Abstract
AIM
To study the effects of danhong huayu koufuye (DHK) on fasting blood glucose (FBG) and diabetic retinopathy (DR) in streptozotocin (STZ)-induced type 1 diabetic rats to facilitate the rational usage of this drug.
METHODS
Diabetic rats were induced by injection of a single dose of STZ intraperitoneally at 50mg/kg. Flash electroretinogram (FERG) and oscillatory potentials (OPs) were used to measure retinal function. The microvascular perfusion of ears was performed to study the microcirculation in rats. FBG, body-weight, and 24-h urine volume, water intake and diet intake were also assessed.
RESULTS
DHK had no effect on FBG in normal rats. However, STZ + DHK group were significantly different from those of Model and moved toward those of normal control. It reversed the increase in diet intake (P≤0.05 vs model control) and the loss in body-weight (P≤0.05 vs model control) in diabetic rats. DHK decreased the FBG of diabetic rats by 25.6% (P≤0.05) and 37.9% (P≤0.01) after 14 and 21 days administration as compared with the model control, respectively. Moreover, DHK significantly increased the FERG b-wave amplitude by 80% (P≤0.05 vs model control) and decreased the FERG b-wave latency by 15.3% (P≤0.01 vs model control) after 24 days administration. The OP1 and OP2 amplitudes in DHK group were 2.6 (P≤0.01) and 2.0 (P≤0.01) times of model group after 24 days of DHK treatment, respectively. At the same time, OP1 and OP2 latencies in DHK group reduced by 16.0% (P≤0.001) and 14.7% (P≤0.001) as compared with the model control, respectively. Furthermore, the microvascular perfusion of DHK group was 2.4 times of model group (P≤0.001) after 21 days administration.
CONCLUSION
DHK had no effect on normal FBG. But it had antihyperglycemic activity, and had a preventive and therapeutic effect on DR in diabetic rats.
Keywords: danhong huayu koufuye, diabetic retinopathy, streptozotocin, fasting blood glucose, microcirculation
INTRODUCTION
Diabetic retinopathy (DR), a kind of microvascular complications of diabetes mellitus, is the leading cause of severe vision loss and irreversible blindness in developed countries[1]. The prevalence of diabetes is increasing dramatically. In China, morbidity had dramatically climbed up to 9.6% in 2008 from 6.1% in 1980. Moreover, the onset age of diabetes is younger than that of before[2]. The prevalence of DR is strongly related to the type and duration of diabetes. For insulin-taking, younger-onset diabetic persons, the prevalence of DR (1979-80) was 17% and 97.5% in persons with diabetes for less than 5 years and 15 or more years, respectively[3]. For patients given diagnoses of diabetes at 30 years or older, the prevalence were 28.8% and 77.8%, respectively[4].
Despite evidences indicate that intensive glycemic therapy is effective in the control of DR, it is difficult to maintain strict blood glucose levels for many diabetic patients, and the risk of hypoglycemia is increasing under such treatment regimen. Furthermore, strict blood glucose regimens do not completely eliminate DR. Therefore, some diabetes patients will still become visually impaired or blind and will require photocoagulation or surgical vitrectomy[1]. So it is pivotal to find out an effective drug for the preventive treatment of DR.
Danhong Huayu Koufuye (DHK), a traditional Chinese prescription, contains Salvia miltiorrhiza, Angelicae sinensis radix, Chuanxiong rhizoma, Persicae semen, Carthami flos, Bupleuri Radix, and Aurantii fructus. The quantities of the ingredients are 29%, 11.5%, 15%, 11.5%, 11.5%, 11.5% and 10% of the total weight, respectively. It possesses the effects of promoting blood circulation to remove blood stasis and promoting qi circulation to remove meridian obstruction. So it is applied for the treatment of blurred vision induced by stagnation of qi and blood stasis, and central retinal vein occlusion. Although DHK showed some therapeutic effects on DR in clinical, doctors still hesitated to prescribe the drug because of few related experimental evidences. Therefore, the efficiencies of DHK on both fasting blood glucose (FBG) and DR in streptozotocin (STZ)-induced type 1 diabetic rats were observed in order to facilitate the rational usage of this drug.
MATERIALS AND METHODS
Materials
Ninety-seven male SD rats (200-260g) were obtained from Experimental Animal Center, Guangzhou University of Chinese Medicine. All rats had free access to a standard diet and drinking water and were housed in an animal room maintained at 24.0°C±0.5°C and with a 12:12h cyclic lighting schedule. The experiments were performed in accordance with the Animal Ethics Committee of Guangzhou University of Chinese Medicine and conformed to the ARVO Resolution for the use of animals in ophthalmic and vision research. STZ was purchased from Sigma-Aldrich Chemical Co. DHK, Isophane Protamine Recombinant Human Insulin Injection (Insulin) and Hypromellose Eye Drops was kindly donated by Guangzhou Baiyunshan Hutchison Whampoa Chinese Medicine Co., Ltd, Zhuhai United Laboratories Co., Ltd., and Zhongshan Ophthalmic Center of Sun Yet-Sen University, respectively. Calcium Dobesilate Dispersible Tablets (CD), Xylazine Hydrochloride Injection, Compound Tropicamide Eye Drops, and Oxybuprocaine Hydrochloride Eye Drops were from Hainan Linheng Pharmaceutical Co., Ltd., Dunhua Shengda Pharmaceutical Co., Ltd., Shenyang Sinqi Pharmaceutical Co., Ltd., and Santen Pharmaceutical Co., Ltd., respectively.
Methods
Induction of diabetes mellitus
Rats were fasted for 13 hours before experiments. Seventy-five fasted rats were injected intraperitoneally with a single dose of 10g/L STZ (5mL/kg) dissolved in normal saline solution (0.1mmol citric acid/L). FBG were determined 7 days later. Rats having FBG concentration between 16.7 and 33mmol/L were selected for the type 1 diabetes mellitus model. Seventy-two diabetic rats were randomly divided into four groups of model group, STZ+Insulin positive group, STZ+CD positive group and STZ+DHK group with eighteen rats in each group. An additional twenty-two rats without STZ induction were selected as the normal group and normal+DHK group with eleven rats in each group.
Method of drug administration
Normal group was administered orally with vehicle (water, 3.2mL/kg) alone without STZ injection. Normal+DHK group was administered orally with DHK (3.2mL/kg) alone without STZ injection. Model group, STZ+CD group and STZ+DHK group were administered orally with vehicle (water, 3.2mL/kg), CD (156.3mg/kg) and DHK (3.2mL/kg) plus 50mg/kg STZ injection, respectively. STZ+Insulin group was given a subcutaneous injection of insulin (6.0U/kg) plus 50mg/kg STZ injection. All rats were taken corresponding drugs one time per day for 24 days from 8th day after STZ injection.
Methods of measurement
The non-fasting weight and FBG of all rats were measured weekly. FBG was performed with FreeView Blood Glucose Monitoring Meter and Test Strips (Guangzhou Wondfo Biotech, Co., Ltd.) after rats have fasted for 8 hours. Ten rats randomly chosen from each group were housed in metabolic cages for collecting 24-hour urine to measure the urine volume, and assaying 24-hour water intake and 24-hour diet intake after 21 days administration of drugs.
FER G and OPs recording
Flash electroretinogram (FERG) and oscillatory potentials (OPs) was performed on 10 rats randomly chosen from each group except normal group after 12 and 24 days administration of drugs. These analyses were done to evaluate retinal function and as a measure of drug effectiveness. SD rats were dark adapted overnight, and then anesthetized with Xylazine Hydrochloride Injection at 0.19mL/kg by intramuscular injection. Half of the initial dose was given each one hour thereafter. Pupils of all rats were dilated with Compound Tropicamide Eye Drops. Before recording, Oxybuprocaine Hydrochloride Eye Drops was given for surface anesthesia. To protect the eye and assist in maintaining a good electrical connection, Hypromellose Eye Drops was added to each eye. All rats were kept warm during FERG measurement.
FERG and OPs were recorded from both eyes simultaneously using silver ring corneal electrodes, two forehead reference electrodes, and ground electrode in the tail. The ERG machine (APS-2000AER) was purchased from Kanghua Rui Ming Technology Co., Ltd. No animals with cataract were used for FERG and OPs analyses.
FERG were recorded from 5 responses to flashes of unattenuated white light (20ms, 0.05Hz) from a photic stimulator (light-emitting diodes) set at brightness of 0.006325cd*s/m2 and filtered by a digital band-pass filter from 0.1 to 300Hz. OPs were evaluated from 10 responses with an inter-stimulus interval of 20ms by filtering the original responses elicited by a stimulus luminance of 0.006325cd*s/m2 by a frequency band-pass filter (50-200Hz).
Measurement of microvascular perfusion
Microvascular perfusion was measured noninvasively by using a PERIMED laser Doppler flowmeter with a right-angle probe (PeriFulx system 5000, Sweden) to determine whether DHK had the effect of promoting blood circulation for removing blood stasis after 24 days administration of drugs. Ten rats were randomly chosen from each group. The probe was fastened to a shaved ear at a site that was devoid of macroscopically visible vessels and the probe surface contact with rat ear directly. Microvascular perfusion was measured after under the identical conditions anesthesia. Blood perfusion data were collected for 1 minute when the wave was stable.
Statistical Analysis
All data were presented as mean ± SD and analyzed by the Statistical Package for the Social Sciences version 11.5 (SPSS 11.5). One-way analysis of variance (ANOVA) test was performed and post hoc multiple comparisons were conducted with LSD. P≤0.05 was assumed to be significant.
RESULTS
Effects of DHK on FBG, Body-Weight, 24-hour Water, 24-hour Diet Intake, and 24-hour Urine Volume
The FBG, 24-hour water intake, 24-hour diet intake and 24-hour urine volume increased significantly, while body-weight decreased remarkably in diabetic rats (P≤0.001 vs normal control). Insulin, a positive control, markedly reversed all of these parameters (P≤0.01 or 0.001 vs model control). Although DHK had no effect on FBG in normal rats, it gradually reduced FBG in diabetic rats. DHK significantly reduced the FBG of diabetic rats by 25.6% (P≤0.05) and 37.9% (P≤0.01) after 14 and 21 days administration as compared with the model group, respectively (Table 1). Moreover, DHK significantly reversed the increase of diet intake (P≤0.05 vs model control, Table 2) and reduction of body-weight (P≤0.05 vs model control, Table 3) in diabetic rats.
Table 1. Effect of DHK on FBG in rats.
| Groups | Fasting blood glucose (mmol/L) |
|||
| Day 0 | Day 7 | Day 14 | Day 21 | |
| Normal | 5.7 ± 1.1c | 6.8 ± 2.1c | 6.5 ± 1.2c | 6.0 ± 2.1c |
| Normal+DHK | 5.5 ± 0.5c | 5.2 ± 0.6c | 4.3 ± 0.4c | 5.1 ± 0.4c |
| Model | 27.2 ± 6.3*** | 28.3 ± 7.4*** | 27.7 ± 5.3*** | 31.4 ± 2.6*** |
| STZ+Insulin | 27.3 ± 7.2*** | 10.0 ± 5.5c | 6.9 ± 3.5c | 4.0 ± 2.0c |
| STZ+CD | 27.7 ± 5.8*** | 25.8 ± 9.7*** | 23.8 ± 11.0*** | 24.3 ± 8.6*** |
| STZ+DHK | 27.4 ± 6.1*** | 23.50 ± 6.9*** | 18.4 ± 7.5***b | 17.1 ± 9.9***c |
*P≤0.05, **P≤0.01 and ***P≤0.001 vs normal group; aP≤0.05, bP≤0.01 and cP≤0.001 vs model control
(mean±SD, n=11-18)
Table 2. Effects of DHK on 24-hour water intake, 24-hour diet intake and 24-hour urine volume in rats.
| Groups | Water intake(mL) | Diet intake(g) | Urine volume(ml) |
| Normal control | 17.0 ± 10.5c | 10.1 ± 2.5c | 3.4 ± 2.0c |
| Model control | 98.6 ± 48.9*** | 27.4 ± 7.3*** | 76.5 ± 38.5*** |
| STZ+Insulin | 35.6 ± 16.5***c | 16.2 ± 4.4b | 16.1 ± 12.8*c |
| STZ+CD | 67.3 ± 45.3*** | 24.8 ± 9.8*** | 62.9 ± 36.6*** |
| STZ+ DHK | 48.1 ± 43.3 | 18.9 ± 10.7*a | 49.3 ± 36.7*** |
*P≤0.05, **P≤0.01 and ***P≤0.001 vs normal group; aP≤0.05, bP≤0.01 and cP≤0.001 vs model control
(mean±SD, n=10)
Table 3. Effects of DHK on body weight of rats.
| Groups | Weight (g) |
|||
| Day 0 | Day 7 | Day 14 | Day 21 | |
| Normal | 209.0 ± 6.3 | 220.3 ± 10.0b | 234.4 ± 10.3b | 247.1 ± 9.1b |
| Normal+ DHK | 218.0 ± 3.1 | 223.1 ± 9.0b | 236.9 ± 14.5b | 250.1 ± 10.2b |
| Model | 196.6 ± 5.7 | 171.9 ± 8.8** | 177.0 ± 7.7** | 183.8 ± 11.9** |
| STZ+Insulin | 205.9 ± 7.1 | 217.1 ± 11.7b | 236.4 ± 8.7b | 253.0 ± 12.3b |
| STZ+CD | 206.4 ± 8.7 | 189.6 ± 13.0 | 199.4 ± 19.9 | 219.8 ± 22.8 |
| STZ+ DHK | 214.9 ± 8.5 | 223.1 ± 14.2b | 228.7 ± 16.5a | 237.3 ± 15.1a |
*P≤0.05, **P≤0.01 and ***P≤0.001 vs normal group; aP≤0.05, bP≤0.01 and cP≤0.001 vs model control
(mean±SD, n=11-18)
Effects of DHK on FERG and OPs
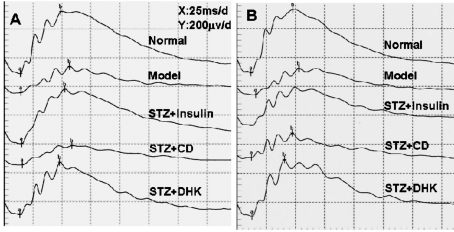
Reduction of FERG b-wave amplitudes and increase of latencies were observed in model group (P≤0.001 vs normal group). DHK remarkably reversed these parameters. After 24 days of DHK treatment, it significantly increased the FERG b-wave amplitude by 80% (P≤0.05 vs model group) and decreased the FERG b-wave latency by 15.3% (P≤0.01 vs model group) (Table 4, 5; Figure 1).
Table 4. Effects of DHK on the amplitude of FERG.
| Amplitude (μV) |
||||||
| Normal | Model | STZ+ Insulin | STZ+ CD | STZ+ DHK | ||
| a-wave | Day 12 | 79.2±39.7a | 52.0±32.1* | 70.3±31.8 | 45.0±39.0** | 87.4±34.3b |
| Day 24 | 74.5±37.3 | 53.5±30.5 | 40.9±17.2* | 43.5±27.3 | 73.3±41.2 | |
| b-wave | Day 12 | 375.0±161.0c | 189.8±100.3*** | 324.3±122.7c | 247.3±90.4** | 354.8±111.6c |
| Day 24 | 403.9±183.4c | 172.6±94.5*** | 209.0±49.4* | 236.2±114.6** | 317.6±190.0a | |
*P≤0.05, **P≤0.01 and ***P≤0.001 vs. normal group; aP≤0.05, bP≤0.01 and cP≤0.001 vs model control
(mean±SD, n=20)
Table 5. Effects of DHK on the latency of FERG.
| Latency (ms) |
||||||
| Normal | Model | STZ+ Insulin | STZ+ CD | STZ+ DHK | ||
| a-wave | Day 12 | 13.6±1.1b | 15.7±0.9** | 14.3±1.1 | 12.0±4.8*c | 14.2±0.7a |
| Day 24 | 12.3±0.9c | 16.2±1.5*** | 12.4±1.0c | 13.4±1.1*c | 13.4±1.4*c | |
| b-wave | Day 12 | 48.6±2.7c | 64.6±7.8*** | 53.2±6.6c | 56.0±14.2*b | 56.0±8.3*b |
| Day 24 | 47.6±5.1c | 66.0±12.8*** | 47.9±1.7c | 49.1±2.2c | 55.9±8.2**b | |
*P≤0.05, **P≤0.01 and ***P≤0.001 vs. normal control; aP≤0.05, bP≤0.01 and cP≤0.001 vs model control
(mean±SD, n=20)
Figure 1. Representative FERG after treatment of corresponding drugs in rat's right eyes.
A: 12 days postoperatively; B: 24 days postoperatively
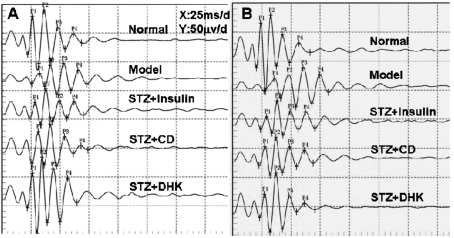
There were visible differences in the OPs of normal and model groups. Firstly, the largest OP wavelet in normal rats was OP2, while the largest one in some eyes of model control changed to OP3. Secondly, the OP1, OP2 and OPS amplitudes were significantly decreased, while OP1, OP2, OP3 and OP4 latencies were prolonged in model group as compared with normal group. DHK not only corrected the largest OP wavelet but also markedly reversed the changed amplitudes and latencies. The OP1, OP2 and OPS amplitudes in STZ+DHK group were 2.6 (P≤0.01), 2.0 (P≤0.01) and 1.7 (P≤0.05) times of model group after 24 days of DHK treatment, respectively. At the same time, the OP1, OP2, OP3 and OP4 latencies in STZ+DHK group were reduced by 16.0% (P≤0.001 vs model group), 14.7%(P≤0.001 vs model group), 12.6% (P≤0.001 vs model group) and 12.6% (P≤0.001 vs model group), respectively (Table 6, 7; Figure 2).
Table 6. Effects of DHK on the amplitude of Ops.
| Amplitude (μV) |
||||||
| Normal | Model | STZ+Insulin | STZ+CD | STZ+DHK | ||
| OP1 | Day 12 | 72.4±34.9c | 26.1±20.4*** | 31.8±13.4*** | 41.1±24.1*** | 36.8±2.7c |
| Day 24 | 80.6±49.8c | 31.1±16.1*** | 49.3±8.9 | 52.3±26.6 | 79.4±47.7b | |
| OP2 | Day 12 | 97.9±38.9c | 36.4±31.7*** | 57.6±23.8* | 58.2±36.2**a | 73.6±40.1b |
| Day 24 | 92.2±47.1b | 49.9±34.5** | 74.6±8.4 | 77.0±25.9 | 100.5±48.3b | |
| OP3 | Day 12 | 59.8±19.4c | 33.9±22.1*** | 46.5±16.5* | 39.0±19.1* | 50.0±28.7a |
| Day 24 | 62.7±36.6 | 43.6±31.4 | 47.3±9.9 | 50.2±14.5 | 52.4±30.7 | |
| OP4 | Day 12 | 60.9±1.0c | 72.13±1.0*** | 62.3±1.0c | 63.1±1.9c | 62.5±1.0c |
| Day 24 | 26.4±12.2 | 23.1±19.8 | 29.2±8.9 | 33.8±12.9 | 26.0±20.6 | |
| OPS | Day 12 | 263.8±93.7c | 113.5±76.7*** | 158.3±65.3*** | 169.8±77.1***a | 202.6±101.3*b |
| Day 24 | 261.9±127.5a | 147.7±98.7* | 200.8±26.2 | 213.3±77.1 | 258.3±143.1a | |
*P≤0.05, **P≤0.01 and ***P≤0.001 vs. normal group; aP≤0.05, bP≤0.01 and cP≤0.001 vs model control
(mean±SD, n=20)
Table 7. Effects of DHK on the latency of Ops.
| Latency (ms) |
||||||
| Normal | Model | STZ+Insulin | STZ+CD | STZ+DHK | ||
| OP1 | Day 12 | 27.5±0.6c | 33.6±0.5*** | 29.6±0.7*b | 27.8±1.6c | 29.5±0.8c |
| Day 24 | 24.9±1.1c | 32.6±0.7*** | 25.1±0.8c | 26.7±0.6c | 27.4±0.6*c | |
| OP2 | Day 12 | 36.8±0.7c | 44.8±0.9*** | 38.5±0.7c | 37.8±1.7c | 38.6±0.9c |
| Day 24 | 33.5±1.1c | 43.4±1.2*** | 33.5±0.8c | 35.4±0.6c | 37.0±0.7**c | |
| OP3 | Day 12 | 48.0±0.9c | 57.7±0.9*** | 49.4±0.8c | 49.9±1.8c | 50.0±0.9c |
| Day 24 | 43.3±1.3c | 56.4±1.3*** | 43.6±0.7c | 45.7±0.8c | 49.3±1.0***c | |
| OP4 | Day 12 | 60.9±1.0c | 72.1±1.0*** | 62.3±1.0c | 63.1±1.9c | 62.5±1.0c |
| Day 24 | 57.0±1.4c | 71.5±1.4*** | 56.8±0.5c | 58.5±0.8c | 62.5±1.3**c | |
*P≤0.05, **P≤0.01 and ***P≤0.001 vs normal group; aP≤0.05, bP≤0.01 and cP≤0.001 vs model control
(mean±SD, n=20)
Figure 2. Representative OPs after treatment of corresponding drugs in rat's right eyes.
A: 12 days postoperatively; B: 24 days postoperatively
Effect of DHK on Microvascular Perfusion
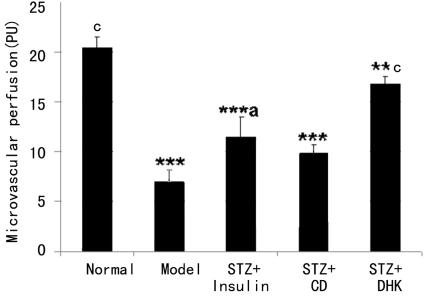
As shown in Figure 3, Diabetes mellitus was associated with a remarkable decrease in microvascular perfusion of ear in rats (P≤0.001 vs normal group). Insulin reversed the decrease significantly (P≤0.001 vs model group). However, CD, another positive control, had no effect on microvascular perfusion. The microvascular perfusion of STZ+DHK group was 2.4 times of model group (P≤0.001) after 24 days administration of drug.
Figure 3. Effect of DHK on microvascular perfusion of ear in diabetic rats.
*P≤0.05, **P≤0.01 and ***P≤0.001 vs normal group; aP≤0.05, bP≤0.01 and cP≤0.001 vs model group. Data were expressed as mean±SD, n=20
DISCUSSION
FERG is widely used to assess electrical responses of various cell types in the retina, including photoreceptors (rods and cones), inner retinal cells (bipolar and amacrine cells), and ganglion cells. OPs are high frequency, low amplitude wavelets embedded on the ascending limb of the FERG b-wave. OPs might be generated from the proximal retina by neural interactions among bipolar cells, amacrine cells, and ganglion cells. The oscillations may be initiated by activities of inhibitory feedback circuits in the inner plexiform layer[5]. Because such changes occur earlier than other ocular symptoms such as abnormal vision and other pathological signs, FERG recordings, particularly OPs, become useful in early diagnosis of DR[6],[7]. As shown in Tables 4-7, DHK significantly reversed the reduction in FERG b-wave and OPs OP1- and OP2-wave amplitudes and increase in FERG b-wave and OPs OP1- and OP2-wave latencies as well, which indicate that the results of STZ + DHK were significantly different from those of Model and moved toward those of Normal Control. The results suggest that DHK markedly improves the retinal function.
Improved glycemic controls help reduce the development and progression of DR[1],[8]. DHK had antihyperglycemic activity (Table 1), which might contribute to the preventive treatment of DR.
The prevalence of DR increases with the duration and severity of diabetes. Vascular injury is the hallmark of DR. Increased vascular permeability leads to diabetic macular edema, and vascular occlusion causes retinal ischemia and finally causes proliferative DR[9]. Some researchers found that retinal blood flow reduced in patients with diabetes mellitus without DR or with background DR[10],[11]. Results of this study also showed diabetes mellitus was associated with a remarkable decrease in microvascular perfusion of ear in rats (P≤0.001 vs normal group). DHK significantly increased microvascular perfusion and then improved the ischemia (Figure 3), which suggests that the effect on promoting blood circulation for removing blood stasis of DHK might be another mechanism of preventive treatment of DR.
In conclusion, DHK had no effect on FBG in normal rats. However, it had antihyperglycemic activity, and improved functional dysfunction of the retina in STZ-induced type 1 diabetic rats as well. The mechanisms of the preventive and therapeutic effect of DHK on DR maybe partly attribute to the antihyperglycemic activity and improvement of microvascular perfusion. DHK may be as an agent intended for use in the prevention and treatment of DR in humans.
REFERENCES
- 1.Williams R, Airey M, Baxter H, Forrester J, Kennedy-Martin T, Girach A. Epidemiology of diabetic retinopathy and macular oedema: a systematic review. Eye (Lond) 2004;18(10):963–983. doi: 10.1038/sj.eye.6701476. [DOI] [PubMed] [Google Scholar]
- 2.Weng JP. Epidemiology, evidence, and basic research of diabetes mellitus. J SUN Yat-sen Univ (Med Sci) 2010;31(2):166–171. [Google Scholar]
- 3.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102(4):520–526. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102(4):527–532. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 5.Lei B, Yao G, Zhang K, Hofeldt KJ, Chang B. Study of rod- and cone-driven oscillatory potentials in mice. Invest Ophthalmol Vis Sci. 2006;47(6):2732–2738. doi: 10.1167/iovs.05-1461. [DOI] [PubMed] [Google Scholar]
- 6.Tzekov R, Arden GB. The electroretinogram in diabetic retinopathy. Surv Ophthalmol. 1999;44(1):53–60. doi: 10.1016/s0039-6257(99)00063-6. [DOI] [PubMed] [Google Scholar]
- 7.Li Q, Zemel E, Miller B, Perlman I. Early retinal damage in experimental diabetes: electroretinographical and morphological observations. Exp Eye Res. 2002;74(5):615–625. doi: 10.1006/exer.2002.1170. [DOI] [PubMed] [Google Scholar]
- 8.Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000;23(Suppl 2):B21–29. [PubMed] [Google Scholar]
- 9.Kovach JL, Schwartz SG. Novel pharmacologic approaches for the management of diabetic retinopathy. Mol Cell Pharmacol. 2009;1(4):222–227. doi: 10.4255/mcpharmacol.09.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bursell SE, Clermont AC, Kinsley BT, Simonson DC, Aiello LM, Wolpert HA. Retinal blood flow changes in patients with insulin-dependent diabetes mellitus and no diabetic retinopathy. Invest Ophthalmol Vis Sci. 1996;37(5):886–897. [PubMed] [Google Scholar]
- 11.Feke GT, Buzney SM, Ogasawara H, Fujio N, Goger DG, Spack NP, Gabbay KH. Retinal circulatory abnormalities in type 1 diabetes. Invest Ophthalmol Vis Sci. 1994;35(7):2968–2975. [PubMed] [Google Scholar]