Abstract
AIM
To establish an untransfected human corneal epithelial (HCEP) cell line and characterize its biocompatibility with denuded amniotic membrane (dAM).
METHODS
The torn HCEP pieces were primarily cultured in DMEM/F12 media (pH 7.2) supplemented with 20% fetal bovine serum and other necessary factors, yielding an HCEP cell line which was its growth performance, chromosome morphology, tumorigenicity and expression of marker proteins analyzed. In addition, the biocompatibility of HCEP cells with dAM was evaluated through histological and immunocytochemistry analyses and with light, electron and slit-lamp microscopies.
RESULTS
HCEP cells proliferated to confluence in 3 weeks, which have been subcultured to passage 160. A continuous untransfected HCEP cell line, designated as utHCEPC01, was established with a population doubling time of 45.42 hours as was determined at passage 100. The cells retained HCEP cell properties as were approved by chromosomal morphology and the expression of keratin 3. They, with no tumorigenicity, formed a multilayer epithelium-like structure on dAMs through proliferation and differentiation during air-liquid interface culture, maintained expression of marker proteins including keratin 3 and integrin β1 and attached tightly to dAMs. The reconstructed HCEP was highly transparent and morphologically and structurally similar to the original.
CONCLUSION
An untransfected and non-tumorigenic HCEP cell line was established in this study. The cells maintained expression of marker proteins. The cell line was biocompatible with dAM. It holds the potential of being used for in vitro reconstruction of tissue-engineered HCEP, promising for the treatment of diseases caused by corneal epithelial disorders.
Keywords: human corneal epithelial cell, cell line, untransfected, biocompatibility, denuded amniotic membrane
INTRODUCTION
The human corneal epithelium (HCEP) is a multi- cellular membrane locating at the anterior end of cornea. It is crucial for maintaining corneal transparency, absorption of oxygen and nutrients and protecting the eye from infections of foreign microorganisms[1]. Cell homeostasis of a healthy functional HCEP is maintained by a unique subpopulation of stem cells (limbal epithelial stem cells, LESCs) located in the limbal region of corneas[2],[3]. Chemical or thermal burns, contact lenses, keratitis and so on often cause HCEP cell deficiencies including edema and turbidity of cornea[4],[5]. LESCs[6] and amniotic membranes (AMs)[7] have been clinically applied; however tissue-engineered HCEP (TE-HCEP) is considered as an ideal HCEP equivalent for repairing HCEP cell deficiencies[8].
HCEP cell lines are perfect models for studying cell differentiation, cellular signaling, immunology of HCEP graft rejection, molecular pathways regulating normal HCEP cell homeostasis, drug testing and in vitro reconstruction of TE-HCEP[9]. However, in vitro culture of HCEP cells is very difficult and time-consuming due to their short life span, rapid differentiation and limited availability of donor corneal tissues[10]. Numerous attempts have been made to cultivate HCEP cells in vitro for protracted periods; however cultured HCEP cell lines have only been established by transfection with SV40 T oncogenes[11],[12] and human telomerase reverse transcriptase genes[13]. These immortalized cell lines cannot be used for biological study of HCEP cells and reconstruction of TE-HCEPs due to their abnormal phenotypes, latent risk of tumorigenicity and decreased potency to reconstruct multilayered epithelia[8],[14]. Till now, only a spontaneously derived HCEP cell line was established through serial culture of limbal cells from a normal human limbus[10]. Unfortunately, there is no report on its functional characteristics and applications. To make a model for studies of HCEP cells and TE-HCEP reconstruction available, a continuous untransfected HCEP cell line was established and evaluated in this study.
MATERIALS AND METHODS
Materials
Corneas of a woman (26 years old, died from cerebral hemorrhage) were obtained from The Affiliated Hospital of Medical College, Qingdao University, Qingdao, China with a permission from her next of kin. The usage of the corneas as the source of HCEP cells for in vitro culture was approved by the Medical Ethics Committee of the hospital and the privacy of the patient was protected in compliance with the Declaration of Helsinki. SPF BalB/c nude mice (male, 18 to 22g in body weight) were purchased from Slaccas Experimental Animal Co., Ltd. (Shanghai, China). All animals were treated in accordance with the ARVO Statement for usage in tumorigenesis assay, and were approved by the Clinical Research Ethics Committee of New Drug Evaluation Center, Shandong University, Jinan, China. Fresh amniotic membranes (AMs) were obtained from Shandong Eye Institute of Shandong Medical Academy, Qingdao, China and denuded to obtain dAMs according to Fan et al[15].
Methods
In vitro culture of HCEP cells
The cornea epithelia along with Bowman's membrane were torn off and placed flatly onto a 35 mm culture dish and immerged with 0.5mL of 0.25% trypsin (Sigma-Aldrich, St. Louis, MO) with the epithelial side down for 2 minutes. The epithelia were rinsed with Dulbecco's Modified Eagle's Medium/Ham's Nutrient Mixture F12 (DMEM/F12, 1:1) medium (pH 7.2) (Invitrogen, Carlsbad, CA) and cut into eight pieces which were then attached directly to a 0.01% gelatin (Sigma-Aldrich)-coated wells of a 24-well culture plate with the epithelial side down and cultured in 0.2mL of DMEM/F12 medium containing 5% fetal bovine serum (FBS) (HyClone, Logan, Utah) at 37°C with 5% CO2. After about 12 hours, the medium was replaced with 20% FBS-DMEM/F12 medium (pH 7.2) supplemented with 10ng/mL basic fibroblast growth factor (bFGF) (Sigma-Aldrich), 40ng/mL epidermal growth factor (EGF) (Sigma-Aldrich), 0.8mg/mL chondroitin sulfate(Sigma-Aldrich), 50µg/mL carboxymethyl-chitosan (AK Scientific, Mountain, CA) and 100µg/mL collagen IV (Sigma-Aldrich). The primary culture was carried out at 37°C with 5% CO2 with medium refreshed every 4 days. Once a confluent monolayer formed, HCEP cells were collected by trypsinization and subcultured at a ratio of 1:2 as described previously[15]. From passage 20, the HCEP cells were subcultured in 10% FBS-containing DMEM/F12 medium (pH 7.2).
Growth properties
HCEP cells at passage 100 were collected by trypsinization and suspended in 10% FBS-DMEM/F12 medium (pH 7.2) to a density of 2.0×105 cell/mL with their growth properties measured as previously described[15]. HCEP cells in every 3 wells were numbered every 12 hours and averaged (mean±SD, 3 parallel experiments). The cell density was plotted against the time, yielding the growth curve on which the population doubling time of HCEP cells based.
Chromosome assay
At passage 100, the HCEP cells at logarithmic phase were treated with 20µg/mL of colchicine at 37°C for 10 hours, harvested, treated with 0.3% KCl hypotonic solution fixed and stained with Giemsa for 30 minutes as previously described[15]. Chromosomes were counted for 300 metaphase cells.
Immunocytochemistry assay
At passage 100, HCEP cells were collected with trypsinization and inoculated into the wells of a 24-well culture plate. At the logarithmic phase, the cells were washed with D-Hanks solution, fixed with 4% paraformaldehyde for 10 minutes and treated with 0.25% Triton X-100 at room temperature for 10 minutes. The wells were blocked with 5% bovine calf serum (BCS, Invitrogen) containing D-Hanks solution at 37°C for 30 minutes and incubated with mouse anti-human keratin 3 monoclonal antibody (Santa Cruz Biotechnology, Heidelberg, Germany) at 4°C overnight according to manufacturer's instructions. After incubated with fluorescent isothiocyanate (FITC)-conjugated goat anti-mouse IgG antibody (Biosynthesis Biotechnology, Beijing, China) at 37°C for 1 hour, the wells were analyzed with a Ti-S inverted fluorescent microscope (Nikon, Tokyo, Japan). Omission of primary antibodies was used as controls.
Tumorigenesis assay
Tumorigenesis assay of the HCEP cells was performed at passage 100 as previously described[15]. Ten SPF BalB/c nude mice were inoculated subcutaneously with 1.0×107 HCEP cells at logarithmic phase, one of the forehand oxters each mice, the other 10 with 1.0×107 HeLa cells. The tumorigenic status of the inoculated mice was monitored daily. The skin of the oxter of inoculated mice was surgically opened with tumorigenic status examined 60 days later.
Biocompatibility with dAMs
At passage 100, HCEP cells at logarithmic phase were collected with trypsinization with the density of cell suspension adjusted to 3.0×106 cell/mL with 10% FBS-DMEM/F12 medium (pH 7.2). Into each of a dAM-paved culture insert in a 24-well plate, 100µL cell suspension was plated and cultured at the same conditions as described above for 48 hours. Then the culture inserts were transferred into a 6-well plate containing 0.8mL 10% FBS-DMEM/F12 medium (pH 7.2) each well for air-liquid interface culture. The medium was refreshed and the morphology and growth status of the cells were monitored daily. The surface morphology of reconstructed HCEP was examined with an Eclipse TS100 inverted microscope (Nikon, Tokyo, Japan) and a JSM2840 scanning electron microscope (SEM) (JEOL, Tokyo, Japan). The histology of reconstructed HCEP was examined with paraffin section and hematoxylin-eosin (HE) staining. The multilayer structure of reconstructed HCEP and its dAM attachment status were examined with a H700 transmission electron microscope (TEM) (Hitachi, Tokyo, Japan). The expression of keratin 3 and integrin β1 was examined with freeze section and incubation with mouse anti-human keratin 3 and integrin β1 monoclonal antibodies(Santa Cruz Biotechnology), respectively, and FITC-conjugated goat anti-mouse IgG antibody (Biosynthesis Biotechnology) as described above. The freeze sections on glass slides were observed under a Ti-S fluorescent microscope (Nikon). The transparency of reconstructed TE-HCEP was examined with a macroscopy and a slit-lamp microscopy.
Statistical Analysis
Data were expressed as mean±SD (triplicates or decuplicates) and tested for statistical significance with ANOVA single factor.
RESULTS
In vitro Culture of HCEP Cells
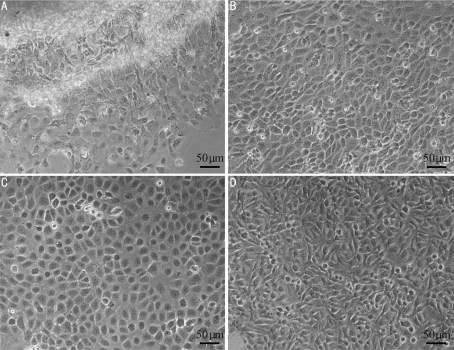
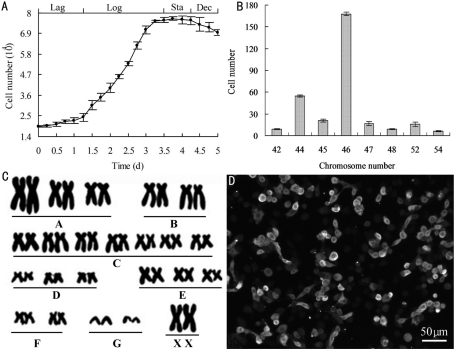
During primary culture, HCEP cells migrated from cornea pieces on day 3 (Figure 1A), growing into a confluent monolayer in 3 weeks (Figure 1B). They were highly transparent and polygonal in shape. During subculture, the HCEP cells maintained their polygonal shape (Figure 1C), and grew and proliferated at a steady rate. Their population doubling time was 45.42 hour as was determined at passage 100 (Figure 2A). After being subcultured for 3 years, a novel continuous untransfected HCEP cell line, designated as utHCEPC01, was established, which has been subcultured to passage 160 by now (Figure 1D).
Figure 1. In vitro culture of HCEP cells.
A: Primary cultures at day 3; B: A confluent monolayer formed 3 weeks later; C: At passage 100; D: At passage 160
Figure 2. Properties of HCEP cell line at passage 100.
A: The growth curve (Lag: Lag phase; Log: Logarithmic phase; Sta: Stationary phase; Dec: Decline phase); B: Chromosome aneuploidy; C: Normal diploid karyotype; D: Keratin 3 expression
Chromosome Analysis
At passage 100, HCEP cells exhibited chromosomal aneuploidy; the number of chromosomes ranged from 42 to 54. The proportion of HCEP cells with 46 chromosomes was about 56.0% (Figure 2B), indicating that the modal chromosome number of established HCEP cell line was 46 (Figure 2C).
Immunocytochemistry
At passage 100, HCEP cells maintained expression of keratin 3 gene, a specific marker of HCEP cells (Figure 2D), proving their HCEP origin.
Tumorigenicity
No solid tumor was found in 10 SPF BalB/c nude mice on day 60 after being inoculated with HCEP cells (passage 100) (Table 1). However, solid tumor was found in all 10 SPF BalB/c nude mice in 7 days after being inoculated with HeLa cells, which grew progressively thereafter. Therefore, the established HCEP cell line was not tumorigenic.
Table 1. Tumorigenesis assay of Passage 100 HCEP cells in BALB/c nude mice.
| Inoculated cells | Total dose (cell/mouse) | Number of mice | Mortality (%) | Number of mice with tumor |
| HCEP cells | 1.0×107 | 10 | 0 | 0 |
| HeLa cells | 1.0×107 | 10 | 0 | 10 |
Note: tumors were identified surgically
Biocompatibility of HCEP Cells with dAMs
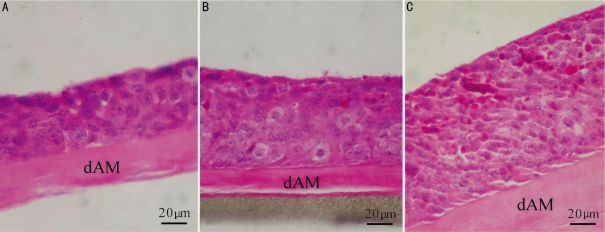
The passage 100 HCEP cells grew very well on dAMs and a well-stratified epithelium was reconstructed (Figure 3). After air-liquid interface cultured for 3 days, the HCEP cells formed a 4-5 layer epithelium-like structure (Figure 3A), and for 5 days and 7 days,7-8 layers and 10-11 layers epithelium-like structures, with a continuous layer of flattened apical cells, were formed, respectively (Figure 3B, C). The HCEP cells differentiated into flattened epidermal cells on its apical surface and cobblestone epithelial cells inside the multilayer epithelium at day 5 (Figure 3B). And most of epithelial cells inside the multilayer epithelium differentiated into elongated column-like epithelial cells at day 7 (Figure 3C).
Figure 3. HE staining of HCEP cells growing on dAM in air-liquid interface culture.
A: At day 3; B: At day 5; C: At day 7
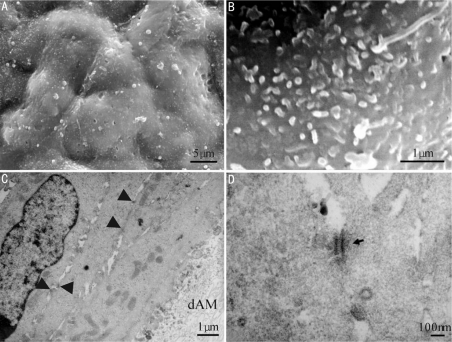
HCEP cells formed a continuous apical epithelial layer in 7 days of air-liquid interface culture (Figure 4A). They were cobblestone in shape and rich in microvilli on apical surface (Figure 5B). HCEP cells constructed very tight cell junctions in 7 days after air-liquid interface culture (arrows) and those adjacent to dAM on the boundary of cell and dAM secreted a lot of vesicles (Figure 4C). Desmosomes can be visualized between HCEP cells (arrow) (Figure 4D).
Figure 4. Electron microscopic images of HCEP cells growing on dAM in air-liquid interface culture.
A, B: SEM; C, D: TEM
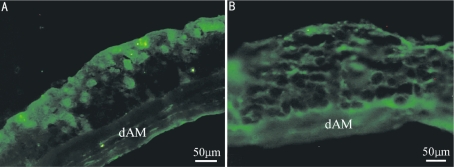
Figure 5. Immunohistochemical staining of HCEP cells growing on dAM in air-liquid interface culture.
A: Keratin 3; B: Integrin β1
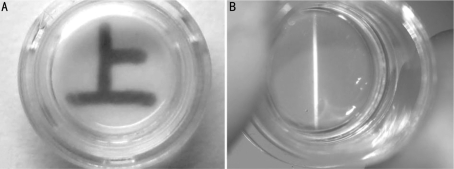
HCEP cells expressed keratin 3 and integrin β1 genes in air-liquid interface culture on dAM (Figure 5, day 7), indicating that they reserved the properties of HCEP cells and abilities of forming cell-dAM junctions. The reconstructed TE-HCEPs were highly transparent (Figure 6).
Figure 6. The transparency status of reconstructed HCEPs from Passage 100 HCEP cells and dAMs.
A: Macroscopic view; B: Slit-lamp microscopic view
DISCUSSION
Untransfected HCEP cell lines can serve as the cell banks of normal HCEP cells for TE-HCEP reconstruction, theoretic study and so on[9]. Numerous attempts have been made to obtain HCEP cells in long-term culture. A spontaneously derived HCEP cell line was established form human limbal cells[10]; however its function was not characterized and its application was not reported later. Based on our previous experience in corneal cell line establishments[16]-[18], tearing of cornea epithelium, controlled trypsin digestion and direct attaching of corneal pieces were used to obtain HCEP cells for primary culture in this study. Proliferation of HCEP cells has been successfully induced by replenishing culture medium with various supplements including bFGF, EGF, chondroitin sulfate, carboxymethyl-chitosan and collagen IV. Our success in the induction of proliferation of HCEP cells was consistent with the reported from cultured HCEP cells[10] and human corneal endothelial cells[18].
From pure HCEP cells and with supplement-induced proliferation, a continuous untransfected HCEP cell line, designated as utHCEPC01, with the modal chromosome number of 46 was successfully established and subcultured to passage 160 in this study. The cells proliferated actively and constantly with a population doubling time of 45.42 hours as was determined at passage 100, which was longer than those of spontaneously derived HCEP cell line (19.6 hours)[10] and primary corneal epithelial cells (24 hours)[19].
HCEP specific markers, such as keratin 3 and integrin β1, have often been used in HCEP cell characterization[10],[20]. In combination with the modal chromosome number, the expression of keratin 3 proved the human corneal epithelial origin of our HCEP cell line established in this study, which was consistent with the obtained in previous studies of HCEP cells[10],[20].
Oncogene transfected HCEP cell lines cannot be used in HCEP cell studies and reconstruction of TE-HCEP due to their latent potencies of tumorigenicity and abnormal phenotypes[8],[14]. The HCEP cell line we established was not tumorigenic; it can be safely used in studies of HCEP cells and in vitro reconstruction of TE-HCEPs.
Only those corneal cells with high biocompatibilities with scaffold carriers can be used for the reconstruction of tissue-engineered corneas[17],[18],[21]. Since dAM has been successfully used in TE-HCEP reconstruction[22],[23], the biocompatibility of the untransfected and non-tumorigenic HCEP cells with dAM was evaluated. The HCEP cells grew and differentiated very well on dAMs, forming a 10-11 layers epithelium-like structure and a continuous apical epithelial layer in 7 days. The apical HCEP cells were cobblestone in shape and secreted a lot of microvilli onto apical cell surface, which was similar in characteristics to those of squamous cells from HCEP in vivo[9],[10]. A lot of desmosomes were established between HCEP cells and vesicles secreted by HCEP cells onto the boundary of cells and dAM. The materials contained in these vesicles might be the precursors for the basal membrane formation. HCEP cells maintained expression of keratin 3 and integrin β1, indicating that they reserved the properties of HCEP cells and abilities of forming cell-dAM junctions. The reconstructed TE-HCEPs showed similar morphology and structure to those of innate HCEP, coinciding with those described in previous reports[6],[7],[22],[23]. It can be concluded that the HCEP cells were highly biocompatible with dAM and they can be used for in vitro reconstruction of TE-HCEP.
In conclusion, a continuous untransfected and non-tumorigenic HCEP cell line, utHCEPC01, was established in this study. The cell line provided a powerful tool for studies on proliferation, differentiation, renewal of HCEP cells and so on. It can be used for the reconstruction of TE-HCEP, promising for the treatment of diseases caused by HCEP cell deficiency. Studies on TE-HCEP reconstruction and its animal transplantation are our ongoing trials.
Acknowledgments
We thank Ai Sun, Qian-Qian Feng and Xi-Ya Ma for their technical assistances, and Dr. Guan-Pin Yang for his polishing of the manuscript.
Footnotes
Foundation item: Supported by National High Technology Research and Development Program (“863” Program) of China (No. 2006AA02A132)
REFERENCES
- 1.Kinoshita S, Adachi W, Sotozono C, Nishida K, Yokoi N, Quantock AJ, Okubo K. Characteristics of the Human Ocular Surface Epithelium. Prog Retin Eye Res. 2001;20(5):639–673. doi: 10.1016/s1350-9462(01)00007-6. [DOI] [PubMed] [Google Scholar]
- 2.Dua HS, Shanmuganathan VA, Powell-Richards AO, Tighe PJ, Joseph A. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol. 2005;89(5):529–532. doi: 10.1136/bjo.2004.049742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stepp MA, Zieske JD. The corneal epithelial stem cell niche. Ocul Surf. 2005;3(1):15–26. doi: 10.1016/s1542-0124(12)70119-2. [DOI] [PubMed] [Google Scholar]
- 4.Daniels JT, Notara M, Shortt AJ, Secker G, Harris A, Tuft SJ. Limbal epithelial stem cell therapy. Expert Opin Biol Ther. 2007;7(1):1–3. doi: 10.1517/14712598.7.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Notara M, Alatza A, Gilfillan J, Harris AR, Levis HJ, Schrader S, Vernon A, Daniels JT. In sickness and in health: Corneal epithelial stem cell biology, pathology and therapy. Exp Eye Res. 2010;90(2):188–195. doi: 10.1016/j.exer.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Shortt AJ, Secker GA, Notara MD, Limb GA, Khaw PT, Tuft SJ, Daniels JT. Transplantation of ex vivo cultured limbal epithelial stem cells: a review of techniques and clinical results. Surv Ophthalmol. 2007;52(5):483–502. doi: 10.1016/j.survophthal.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Andri KR, Roger WB, Laurence SL, Jodhbir SM. Preservation, sterilization and de-epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biom. 2010;31(2):216–225. doi: 10.1016/j.biomaterials.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 8.Fan TJ, Yang HS, Hu XZ, Zhao J. Research advances on in vitro reconstruction of tissue-engineered human corneal epithelium. J Shandong Univ Med. 2010;48(7):7–13. [Google Scholar]
- 9.Federico CM. Corneal epithelial cell cultures as a tool for research, drug screening and testing. Exp Eye Res. 2008;86:459–469. doi: 10.1016/j.exer.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Song G, Wang Z, Huang B, Gao Q, Liu B, Xu Y, Liang X, Ma P, Gao N, Ge J. Establishment of a corneal epithelial cell line spontaneously derived from human limbal cells. Exp Eye Res. 2007;84(3):599–609. doi: 10.1016/j.exer.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Araki-Sasaki K. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36(3):614–621. [PubMed] [Google Scholar]
- 12.Yamasaki K, Kawasaki S, Young RD, Fukuoka H, Tanioka H, Nakatsukasa M, Quantock AJ, Kinoshita S. Genomic Aberrations and Cellular Heterogeneity in SV40-Immortalized Human Corneal Epithelial Cells. Invest Ophthalmol Vis Sci. 2009;50(2):604–613. doi: 10.1167/iovs.08-2239. [DOI] [PubMed] [Google Scholar]
- 13.Robertson DM, Li L, Fisher S, Pearce VP, Shay JW, Wright WE, Cavanagh HD, Jester JV. Characterization of growth and differentiation in a telomerase- immortalized human corneal epithelial cell line. Invest Ophthalmol Vis Sci. 2005;46(2):470–478. doi: 10.1167/iovs.04-0528. [DOI] [PubMed] [Google Scholar]
- 14.Yamasaki K, Kawasaki S, Young RD, Fukuoka H, Tanioka H, Nakatsukasa M, Quantock AJ, Kinoshita S. Genomic aberrations and cellular heterogeneity in SV40-immortalized human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2009;50(2):604–613. doi: 10.1167/iovs.08-2239. [DOI] [PubMed] [Google Scholar]
- 15.Fan T, Zhao J, Ma X, Xu X, Zhao W, Xu B. Establishment of a continuous untransfected human corneal endothelial cell line and its biocompatibility to denuded amniotic membrane. Mol Vis. 2011;17:469–480. [PMC free article] [PubMed] [Google Scholar]
- 16.Fan T, Zhao J, Fu Y, Cong R, Guo R, Liu W, Han B, Yu Q, Wang J. Establishment of a novel corneal endothelial cell line from domestic rabbit, Oryctolagus curiculus. Sci China C. 2007;50(2):161–169. doi: 10.1007/s11427-007-0033-1. [DOI] [PubMed] [Google Scholar]
- 17.Fan T, Wang D, Zhao J, Wang J, Fu Y, Guo R. Establishment and characterization of a novel untransfected corneal endothelial cell line from New Zealand white rabbits. Mol Vis. 2009;15:1070–1078. [PMC free article] [PubMed] [Google Scholar]
- 18.Fan T, Zhao J, Ma X, Xu X, Zhao W, Xu B. Establishment of a continuous untransfected human corneal endothelial cell line and its biocompatibility to denuded amniotic membrane. Mol Vis. 2011;17:469–480. [PMC free article] [PubMed] [Google Scholar]
- 19.Kahn CR, Young E, Lee IH, Rhim JS. Human corneal epithelial primary cultures and cell lines with extended life span: in vitro model for ocular studies. Invest Ophthalmol Vis Sci. 1993;234:3429–3441. [PubMed] [Google Scholar]
- 20.Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vrana NE, Builles N, Justin V, Bednarz J, Pellegrini G, Ferrari B, Damour O, Hulmes DJ, Hasirci V. Development of a reconstructed cornea from collagen-chondroitin sulfate foams and human cell cultures. Invest Ophthalmol Vis Sci. 2008;49:5325–5331. doi: 10.1167/iovs.07-1599. [DOI] [PubMed] [Google Scholar]
- 22.Shortt AJ, Secker GA, Rajan MS, Meligonis G, Dart JK, Tuft SJ, Daniels JT. Ex vivo expansion and transplantation of limbal epithelial stem cells. Ophthalmol. 2008;115(11):1989–1997. doi: 10.1016/j.ophtha.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 23.Nadia Z, Carina K, Viggo VT, Zwi B, Andrew H, Marie-José T. Standardized Limbal Epithelial Stem Cell Graft Generation and Transplantation. Tissue Eng C. 2010;16(5):921–927. doi: 10.1089/ten.TEC.2009.0634. [DOI] [PubMed] [Google Scholar]