Abstract
AIM
To investigate the effect of aminoguanidine (AG) on the expression of caspase-3 in rat retina after ischemia- reperfusion injury.
METHODS
The rats were anesthetized with 30mg/kg sodium pentobarbital introperitoneal(ip) injections. After topical application of 10g/L dicaine, the anterior chamber was punctured with a 5-gauge needle connected to a bottle containing normal saline. Intraocular pressure was raised to 100 mmHg by elevating the saline container. The infusion needle was removed from the anterior chamber 60 minutes later. Reperfusion of the retinal vasculature was confirmed by fundus examination. AG 100mg/kg was ip injected in drug group. The rats were then euthanatized at 6, 24, and 72 hours after reperfusion, and their eyes were enucleated for immunohistochemistry.
RESULTS
No specific staining was detected by using the caspase-3 antibody in the retina of control group. In ischemia group, the protein of caspase-3 was over-expressed at 6 hours and relieved at 24 hours and 72 hours, while with drug treatment, the expression of protein of caspase-3 was decreased at each time point.
CONCLUSION
AG provides retinal protection against ischemia-reperfusion injury in rat retina, probably through an inducible NOS-dependent mechanism.
Keywords: aminoguanidine, ischemia-reperfusion, caspase-3
INTRODUCTION
Glaucoma is a leading cause of blindness in the world. The gradual vision loss seen with glaucoma is associated with a progressive loss of retinal ganglion cells (RGC) and axons leading to deterioration of the optic nerve[1]-[2]. RGC death has been demonstrated in animal models of glaucoma. The pattern of death of retinal ganglion cell(RGC) in glaucoma is apoptosis. The markers of apoptosis have been observed in the human glaucomatous retina[3]-[5]. Inhibiting apoptosis of RGC is the key of neuroprotection of the optic nerve in glaucomatous eyes. The current studies show that the mechanisms that may initiate RGC apoptosis in glaucoma include neurotrophic factor deprivation, hypoperfusion/ischemia, glial cell activation, glutamate excitotoxicity, and abnormal immune response. Some evidences indicated that the aminoguanidine plays a neuroprotection role by inhibition of NOS-2 for the treatment of patients with glaucoma[6]. However, the effects of aminoguanidine on the RGC apoptosis are not clear. In this study, we are going to investigate the anti-apoptosis effects of aminoguanidine on RGC after ischemia reperfusion (I/R) injury.
MATERIALS AND METHODS
Materials
Animals and I/R Injury Model Establishment
Fifty-four adult Wistar rats( 180-220g) were divided at random into 3 groups of 18 animals each: control group, ischemia/reperfusion (I/R) group and aminoguanidine treatment group. The process of ischemia reperfusion (I/R) injury was performed as following: briefly, the rats were anesthetized with ip injection of 30mg/kg sodium pentobarbital. After topical application of 0.2mL oxybuprocaine, the anterior chamber was punctured with a 5-gauge needle connected to a bottle containing normal saline. Intraocular pressure was raised to 100mmHg by elevating the saline container. The infusion needle was removed from the anterior chamber 60 minutes later. Reperfusion of the retinal vasculature was confirmed by fundus examination. AG 100mg/kg was ip in drug group. The rats were then euthanatized at 6, 24, and 72 hours after reperfusion, and their eyes were enucleated for further detection.
Methods
Immunohistochemistry
After euthanasia, the rat eyes were rapidly enucleated and placed in ice-cold BSS. The anterior chamber, lens, and vitreous were then removed and the resultant eyecups were immediately placed into 100g/L neutral formaldehyde. Next, the eyecups were Paraffin embedded, and cut into 10µm thick cross-sections. The eyecups from the three groups were embedded into the same block and sectioned onto the same slide to reduce variability when comparing changes in immunoreactivity. Immunohistochemistry method was as previously described [references]. Briefly, immunocytochemistry was performed on the retinal sections using standard methods on retinal cross-sections mounted on slides. Immunohistochemistry pictures were taken on Olympus BX51. Images were analyzed using the Metamorph Image software. Absorbance value (A) of each view was used for estimation of intensity of staining.
Statistical Analysis
Statistical analysis was performed using SPSS 12.0. P<0.05 was considered statistically significant.
RESULTS
No specific staining was detected by using the caspase-3 antibody in the retina of control group. In I/R group, there were positive staining cells in the RGCL, (inner plexiform layer) IPL and (inner nuclear layer) INL (especially the inner part of the INL).Positive staining materials were scattered in the cytoplasm with brown or light brown colors. The number of positive cells in I/R group was markedly more than that in contral group at 6 hours (P<0.01) and then decreased progressively. Compared with the control group, however, the number of positive cells was significantly different (P<0.01). In drug given group, the number of positive cells was less than that in the ischemia-reperfusion group markedly at 6 hours and 24 hours (P<0.0l), and was less than that of the I/R group at 72 hours (P<0.05). Compared with the control group, the number of positive cells was more in AG group, it showed significant difference at 6 hours (P<0.01) and difference at 24 hours and 72 hours (P<0.05).
Table 1. Caspase-3 positive cells in rat retina.
| Group | The number of caspase-3 positive cells on retina |
||
| 6h | 24h | 72h | |
| Control | 1.17±0.37b | 1.33±0.47b | 1.83±0.98b |
| I/R | 13.67±2.07 | 11.83±2.48 | 5.17±1.60 |
| AG | 3.67±0.82b | 3.00±0.89b | 3.50±1.89a |
aP<0.05, bP<0.01 vs I/R group
(mean±SD, n=6)
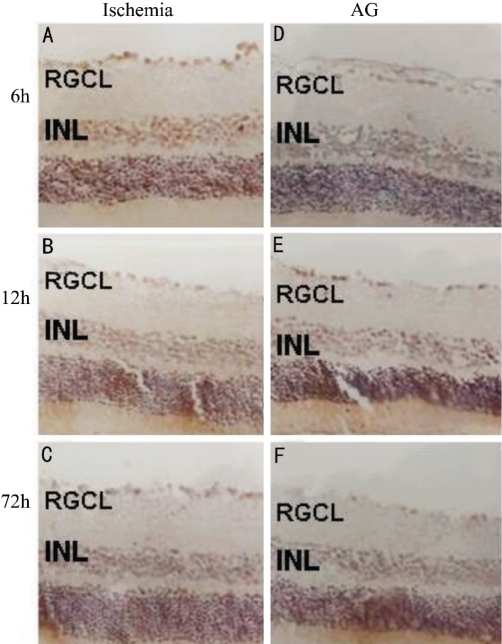
Figure 1. Caspase-3 expression between ischemia rat retina(SABC×200).
Immunohistochemical localization of caspase-3 in the 6h-ischemia retina (A), 24h-ischemia retina (B), 72h-ischemia retina (C), 6h-AG-treament retina (D), 24h-AG-treament retina (E), 72h-AG-treament retina (F). In the A, B, C, caspase-3 is over-expressed. In the D, E, F, caspase-3 is lower expressed. RGCL, ganglion cell layer; INL, inner nuclear layer
DISCUSSION
Neuroprotection of retinal ganglion cell (RGC) from death has recently been emphasized as an important strategy for the management of glaucoma. Some studies indicated that AG appears to be a novel and effective therapeutic approach for retinal NV[5]. Vakili's findings show that AG decrease ischemic brain damage dose-dependently and improve neurological recovery in acute phase of transient focal cerebral ischemia[6]. In this study, we investigate the anti-apoptosis effects of AG on RGC. The detection of activated caspase-3 is a very reliable way to identify cells destined to die by apoptosis even before many of the morphological characteristics are present. All the active caspase then active the substrates, finally induce cell apoptosis. Caspase-3 is the main executor of apoptosis, and is a specific marker of cell process death. In our study, we observed that the expression of caspase-3 was upregulated at protein level in RGCL and INL in early period after ischemia-reperfusion injury. We found that the retinal injury was most serious in early period after ischemia-reperfusion and we could improve the changes of the tissues by using AG to inhibit apoptosis. So it is necessary for us to further understand the mechanisms of the apoptosis understand the time window of the effective treatment against ischemia-reperfusion injury in glaucoma and seek valued drugs to inhibit apoptosis. All these will be very important and good for us to protect against the I/R injury in glaucoma.
REFERENCES
- 1.Weber AJ, Harman CD, Viswanathan S. Effects of optic nerve injury, glaucoma, and neuroprotection on the survival, structure, and function of ganglion cells in the mammalian retina. J Physiol. 2008;586(Pt 18):4393–4400. doi: 10.1113/jphysiol.2008.156729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta N, Ang LC, No?l de Tilly L, Bidaisee L, Yücel YH. Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br J Ophthalmol. 2006;90(6):674–678. doi: 10.1136/bjo.2005.086769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36(5):774–786. [PubMed] [Google Scholar]
- 4.Kerrigan LA, Zack DJ, Quigley HA, Smith SD, Pease ME. TUNELpositive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol. 1997;115:1031–1035. doi: 10.1001/archopht.1997.01100160201010. [DOI] [PubMed] [Google Scholar]
- 5.Okisaka S, Murakami A, Mizukawa A, Ito J. Apoptosis in retinal ganglion cell decrease in human glaucomatous eyes. Jpn J Ophthalmol. 1997;41(2):84–88. doi: 10.1016/s0021-5155(97)00013-0. [DOI] [PubMed] [Google Scholar]
- 6.Ghada Ghanem El-Hossary, Amal Ahmed El-Gohary, Amany Hassan El-Shazly. Topical Instillation of Aminoguanidine Reducing Intraocular Pressure and Improving visual Evoked Potential in Rabbits with Experimental Glaucoma. Res J Medicine & Med Sci. 2010;5(1):18–24. [Google Scholar]
- 7.Yang Y, Di Y, Gui DM, Liu ZL, Liu X, Gao DW. IOP-lowering effects for the application of human umbilical vein in non-penetrating deep sclerostomy in rabbits. Int J Ophthalmol. 2011;4(1):55–57. doi: 10.3980/j.issn.2222-3959.2011.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q, Zhang J, Guan Y, Zhang S, Zhu C, Xu GT, Wang L. Suppression of retinal neovascularization by the iNOS inhibitor aminoguanidine in mice of oxygen-induced retinopathy. Graefes Arch Clin Exp Ophthalmol. 2009;247(7):919–927. doi: 10.1007/s00417-009-1066-x. [DOI] [PubMed] [Google Scholar]
- 9.Vakili A, Zahedi-Khorasani M. Effect of aminoguanidine on post-ischemic damage in rodent model of stroke. Pak J Pharm Sci. 2008;21(1):24–28. [PubMed] [Google Scholar]