anesthesia (100mg/kg i.v.). Eyes were enucleated immediately thereafter. Tissues were harvested from the bleb area (including conjunctiva, Tenon's capsule and sclera) before surgery in the control rabbits and at 7, 14, 21 and 30 days after surgery in the remaining rabbits. For RNA extraction, tissues were immediately frozen and stored at -80°C. Tissues from each time point were homogenized in 1mL extraction reagent (Trizol; Invitrogen, Wuhan Life Technology Co. Ltd, China) according to the manufacturer's suggested protocol. cDNA was synthesized using a cDNA synthesis kit (Toyobo Co. Ltd, Life Science Department, Osaka, Japan). The rabbit mRNA sequences for CTGF and β-actin were downloaded from GenBank (http://www.ncbi.nlm.nih.gov/GenBank/). A computer program (Premier 5.0 Biosoft International, Palo Alto, CA) was used to design the primer sequences. The following primers were used: connective tissue growth factor (CTGF), 5′-CCCTGCGTCTTCGGT GGC-3′ (forward) and 5′-AGGCAGTTGGCTCGCATCAT-3′(reverse); β-actin, 5′-CGAGATCGTGCGGGACAT-3′ (forward)and 5′-CAGGAAGGAGGGC-TGGAAC-3′(reverse). Real-time quantitative RT-PCR was performed in a real-time PCR detection system (SLAN Hongshi Medical Technology Co. Ltd, Shanghai, China). The amplification reaction mixture contained cDNA, sense and antisense primers and SYBR green master mix (Toyobo Co. Ltd, Life Science Department, Osaka, Japan) in a total volume of 25µL. The real-time PCR detection system parameters were as follows: an initial ramp to 95°C followed by denaturation at 95°C for 2 minutes, 40 cycles of denaturation at 95°C for 15 seconds and annealing at 60°C for 15 seconds. At the end of each of the 40 cycles, fluorescence of SYBR green was measured at an excitation wavelength of 470nm and an emission wavelength of 530nm. To analyze results after real-time RT-PCR experiments, we performed relative quantitation of the target gene transcript in comparison to the reference gene transcript β-actin using the 2−▵▵Ct mathematical model, as previously described[4].
Statistical Analysis
Two-factor variance analysis was used to compare survival analysis and IOP; single-factor variance analysis was used to detect CTGF mRNA. Survival analyses were performed for bleb failure. A log-rank test was used to test for overall survival differences.A Kaplan-Meier survival plot was used to represent the survival data. P<0.05 was considered significant.
RESULTS
Survival Analysis
Survival time of filtration blebs was analyzed with survival analysis. Differences among groups were analyzed using the log-rank test. A Kaplan Meier survival curve was plotted. In the blank and control groups, 50% and 80% of filtration blebs disappeared on postoperative 5 days, respectively; 100% had disappeared by post-operation days 15 and 20, respectively. In the experimental group, survival time of filtration blebs was prolonged significantly until post-operation day 10 to 25. Log-rank test revealed statistical differences in survival time of filtration blebs among the three groups (P=0.002), with the maximum value observed in the experimental group (Figure 2).
Figure 2. Survival analysis in three groups.
Blue: trabeculectomy in combination with freeze-dried bilayered fibrin-binding amniotic membrane transplantation; Yellow: trabeculectomy and natural bilayered fibrin-binding amniotic membrane; Grey: single trabeculectomy
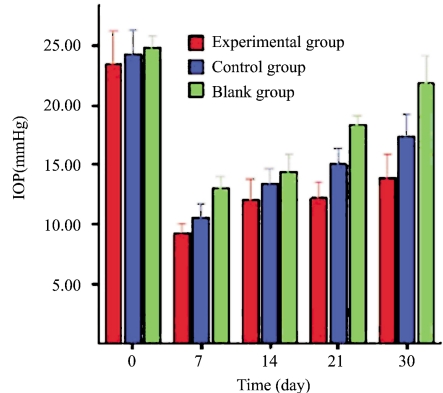
IOP Measurement
IOP was measured at the same time points before the surgery and on days 7, 14, 21 and 30 after filtering surgery using an applanation tonometer (Tono-Pen XL, Mentor, USA). The average IOP of the three measurements was recorded (Figure 3). Bivariate analysis revealed significant differences among the experimental, control and blank groups (P<0.001). Further pairwise comparison using the LSD method demonstrated statistical differences between the experimental and control groups (P<0.001), between the experimental and blank groups (P<0.001) and between the control and blank groups (P<0.001).
Figure 3. IOP measurement in three groups (pairwise comparison using the LSD method).
Red: trabeculectomy in combination with freeze-dried bilayered fibrin-binding amniotic membrane transplantation; Blue: trabeculectomy in combination with natural bilayered fibrin-binding amniotic membrane; Green: single trabeculectomy
HE Staining
Fourteen days after surgery light microscopy revealed a patent filtration passageway, filtration blebs and partly lytic amniotic membrane, as well as a lack of significant granulation tissue and fibroblasts, in the experimental group. In the control group, only a small quantity of fibroblasts was observed, with the majority of the amniotic membrane having dissolved. In the blank group, a great deal of granulation tissue filled in the gap between the filtration passageway, conjunctiva and sclera with many fibroblasts visible (Figure 4).
Figure 4. HE staining on day 14 post-surgery.
A and B: HE staining on pathological slices of surgical corneosclera on day 14 following trabeculectomy in combination with freeze-dried bilayered fibrin-binding amniotic membrane transplantation; C and D: HE staining on pathological slices of surgical corneosclera on day 14 following trabeculectomy in combination with natural bilayered fibrin-binding amniotic membrane transplantation; E and F: HE staining on pathological slices of surgical corneosclera on day 14 following single trabeculectomy (significant filtration blebs and passageway in images A, B, C and D with the amniotic membrane present; E and F depict scar formation in the filtration passageway).
Massion Staining
Under light microscopy, a small quantity of collagen was seen in the filtration passageway and conjunctiva. The amniotic membrane was integrated 14 days after filtrating surgery in the experimental and control groups. A great deal of collagen was observed on day 14 in the blank group (Figure 5). On day 30 after surgery, a limited amount of collagen was found in the filtration passageway and conjunctiva in the experimental and control groups, with amniotic membrane present in the experimental group and having dissolved completely in the control group. Abundant collagen was present in the filtration passageway and conjunctiva (Figure 6).
Figure 5. Massion staining on day 14 post-surgery.
A and B: Massion trichrome staining on pathological slices of surgical corneosclera on day 14 following trabeculectomy in combination with freeze-dried bilayered fibrin-binding amniotic membrane transplantation; C and D: Massion trichrome staining on pathological slices of surgical corneosclera on day 14 following trabeculectomy in combination with natural bilayered fibrin-binding amniotic membrane transplantation; E and F: Massion trichrome staining on pathological slices of surgical corneosclera on day 14 following single trabeculectomy (a small quantity of collagen is seen in filtration blebs and passageways in integrated amniotic membrane in images A, B, C and D; abundant collagen is observed in the filtration passageway in images E and F)
Figure 6. Massion staining on day 30 post-surgery.
A and B: Massion trichrome staining on pathological slices of surgical corneosclera on day 30 following trabeculectomy in combination with freeze-dried bilayered fibrin-binding amniotic membrane transplantation; C and D: Massion trichrome staining on pathological slices of surgical corneosclera on day 30 following trabeculectomy in combination with natural bilayered fibrin-binding amniotic membrane transplantation; E and F: Massion trichrome staining on pathological slices of surgical corneosclera on day 30 following single trabeculectomy (the amniotic membrane and significant filtration blebs and passageways in images A and B, lack of an amniotic membrane and significant filtration blebs and passageways in images C and D and abundant collagen deposited in the subconjunctival filtration passageway in images E and F)
Immunohistochemistry for α-SMA
Light microscopy revealed α-SMA expression on day 14 following surgery to be rare in the experimental group, limited in the control group and abundant in the blank group (Figure 7).
Figure 7. Immunohistochemical staining : expression of α-SMA on day 14 post-surgery.
A: Weak α-SMA expression after trabeculectomy in combination with freeze-dried bilayered fibrin-binding amniotic membrane transplantation; B: Limited α-SMA expression after trabeculectomy in combination with natural bilayered fibrin-binding amniotic membrane; C: Abundant α-SMA expression after single trabeculectomy
mRNA expression of CTGF
Figure 8 presents peak expression on day 14 in all groups; the experimental group <control group <blank group at every time point. The difference was statistically significant (P<0.05).
Figure 8. mRNA expression of CTGF in the ocular surgical area of rabbits in three groups on day 14 post-surgery.
Red: trabeculectomy in combination with freeze-dried bilayered fibrin-binding amniotic membrane transplantation; Blue: trabeculectomy in combination with natural bilayered fibrin-binding amniotic membrane transplantation; Green: single trabeculectomy
DISCUSSION
In our experiment, freeze-dried technology and fibrin-binding bilayered amniotic membrane were combined to investigate the effect on patency of the filtration passageway after trabeculectomy. Currently, the most commonly applied therapy for glaucoma is filtration surgery. The long-term success rate of this approach is only 75%, which is mainly attributed to the following reasons: first, in the early wound-healing stage, direct damage to vessels by surgery and local inflammation increases the permeability of vessels, leading to leakage of plasma proteins, platelets and blood cells, and leakage and deposition in the filtration passageway, leading to the creation of a blood clot and obstructed drainage of aqueous humor[5]. Second, surgical trauma induces the proliferation of multiple fibroblasts and cytokines during scar formation. Surgical trauma is also directly responsible for fibroblast proliferation, scar formation and blocked drainage of the aqueous humor[6]. The amniotic membrane is the innermost layer of the placenta and contains no blood or lymphatic vessels. Amniotic membrane is an excellent biological membrane due to the low degree of immunogenicity[7],[8]. Recent studies showed that the amniotic membrane plays an important role in the repair of ocular defects[9],[10]. Kim and Tseng first reported its application in an experimental alkaline burn model[11]-[15].
Survival time of filtration blebs is the key ensuring successful ocular trabeculectomy. The log-rank test and Kaplan-Meier survival curve revealed significant differences among groups in survival time of filtration blebs (P=0.002). Filtration blebs survived for the longest (from post-operation day 10 to 25) in the experimental group. This proves that fibrin-binding amniotic membrane can inhibit inflammation as well as scar formation[16]. IOP control determines the success or failure of glaucoma filtration surgery. Our experiment proves that trabeculectomy in combination with subconjunctival flap bilayered freeze-dried fibrin-binding amniotic membrane transplantation can yield desirable IOP. Effects of freeze-dried bilayered amniotic membrane following trabeculectomy were investigated using further histology experiments. Fourteen-day HE staining revealed a potent filtration passageway, filtration blebs, and no significant inflammatory cells or neutrophils in the surgical area in the experimental group, while fibroblast proliferation in the sclera and conjunctiva was seen in the control group and in the blank group; plentiful fibroblasts were seen in conjunctiva; and scar formation by granulation tissues was observed in sclera. Two-week Massion staining revealed limited collagen deposition in the surgical area of the experimental and control groups and abundant staining in the corresponding area of the blank group. Massion staining on day 30 after surgery revealed limited collagen deposition in the filtration passageway and conjunctiva in the experimental and control groups, with amniotic membrane present in the experimental group and dissolving completely in the control group; abundant collagen was present in the filtration passageway and conjunctiva. Light microscopy revealed α-SMA expression on day 14 following surgery to be rare in the experimental group, limited in the control group and strong in the blank group. CTGF plays a key role in scar formation after glaucoma filtration surgery. Recent studies have suggested that CTGF promotes wound contraction and scar formation after surgery[17]. We observed peak CTGF values 14 days after surgery. With regard to mRNA expression, the experimental group < control group < blank group in every time period. The differences were significant (P<0.05), illustrating that freeze-dried fibrin-binding amniotic membrane, as an excellent biological membrane substitute, has no blood or lymphatic vessels and low immunogenicity, separates the conjunctiva from the sclera, effectively prevents fibroblast proliferation and scar formation, inhibits inflammation, prolongs degradation time of the amniotic membrane and is more beneficial with regard to post-operative effects.
The amniotic membrane can be preserved in several ways (e.g., glycerol or cryopreservation)[18]. However, there are disadvantages, including complicated processing, harsh conditions, limited duration and rapid degradation. In recent years, amniotic membrane has been applied more frequently in ophthalmology[19]-[21]. However, high water content and poor ductility necessitate an elaborate surgical operation, and use of a multilayered membrane further increases surgical difficulty. Fibrin glue is a type of fluid glue with high viscoelasticity and plasticity and is able to improve the mechanical properties of tissues[22]. We first bound fibrin glue and amniotic membrane to yield freeze-dried fibrin-binding bilayered amniotic membrane. The procedure was performed in a lyophilizer, followed by disinfection and vacuum preservation, allowing for storage at room temperature, maintenance of the material's properties and prolonged degradation. This approach not only avoids fluidification and pneumatosis between the layers but also overcomes disadvantages in unilayered amniotic membrane transplantation. For example, the amniotic membrane is thin enough to be easily ruptured during the surgery and dissolved and ablated in the early stage after transplantation and is not easily fixed. These factors increase the difficulty of the operation[23]. Reportedly, rehydrated amniotic membrane has many similarities to fresh membrane, as far as physical and biological properties are concerned, and has a good biocompatibility when insert under the conjunctival flap[24]. Further problems have not been resolved, including the feasibility of multilayered freeze-dried fibrin-binding amniotic membrane in clinical practice, the solid state of multilayered amniotic membrane, the stability of the aridity and anti-inflammatory mechanisms, as well as whether aseptic technique can be ensured.
In conclusion, the bilayered fibrin-binding amniotic membrane prolonged the survival time of filtration blebs, reduced conjunctival congestion, inhibited tissue fibrosis and decreased IOP. This novel material may be used routinely for glaucoma filtration surgery.
Footnotes
Foundation item: Natural Science Foundation of Hubei Province, China (No. 2008cda055)
REFERENCES
- 1.Palanca-Capistrano AM, Hall J, Cantor LB, Morgan L, Hoop J, WuDunn D. Long-term outcomes of intraoperative 5-fluorouracil versus intraoperative mitomycin C in primary trabeculectomy surgery. Ophthalmology. 2009;116(2):185–190. doi: 10.1016/j.ophtha.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Ciancaglini M, Carpineto P, Agnifili L, Nubile M, Fasanella V, Mattei PA, Mastropasqua L. Conjunctival characteristics in primary open-angle glaucoma and modifications induced by trabeculectomy with mitomycin C: an in vivo confocal microscopy study. Br J Ophthalmol. 2009;93(9):1204–1209. doi: 10.1136/bjo.2008.152496. [DOI] [PubMed] [Google Scholar]
- 3.Reibaldi A, Uva MG, Longo A. Nine-year follow-up of trabeculectomy with or without low-dosage mitomycin-c in primary open-angle glaucoma. Br J Ophthalmol. 2008;92(12):1666–1670. doi: 10.1136/bjo.2008.140939. [DOI] [PubMed] [Google Scholar]
- 4.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Occleston NL, Daniels JT, Tarnuzzer RW, Sethi KK, Alexander RA, Bhattacharya SS, Schultz GS, Khaw PT. Single exposures to antiproliferatives: long-term effects on ocular fibroblast wound-healing behavior. Invest Ophthalmol Vis Sci. 1997;38:1998. [PubMed] [Google Scholar]
- 6.Sherwood MB. A sequential, multiple-treatment, targeted approach to reduce wound healing and failure of glaucoma filtration surgery in a rabbit model (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:478–492. [PMC free article] [PubMed] [Google Scholar]
- 7.Murube J. Early clinical use of amniotic membrane in medicine and ophthalmology. Ocul Surf. 2006;4(3):114–118. doi: 10.1016/s1542-0124(12)70037-x. [DOI] [PubMed] [Google Scholar]
- 8.Seitz B, Das S, Sauer R, Mena D, Hofmann-Rummelt C. Amniotic membrane transplantation for persistent corneal epithelial defects in eyes after penetrating keratoplasty. Eye (Lond) 2009;23(4):840–848. doi: 10.1038/eye.2008.140. [DOI] [PubMed] [Google Scholar]
- 9.Yoon KC, Im SK, Kim JC, Yoon KW, Choi SK. Prognosis of paraquat-induced ocular surface injury: therapeutic effect of amniotic membrane transplantation. Cornea. 2009;28(5):520–523. doi: 10.1097/ICO.0b013e3181914316. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura T, Yoshitani M, Rigby H, Fullwood NJ, Ito W, Inatomi T, Sotozono C, Shimizu Y, Kinoshita S. Sterilized, freeze-dried amniotic membrane: a useful substrate for ocular surface reconstruction. Invest Ophthalmol Vis Sci. 2004;45(1):93–99. doi: 10.1167/iovs.03-0752. [DOI] [PubMed] [Google Scholar]
- 11.Tsubota K, Satake Y, Ohyama M, Toda I, Takano Y, Ono M, Shinozaki N, Shimazaki J. Surgical reconstruction of the ocular surface in advanced ocular cicatricial pemphigoid and Stevens-Johnson syndrome. Am J Ophthalmol. 1996;122:38–52. doi: 10.1016/s0002-9394(14)71962-2. [DOI] [PubMed] [Google Scholar]
- 12.Shimazaki J, Yang HY, Tsubota K. Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmology. 1997;104:2068–2076. doi: 10.1016/s0161-6420(97)30057-8. [DOI] [PubMed] [Google Scholar]
- 13.Tseng SC, Prabhasawat P, Barton K, Gray T, Meller D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell defi ciency. Arch Ophthalmol. 1998;116:431–441. doi: 10.1001/archopht.116.4.431. [DOI] [PubMed] [Google Scholar]
- 14.Chen HJ, Pires RT, Tseng SC. Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br J Ophthalmol. 2000;84:826–833. doi: 10.1136/bjo.84.8.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meller D, Pires RT, Mack RJ, Figueiredo F, Heiligenhaus A, Park WC, Prabhasawat P, John T, McLeod SD, Steuhl KP, Tseng SC. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000;107:980–989. doi: 10.1016/s0161-6420(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 16.Shaunak S, Thomas S, Gianasi E, Godwin A, Jones E, Teo I, Mireskandari K, Luthert P, Duncan R, Patterson S, Khaw P, Brocchini S. Polyvalent dendrimer glucosamine conjugates prevent scar tissue formation. Nat Biotechnol. 2004;22:977–984. doi: 10.1038/nbt995. [DOI] [PubMed] [Google Scholar]
- 17.Esson DW, Neelakantan A, Iyer SA, Blalock TD, Balasubramanian L, Grotendorst GR, Schultz GS, Sherwood MB. Expression of connective tissue growth factor after glaucoma filtration surgery in a rabbit model. Invest Ophthalmol Vis Sci. 2004;45:485–491. doi: 10.1167/iovs.03-0485. [DOI] [PubMed] [Google Scholar]
- 18.Libera RD, Melo GB, Lima Ade S, Haapalainen EF, Cristovam P, Gomes JA. Assessment of the use of cryopreserved x freeze-dried amniotic membrane (AM) for reconstruction of ocular surface in rabbit model. Arq Bras Oftalmol. 2008;71(5):669–673. doi: 10.1590/s0004-27492008000500011. [DOI] [PubMed] [Google Scholar]
- 19.Madhira SL, Vemuganti G, Bhaduri A, Gaddipati S, Sangwan VS, Ghanekar Y. Culture and characterization of oral mucosal epithelial cells on human amniotic membrane for ocular surface reconstruction. Mol Vis. 2008;14:189–196. [PMC free article] [PubMed] [Google Scholar]
- 20.Liang L, Li W, Ling S, Sheha H, Qiu W, Li C, Liu Z. Amniotic membrane extraction solution for ocular chemical burns. Clin Experiment Ophthalmol. 2009;37(9):855–863. doi: 10.1111/j.1442-9071.2009.02159.x. [DOI] [PubMed] [Google Scholar]
- 21.Nubile M, Carpineto P, Lanzini M, Ciancaglini M, Zuppardi E, Mastropasqua L. Multilayer amniotic membrane transplantation for bacterial keratitis with corneal perforation after hyperopic photorefractive keratectomy: case report and literature revie. J Cataract Refract Surg. 2007;33(9):1636–1640. doi: 10.1016/j.jcrs.2007.04.040. [DOI] [PubMed] [Google Scholar]
- 22.Panda A, Kumar S, Kumar A, Bansal R, Bhartiya S. Fibrin glue in ophthalmology. Indian J Ophthalmol. 2009;57(5):371–379. doi: 10.4103/0301-4738.55079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dekaris I, Gabric N, Mravicic I, Karaman Z, Katusic J, Lazic R, Spoljaric N. Multilayer vs Monolayer amniotic membrane transplantation for deep corneal ulcer treatment. J Coll Antropol. 2001;25 Suppl:23–28. [PubMed] [Google Scholar]
- 24.Nakamura T, Sekiyama E, Takaoka M, Bentley AJ, Yokoi N, Fullwood NJ, Kinoshita S. The use of trehalose-treated freeze-dried amniotic membrane for ocular surface reconstruction. Biomaterials. 2008;29(27):3729–3737. doi: 10.1016/j.biomaterials.2008.05.023. [DOI] [PubMed] [Google Scholar]