Abstract
AIM
To establish and compare serum proteomic of diabetic retinopathy (DR) patients in various phases and discuss pathogenesis of DR so as to find out possible serum specific molecular markers for early diagnosis of DR.
METHODS
Thirty-two subjects were divided into four groups: one group of eight type 2 diabetes mellitus (T2DM) patients without apparent DR (No-DR, NDR), one group of eight T2DM patients with non-proliferative diabetic retinopathy (NPDR), one group of eight T2DM patients with proliferative diabetic retinopathy (PDR) and one group of eight healthy volunteer participants. Two dimensional fluorescence difference gel electrophoresis (2D-DIGE) was applied to establish differential protein expression profiles in four groups. Matrix-assisted laser desorption/ionization time of flight tandem mass spectrometry (MALDI-TOF-TOF MS) was applied to identify mass spectrometry of differential proteins and analyze follow-up bioinformatics.
RESULTS
2D-DIGE maps of serum protein were satisfactory obtained from NDR, NPDR, PDR and normal control groups. Twenty-six different proteins spots were screened (the volume ratio was >1.5 based on DeCyder software analysis). Twenty-four of them were verified and two of them were not. Fifteen proteins were verified. Most of them were high-abundant proteins in serum. The four relatively low-abundant ones were beta 2-glycoprotein I (β2-GPI), alpha2-HS-glycoprotein(AHSG), alpha1-acid glycoprotein(α1-AGP) and apolipoprotein A-1(apo A-1). β2-GPI expression was gradually increased in the development of DR but unrelated to the severity of DR. The volume ratio of β2-GPI is 1.54, 2.43, and 2.84 in NDR, NPDR and PDR group respectively compared with normal control group.
CONCLUSION
Serum proteomic analysis of 2D-DIGE combined with MALDI-TOF-TOF MS is feasible to be applied in the study of DR. β2-GPI probably takes part in the process of DR occurrence and development and it could be a candidate biomarker on DR diagnosis in early phase.
Keywords: diabetic retinopathy, difference gel electrophoresis, β2-glycoprotein I, proteomics, serum, type 2 diabetes
INTRODUCTION
Diabetic retinopathy (DR) is one of the most common and severe microvascular complications of type 2 diabetes mellitus (T2DM). It mainly manifests with decreased sight, progressive retina impairment, and permanent loss of sight eventually[1]. There are non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR) according to the progression of DR. NPDR is the early stage of DR. Its manifestations could be microaneurysms, hemorrhage, exudation and edema on retina. Many DR patients have mild NPDR, but their vision usually does not get affected. In PDR phase, new vessels grow on posterior surface of retina or vitreous body. The new vessels bleed easily and cause scar formation. Retinal folds and detachment afterwards lead to vision loss eventually. In the progression from NPDR to PDR, a large number of patients don't have obvious symptoms. Therefore, it is with great significance to unfold DR pathogenesis, and verify the protein biomarkers on its early diagnosis and evaluation as well as prognosis. The former studies were convinced that the occurrence and development of DR is related to a variety of pathogenic factors like growth factors over-expression, hyperglycemia, hypertension, hemodynamic abnormalities, protein glycosylation end products, polyol pathway and genetic[2],[3]. However, these outcomes can not explain DR pathogenesis in a further molecular level. Many studies indicate that DR is a complicated biological process including multiple factors and steps. Single molecular research can not fully clarify its mechanism.
In recent years, fast development of proteomic technology has provided a reliable technological stage for high-through molecular marker research. So we are enabled to realize the studies on exploration of DR pathogenesis by multiple level and factors, seeking for DR specific molecular markers for early diagnosis[4],[5]. Today, most proteomic analysis on DR is based on patients' vitreous humor or eye tissue from animal model. However, neither is flawless. Vitreous humor is hard to draw, and blood-retinal barrier impairment may cause biased result. Meanwhile, animal models cannot authentically duplicate the characteristics of DR patients. One the other hand, as the development of serum proteomic technology, proteomic research based on serum samples can convey the protein expression more clearly and authentically. Thus, we use two dimensional fluorescence difference gel electrophoresis(2D-DIGE) separation technology, which is relatively advanced at present, combined with matrix-assisted laser desorption/ionization time of flight tandem mass spectrometry(MALDI-TOF-TOF) technology to screen serum specific molecular markers in type 2 diabetes mellitus (T2DM) patients complicated with DR. No similar literature has been reported so far.
MATERIALS AND METHODS
Materials
Twenty-four T2DM patients in accordance with inclusion and exclusion criteria were recruited from endocrinology and ophthalmology of Yijishan hospital, affiliated with Wannan Medical College. DM was confirmed according to WHO criteria set in 1999[6]. DR grade was confirmed on proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales raised in 2002, which was established by the Global Diabetic Retinopathy Project Group on the basis of two large sample, multi-center clinical researches, Early Treatment Diabetic Retinopathy Study (ETDRS) and Wisconsin Epidemiological Study of Diabetic Retinopathy (WESDR)[7]. Therefrom, the patients were divided into three groups: one group of 8 patients without apparent DR (No-DR, NDR group), one group of 8 patients with non-proliferative diabetic retinopathy (NPDR group) and one group of 8 patients with proliferative diabetic retinopathy (PDR group). All 24 patients were graded according to fundus fluorescein angiography and fundus examination by three ophthalmologists single-blinded. Inclusion criteria: (1) age from 45 to 65 years-old; (2) T2DM course longer than two years; (3) the male-to-female ratio of each group was 1:1. Exclusion criteria: (1) patients with hypertension; (2) patients with severe heart, liver or renal failure; (3) patients with chronic disease other than DM. In the same time, to lessen the medicine effect on results all included participants were taking human insulin to control blood glucose. Table 1 is about the common information of the participants. Clinical data on included participants indicates that there is no statistic difference in groups other than FPG and duration of diabetes. Additionally, 8 healthy volunteer participants from Health Center of Yijishan Hospital with matched age and sex were included in normal control (NC) group. The protocol for sample collection was approved by the ethics committee of Yijishan hospital, abided to Helsinki Declaration. Informed consent was obtained from all subjects.
Table 1. Clinical characteristics and demographic data of the study subjects.
| NC group | T2DM patients |
|||
| NDR group | NPDR group | PDR group | ||
| Number(Cases) | 8 | 8 | 8 | 8 |
| Age(years) | 54.75±3.78 | 56.38±5.18 | 54.38±4.60 | 57.88±3.09 |
| Sex(M/F) | 4/4 | 4/4 | 4/4 | 4/4 |
| FPG(mmol/L) | 5.17±0.50 | 9.22±1.65∗ | 9.15±1.95∗ | 7.94±2.02∗ |
| HbA1c(%) | - | 9.81±1.39 | 8.85±1.13 | 8.06±1.87 |
| TG(mmol/L) | 1.33±0.53 | 1.79±0.79 | 1.59±0.32 | 1.28±0.51 |
| TC(mmol/L) | 4.60±0.68 | 5.00±1.15 | 4.71±0.43 | 4.48±0.57 |
| HDL-c(mmol/L) | 1.34±0.18 | 1.54±1.27 | 1.22±0.21 | 1.11±0.21 |
| LDL-c(mmol/L) | 2.57±0.48 | 2.43±0.43 | 2.89±0.32 | 2.36±0.58 |
| BMI(kg/m2) | 22.88±1.73 | 23.89±2.35 | 24.47±1.10 | 24.74±1.90 |
| Duration of diabetes(years) | - | 5.6±2.0 | 7.2±1.6b# | 9.3±3.4#Δ |
Values are means±SD, ∗P<0.05 compared with NC group, #P<0.05 compared with NDR group, ΔP<0.05 compared with NPDR group. FPG: fasting plasma glucose; HbA1c: glycated Hemoglobin A1c; TG: triglyceride TC: total cholesterol; HDL-c: high density lipoprotein cholesterol; LDL-c: low density lipoprotein cholesterol; BMI: body mass index.
Methods
Serum sample collection
2mL blood drawn from cubital vein with empty stomach was kept standing for 10-20 minutes under room temperature, 4°C, and then was centrifuged at 3 000rpm for ten minutes (Fresco21, Thermo Scientific Heraeus,Germany). Upper serum was collected, charged separately and reserved at -804°C.
Serum high-abundance protein removal
75µL serum diluted by 400µL combined buffer was incubated under room temperature for 10 minutes. The diluted serum was added into ProtroPrep Blue Alumin and IgG Depletion kit conjugaged column (Sigma, USA) eluted with albumin and IgG. The filtered solution without albumin or IgG was collected by centrifugation at 10 000rpm. Eluent was collected by centrifugation at 10 000rpm, charged separately and reserved at -804°C.
Protein sample purification
Protein sample was purified by 2-D Clean-up Kit (GE Healthcare). More details seen in kit instructions.
Detection of protein concentration
Protein concentration was detected by Ettan™ 2-D Quant Kit (GE Healthcare), more details seen in kit instructions. Protein contents were calculated automatically by instrument software according to standard curve drawn by instrument software automatically and the absorbance values of protein samples.
Protein separation by 2D-DIGE
Two DIGE gel were made, A gel and B gel. In each group, eight serum samples treated in above-mentioned way were taken randomizedly and mixed by equivalent volume, and then four samples were made. The four samples were mixed by equivalent volume to establish internal standard (50µg in total). The pH value was adjusted to 8.0-9.0. Three kinds of special fluorescent dye, Cy2, Cy3 and Cy5 were used to conduct labeling reaction by ratio of 50µg protein: 400pmo fluorescent dye, namely 50µg of sample with 1µL 400pmol/µL fluorescent dye. Equivalent volume of sample was drawn out of each group to be internal standard sample, stained with Cy2 stricted to product introduction (GE Healthcare, USA). In A gel, samples of NC group were labeled with Cy3 and sample of NDR group with Cy5. In B gel, samples of NPDR group were labeled with Cy3 and samples of PDR group with Cy5. After fully mixing, labeling reaction went on in black-out ice-bath for 10 minutes before termination. The labeled protein samples were put in 24cm immobilized pH gradient (IPG) gel bars with pH 3-10. (The best condition: 30v 12 hours, 500v lhr, 1000v lhr, 8000v gradient 1hr, 8000v 50000vhr). Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) with gum concentration of 12.5%. Typhoon 9400 scanner (GE, Healthcare) was used to scan image of gel plate after electrophoresis. Cy2, Cy3 and Cy5 were scanned by laser with wavelength of 488/520nm, 532/580nm and 633/670nm. After processed by analytic software DeCyder 2D 7.0 (GE, Healthcare), Cy2, Cy3 and Cy5 appeared blue, green and red correspondingly. The gel with most protein spots was made reference gel and matched with other gels. The matched spots were analyzed in next stage of research. Analytic software DeCyder 2D 7.0 was used to do Differential In-gel Analysis (DIA). The differential protein spots were identified by protein spots difference greater than 1.5-fold in size (1.5-fold up-regulated or down-regulated). After sample volume increase (1mL per gel), ordinary two-dimensional gel electrophoresis was done to get preparation gel. In-gel Coomssie Brilliant Blue staining was carried out after the second electrophoresis. Analytic gel was matched with preparation gel to find out matched differential protein spots. Ettan™ Spot picker automatic workshop was used to dig the gels. The gel particles collected was cryo-preserved in 500µL EP tube under -204°C for following mass spectrometry.
Protein identification by MALDI-TOF-TOF MS
In-enzyme glycolysis of trypsin (Promega) was manipulated to differential protein spots. The prepared samples were done with peptide mass fingerprinting, (PMF) analysis by MALDI-TOF-TOF MS (ABI 4800 Proteomic Analyzer, Applied Biosystems Company, USA). PMF could be collected when protein content is greater than 800-4 000Da. Then ten strongest peaks were selected to obtain tandem mass spectrometry (MS/MS) data. Conjunction search was done with MS and MS/MS. Results with total score >64 and more than four peptide fragments matched (best ion score>30, P<0.05) were accepted. Data of protein PMF by MS and MS/MS were searched by Mascot engine in Swiss-Prot database to get bioinformatics data.
Statistical Analysis
Data were expressed as means± standard deviation (mean±SD). Multiple comparisons were analysed by one-way ANOVA, followed by non-paired Student t-test for comparison between two groups. Statistical significance was set at P<0.05. All statistical analysis was carried out by SPSS software (version 13.0). In-gel difference analysis was carried out by DeCyder v.7.0 automatically.
RESULTS
Average Protein Concentration in each group
NC group: 5.6mg/mL, NDR group: 6.1mg/mL, NPDR group: 5.8 mg/mL, PDR group: 5.5mg/mL.
Analysis on 2D-DIGE Image and Screening of Differential Protein
The paired analysis on image by software DeCyder 2D 7.0 unveiled 1854 protein spots in gel A (Figure 1), and 1613 ones in gel B (Figure 2). Differential protein spots were sought based on the standard ratio >1.5[8],[9]. Twenty-six differential protein spots were detected (Figure 3). Compared to NC group, there were seven up-regulated and six down-regulated differential protein spots in NDR group, eleven up-regulated and four down-regulated ones in NPDR group, nine up-regulated and four down-regulated ones in PDR group.
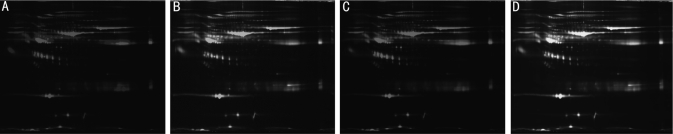
Figure 1. Image of 2D-DIGE gels of the serum proteins from NC and NDR groups.
A: Cy2(blue) image of proteins from an internal standard(equal amount of NC and NDR samples); B: Cy3(green) image of proteins from NC group; C: Cy5(red) image of proteins from NDR group. D: False-colored DIGE gel image of serum proteins from NC and NDR groups. The overlay images showing yellow spots containing proteins that have equal expression levels in the two samples, red spots containing proteins with higher expression, and green spots with proteins down-regulated
Figure 2. Image of 2D-DIGE gels of the serum proteins from NPDR and PDR groups.
A: Cy2(blue) image of proteins from an internal standard (equal amount of NPDR and PDR samples); B: Cy3(green) image of proteins from NPDR group; C: Cy5(red) image of proteins from PDR group. D: False-colored DIGE gel image of serum proteins from NPDR and PDR groups. The overlay images showing yellow spots containing proteins that have equal expression levels in the two samples, red spots containing proteins with a higher expression, and green spots with proteins down-regulated
Figure 3. The 2-DE protein profile of differential protein spots in four groups (Coomssie Brilliant Blue r-250 staining). The circles show the 26 differential protein spots. Spot numbers correspond to those in Table 2.
Spectrometry results of screened differential protein spots
Among the 26 differential protein spots screened by 2D-DIGE technology, 24 were characterized by MALDI-TOF-TOF MS mass spectrometry and two were not. Fifteen kinds of protein were characterized (Table 2). PMFs of all proteins were acquired successfully. However, many proteins spots turned out to be gene expression product from the same genes. So the proteins characterized were less than the protein spots, which could be due to the shearing and post-translation modification of proteins of various subtypes. Most of them were high-abundance proteins in plasma, such as serum albumin, transferrin, immunoglobulins, fibrinogen, haptoglobin, clusterin and ceruloplasmin. There were four proteins with low-abundance and low molecular weight, which were meaningful to the pathogenesis exploration and disease diagnosis. They were β2-GPI (spot #995), alpha2-HS-glycoprotein (AHSG) (spot #1036), alpha1-acid glycoprotein (α1-AGP) (spot #1219) and apolipoprotein A-1 (apo A-1) (spot #1604). Compared with the NC group, β2-GPI and AHSG were both up-regulated in NDR group, NPDR group and PDR group. α1-AGP and apo A-1 were both down-regulated in the three groups. Only β2-GPI of them manifested regularly in expression level, which was up-regulated with the development of DR. Differential protein spots expression level was evaluated on ratio>1.5 (DeCyder 2D 7.0). Compared with NC group β2-GPI increased significantly in NDR group (ratio=1.54), NPDR group (ratio=2.43) and in PDR group (ratio=2.84). Compared with NDR group, β2-GPI increased significantly in NPDR group (ratio=1.58) and PDR group (ratio=1.84). Compare with NPDR group, there was no significant increase found in PDR group (ratio=1.17). The results above indicated that β2-GPI took part in the occurrence and development of DR. It was speculated that β2-GPI was highly concerned with DR pathogenesis.
Table 2. Identification of differential proteins by MALDL-TOF-TOF MSa.
| No.of | Protein name | Accession | Protein | Protein | Protein Score | Average ratio | ||
| spotsb | No. | MW | PI | C.I.% | NDR/NC | NPDR/NC | PDR/NC | |
| 438,760,763 | Serum albumin | P02768 | 71317.2 | 5.92 | 100 | -3.41 | -1.24 | 0.42 |
| 439,617 | Serotransferrin | P02787 | 79280.5 | 6.81 | 99.134 | 1.27 | 2.42 | 3.41 |
| 840,826 | Ig alpha-1 chain C region | P01876 | 38486 | 6.08 | 100 | 0.02 | 9.75 | -0.04 |
| 961 | Fibrinogen beta chain | P02675 | 56576.5 | 8.54 | 100 | 2.31 | 1.03 | 2.51 |
| 995 | Beta-2-glycoprotein 1 | P02749 | 39584.1 | 8.34 | 100 | 1.54 | 2.43 | 2.84 |
| 1,036 | Alpha-2-HS-glycoprotein | P02765 | 40098 | 5.43 | 100 | 1.52 | 1.4 | 1.24 |
| 1,135 | Glial fibrillary acidic protein | P14136 | 49906.7 | 5.42 | 99.974 | 20.13 | -2.91 | 16.67 |
| 1,140 | Fibrinogen gamma chain | P02679 | 52106.1 | 5.37 | 100 | 42.34 | -1.9 | 50.04 |
| 1,219 | Alpha-1-acid glycoprotein | P02763 | 23724.8 | 4.93 | 100 | -2.59 | -1.14 | -2.31 |
| 1292, 1303, 1322, 1349, 1330, 1342 | Haptoglobin | P00738 | 45860.8 | 6.13 | 100 | 7.81 | 0.92 | 8.41 |
| 1,347 | Haptoglobin-related protein | P00739 | 39495.8 | 6.42 | 100 | 1.19 | -1.57 | 1.12 |
| 1,363 | Clusterin | P10909 | 53031.2 | 5.89 | 83.086 | -1.52 | -1.11 | 1.04 |
| 1,478 | Ig kappa chain C region | P01834 | 11772.7 | 5.58 | 99.986 | 1.2 | 10.64 | 1.49 |
| 1,604 | Apolipoprotein A-I | P02647 | 30758.9 | 5.56 | 100 | -1.6 | -1.06 | -1.59 |
| 1,658 | Ceruloplasmin | P00450 | 122982.9 | 5.44 | 99.889 | 1.9 | 1.52 | 1.47 |
aSpots for which the volume ratio was >1.5 based on DeCyder software analysis were identified by MALDL-TOF-TOF MS; bSpots referring to Figure 3.
DISCUSSION
Development of proteomic technology has provided a reliable technological stage for research on pathogenesis and exploration for disease-related bio-molecular markers, which could be used in early diagnosis and course confirmation. These serum molecular markers, mainly low-abundance proteins, are closely related to the disease and promising in diagnosis, accounting for less than 1% in serum total protein[10]. They are difficult to separate and characterize for they are usually covered or interfered by albumin and immunoglobulin which account for 70% in serum total proteins. Therefore, it is very important to select highly sensitive and reliable proteomic technology when doing serum proteomic analysis. So far, there are a few researches on proteomic of DR. These researches mostly were applied with traditional two dimensional gel electrophoresis(2-DE) to separate proteins[11]-[13], with deficiency like limited separation and poor repetition[14]. In this research, we used some new methods to guarantee the reliability of results: (1) Participants included are even in impact factors like age, sex, blood lipid and BMI for a uniform phase; (2) The inclusion criteria and exclusion criteria for participants were strictly complied. (3) 2D-DIGE technology, which is a proteomic technology with higher accuracy and reliability was applied. It's internal standard and automatic software for in-gel and inter-gel analysis allowed a wider dynamic range and more accurate outcome[5],[15].
In the study, 26 differential protein spots were screened by 2D-DIGE technology. 24 of them were characterized by MALDI-TOF-TOF MS mass spectrometry and two were not. Fifteen kinds of proteins were characterized. Many protein spots turned out to be gene expression product from same genes. So the proteins characterized were fewer than the protein spots screened, which could be due to the shearing and post-translation modification of proteins of various subtypes. Two kinds of proteins can't be identified. This is often because of inadequate enzymolysis or protein content. Most of the fifteen proteins were high-abundance proteins in plasma, such as serum albumin, transferrin, immunoglobulins, fibrinogen, haptoglobin, clusterin, and ceruloplasmin. These high-abundance proteins had been separated, characterized and used in clinical diagnosis. For there was no specific biological information, they were not included in the secondary research. There were four proteins with low-abundance and low molecular weight, which were meaningful to the pathogenesis exploration and disease diagnosis. They were β2-GPI, AHSG, α1-AGP and apo A-1. Compared with the NC group, β2-GPI and AHSG were both up-regulated in NDR group, NPDR group and PDR group while α1-AGP and apo A-1 were both down-regulated in the three groups. Only β2-GPI manifested regularly in expression level, which was up-regulated gradually as the development of DR. Differential protein spots expression level was evaluated on ratio>1.5(DeCyder 2D 7.0). Compared with NC group, β2-GP showed a 1.54-fold increase in NDR group. Compared with NDR group, β2-GPI showed a 1.58-fold increase in NPDR group. Compared with NDR group, β2-GPI showed a 1.84-fold increase in PDR group. However, Compare with NPDR group, β2-GPI showed a only 1.17-fold increase, less than 1.5-fold, in PDR group. It was indicated that serum β2-GPI level was higher in people with DM than those ones without DM, higher in patients with DR than those ones with NDR, but its level is unrelated to the severity of DR. Hence, we speculated that: (1) β2-GPI possibly took part in DR pathogenesis; (2) β2-GPI expression difference in different groups suggested that it was event in early phase of DR pathogenesis. It could be a candidate serum molecular marker for DR early diagnosis.
β2-GPI is a kind of plasma glycoprotein with 50kD of molecular weight. It is mainly synthesized in liver and plays a very important role of lipid metabolism regulation in many diseases like atherosclerosis and autoimmune diseases[16],[17], but its physiological function remains unclear. β2-GPI was reported in DR pathogenesis study. Simó et al[18] discovered that β2-GPI level in vitreous humour of PDR patients is increased significantly compared with patients with macular hole. Also, it was pointed out that the abnormalities happened as early as in primary phase in DR. So far, serum β2-GPI level of DR patients and its influence have not been reported. In this study, under the help of DIGE combined with mass spectrometry technology, we found that the changing trend of serum β2-GPI level of DR patients was in consistence with the changing trend in vitreous humour suggested by Simó R. Namely, serum β2-GPI expression level was increased in patients with DR and the increase occurred in early stage of DR. But the reason and mechanism for increase of serum β2-GPI in DR patients remain unclear. Further study will be done in the follow-up experiment.
Additionally, results inconsistent with those reported in former study were also discovered. α1-AGP expression down-regulated. Compared with NC group, α1-AGP level was down-regulated by 2.59-fold in NDR group, 1.14-fold in NPDR group and 2.31-fold in PDR group. Now, most studies consider α1-AGP as a non-specific reactive protein in acute phase. α1-AGP has a low content in human serum and rises obviously under infection, inflammation or immune reaction[19]-[21]. Patients with DM were considered in a chronic inflammatory condition. Study suggested that level of α1-AGP increased in serum of patients with DM[22]. Experiments with more samples are needed to determine if the different results were caused by different procedures of collecting and processing serum samples before mass spectrometry.
This is not a flawless study. Although 2D-DIGE has many advantages like higher sensitivity, uniqueness and wider dynamic range compared with 2-DE, it cannot break the internal limitation based on 2-DE, which means it can hardly differentiate proteins with low molecular weight or low abundance. In this study, proteins with molecular weight under 10kDa were not separated. Even we had eliminated high-abundance proteins in serum samples before DIGE analysis so as to avoid its possible coverage on low-abundance proteins, those low-abundance proteins possibly taking part in DR pathogenesis still remain uncovered. We speculated that two or more proteomic methods used jointly may help this problem. For an example, Multidimensional Liquid Chromatography Technology has a relatively poor separation method and resolution compared to DIGE, but it has advantage in analysis on proteins with different sizes, low-abundance proteins and hydrophobic proteins. So, application of Multidimensional Liquid Chromatography Technology as separation method complementary with 2D-DIGE will help overcoming the defect.
In conclusion, our study indicated that serum proteomic analysis of 2D-DIGE combined with MALDI-TOF-TOF MS was feasible to be applied in the study of DR. β2-GPI possibly took part in DR pathogenesis and was a candidate serum molecular marker, but its specific function in DR pathogenesis asked for further research.
Acknowledgments
The authors thank Dr. Xiao-Ming Li for his constructive comments.
Footnotes
Foundation item: Supported by Natural Science Fund for Colleges and Universities in Anhui Province, China (No. KJ2007B208)
REFERENCES
- 1.Kempen JH, O'Colmain BJ, Leske MC, Haffner SM, Klein R, Moss SE, Taylor HR, Hamman RF. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122(4):552–563. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- 2.Cheung AK, Fung MK, Lo AC, Lam TT, So KF, Chung SS, Chung SK. Aldose reductase deficiency prevents diabetes-induced blood-retinal barrier breakdown, apoptosis, and glial reactivation in the retina of db/db mice. Diabetes. 2005;54(11):3119–3125. doi: 10.2337/diabetes.54.11.3119. [DOI] [PubMed] [Google Scholar]
- 3.Moore TC, Moore JE, Kaji Y, Frizzell N, Usui T, Poulaki V, Campbell IL, Stitt AW, Gardiner TA, Archer DB, Adamis AP. The role of advanced glycation end products in retinal microvascular leukostasis. Invest Ophthmol Vis Sci. 2003;44(10):4457–4464. doi: 10.1167/iovs.02-1063. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Ramirez M, Canals F, Hernandez C, Colome N, Ferrer C, Carrasco E, Garcia-Arumi J, Simo R. Proteomic analysis of human vitreous fluid by fluorescence-based difference gel electrophoresis (DIGE): a new strategy for identifying potential candidates in the pathogenesis of proliferative diabetic retinopathy. Diabetologia. 2007;50(6):1294–1303. doi: 10.1007/s00125-007-0627-y. [DOI] [PubMed] [Google Scholar]
- 5.Merchant ML, Klein JB. Proteomics and diabetic retinopathy. Clin Lab Med. 2009;29(1):139–149. doi: 10.1016/j.cll.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Drouin P, Blickle JF, Charbonnel B, Eschwege E, Guillausseau PJ, Plouin PF, Daninos JM, Balarac N, Sauvanet JP. Diagnosis and classification of diabetes mellitus: the new criteria. Diabetes & metabolism. 1999;25(1):72–83. [PubMed] [Google Scholar]
- 7.Wilkinson CP, Ferris FL. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 8.Liu S, Bai S, Qin Z, Yang Y, Cui Y, Qin Y. Quantitative proteomic analysis of the cerebrospinal fluid of patients with multiple sclerosis. Journal of cellular and molecular medicine. 2009;13(8A):1586–1603. doi: 10.1111/j.1582-4934.2009.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao PV, Lu X, Standley M, Pattee P, Neelima G, Girisesh G, Dakshinamurthy KV, Roberts CT, Jr, Nagalla SR. Proteomic identification of urinary biomarkers of diabetic nephropathy. Diabetes Care. 2007;30(3):629–637. doi: 10.2337/dc06-2056. [DOI] [PubMed] [Google Scholar]
- 10.Tirumalai RS, Chan KC, Prieto DA, Issaq HJ, Conrads TP, Veenstra TD. Characterization of the low molecular weight human serum proteome. Mol Cell Proteomics. 2003;2(10):1096–1103. doi: 10.1074/mcp.M300031-MCP200. [DOI] [PubMed] [Google Scholar]
- 11.Kim SJ, Kim S, Park J, Lee HK, Park KS, Yu HG, Kim Y. Differential expression of vitreous proteins in proliferative diabetic retinopathy. Current Eye Res. 2006;31(3):231–240. doi: 10.1080/02713680600557030. [DOI] [PubMed] [Google Scholar]
- 12.Ahn BY, Song ES, Cho YJ, Kwon OW, Kim JK, Lee NG. Identification of an anti-aldolase autoantibody as a diagnostic marker for diabetic retinopathy by immunoproteomic analysis. Proteomics. 2006;6(4):1200–1209. doi: 10.1002/pmic.200500457. [DOI] [PubMed] [Google Scholar]
- 13.Kim T, Kim SJ, Kim K, Kang UB, Lee C, Park KS, Yu HG, Kim Y. Profiling of vitreous proteomes from proliferative diabetic retinopathy and nondiabetic patients. Proteomics. 2007;7(22):4203–4215. doi: 10.1002/pmic.200700745. [DOI] [PubMed] [Google Scholar]
- 14.Garbis S, Lubec G, Fountoulakis M. Limitations of current proteomics technologies. J Chromatography. 2005;1077(1):1–18. doi: 10.1016/j.chroma.2005.04.059. [DOI] [PubMed] [Google Scholar]
- 15.Timms JF, Cramer R. Difference gel electrophoresis. Proteomics. 2008;8(23-24):4886–4897. doi: 10.1002/pmic.200800298. [DOI] [PubMed] [Google Scholar]
- 16.George J, Harats D, Gilburd B, Afek A, Levy Y, Schneiderman J, Barshack I, Kopolovic J, Shoenfeld Y. Immunolocalization of beta2-glycoprotein I (apolipoprotein H) to human atherosclerotic plaques: potential implications for lesion progression. Circulation. 1999;99(17):2227–2230. doi: 10.1161/01.cir.99.17.2227. [DOI] [PubMed] [Google Scholar]
- 17.Matsuura E, Lopez LR. Autoimmune-mediated atherothrombosis. Lupus. 2008;17(10):878–887. doi: 10.1177/0961203308093553. [DOI] [PubMed] [Google Scholar]
- 18.Simó R, Higuera M, Garcia-Ramirez M, Canals F, Garcia-Arumi J, Hernandez C. Elevation of apolipoprotein A-I and apolipoprotein H levels in the vitreous fluid and overexpression in the retina of diabetic patients. Arch Ophthalmol. 2008;126(8):1076–1081. doi: 10.1001/archopht.126.8.1076. [DOI] [PubMed] [Google Scholar]
- 19.Nishi K, Maruyama T, Halsall HB, Handa T, Otagiri M. Binding of alpha1-acid glycoprotein to membrane results in a unique structural change and ligand release. Biochemistry. 2004;43(32):10513–10519. doi: 10.1021/bi0400204. [DOI] [PubMed] [Google Scholar]
- 20.Christiansen MS, Hommel E, Magid E, Feldt-Rasmussen B. Orosomucoid in urine is a powerful predictor of cardiovascular mortality in normoalbuminuric patients with type 2 diabetes at five years of follow-up. Diabetologia. 2005;48(2):386–393. doi: 10.1007/s00125-004-1630-1. [DOI] [PubMed] [Google Scholar]
- 21.Gupta N, Shankernarayan NP, Dharmalingam K. Alpha1-acid glycoprotein as a putative biomarker for monitoring the development of the type II reactional stage of leprosy. J Med Microbiol. 2010;59(Pt 4):400–407. doi: 10.1099/jmm.0.016394-0. [DOI] [PubMed] [Google Scholar]
- 22.Akbay E, Yetkin I, Ersoy R, Kulaksizoglu S, Toruner F, Arslan M. The relationship between levels of alpha1-acid glycoprotein and metabolic parameters of diabetes mellitus. Diabetes, nutrition & metabolism. 2004;17(6):331–335. [PubMed] [Google Scholar]