Abstract
AIM
To explore the effect of SDF-1α on the development of experimental corneal neovascularization (CRNV).
METHODS
CRNV was induced by alkali injury in mice. The expression of SDF-1α and CXCR4 in burned corneas was examined by Flow Cytometry. Neutralizing anti-mouse SDF-1α antibody was locally administrated after alkali injury and the formation of CRNV 2 weeks after injury was assessed by Immunohistochemistry. The expression of VEGF and C-Kit in burned corneas was detected by RT-PCR.
RESULTS
The number of CRNV peaks at 2 weeks after alkali injury. Compared to control group, SDF-1α neutralizing antibody treatment significantly decreased the number of CRNV. RT-PCR confirmed that SDF-1α neutralizing antibody treatment resulted in decreased intracorneal VEGF and C-Kit expression.
CONCLUSION
SDF-1α neutralizing antibody treated mice exhibited impaired experimental CRNV through down regulated VEGF and C-Kit expression.
Keywords: corneal neovascularization, alkali injury, chemokine
INTRODUCTION
Cornea is physiologically avascular and transparent. The normal transparent state is maintained by blood barrier which contributed to prevent any leukocytes and hematopoietic cells infiltrate into corneal stroma[1], and ensure the optical system to perform clarified visual function. However, under some pathological conditions, such as inflammation, chemical burn and infection which leading to inflammatory cells infiltration and angiogenesis, it would damage the transparent structure and at last cause severe visual impairment[2].
As a chemokine receptor of SDF-1α/CXCL12, CXCR4 is widely expressed in monocytes, lymphocytes, hematopoietic, endothelial progenitor cells and other cells[3]. It involved in cell chemotaxis, adhesion, proliferation, apoptosis and other functions after activation by its ligand of SDF-1α[4]. In addition, the expression of CXCR4 was detected in vascular endothelial cells, suggesting that SDF-1α/CXCR4 axis may play a critical role in angiogenesis[5]. It was concomitantly with recent studies which revealed that SDF-1α can cause posterior segment angiogenesis under pathological conditions. They explained that CXCR4 was expressed on hematopoietic stem cells, and SDF-1α could binding to its receptor, and through the SDF-1α-CXCR4 interaction, the signaling pathway may lead to the occurrence of choroidal neovascularization[6]. Meanwhile, induced by stimulation of inflammatory factors such as FGF2 and VEGF, mature endothelial cells can up-regulate the expression of CXCR4[7], and this function would indirectly enhance the angiogenesis signal.
The signaling pathway of SDF-1α/CXCR4 plays a critical role in ocular neovascular diseases such as choroidal neovascularization, diabetic retinopathy and hypoxia-induced retinopathy[8]. However, their function in other diseases such as corneal neovascularization is yet unclear. In order to delineate their possibly specific mechanism in corneal neovascularization, we have prepared alkali-inducing experimental CRNV model using NaOH, topically administrated neutralizing anti-mouse SDF-1α mAbs, microscopically and immunostaining detected the occurrence of corneal neovascularization, and examined the target gene expression in injury corneas. Through these exploration and resultant statistically analysis, the experiment conclusion will provide us a new thinking about SDF-1α/CXCR4 signaling pathway involvement in ocular neovascularization.
MATERIALS AND METHODS
Materials
Reagents and antibodies
Sodium hyaluronate (HA) and Avertin were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Neutralizing goat anti-mouse SDF-1α (CL9189AP) mAbs were purchased from CEDARLANE Laboratories Ltd. (Burlington, Ontario, Canada), Rabbit anti-mouse CXCR4 Ab (14-6009) was supplied from eBioscience (San Diego, CA, USA). PE-labeled rabbit anti-goat IgG Ab and FITC-labeled rat anti-rabbit IgG Ab were purchased from Pierce Co. (Rockford, IL, USA). Rat anti-mouse CD31 (MEC13.3) mAbs were purchased from BD Pharmingen (San Diego, CA, USA).
Mice
Specific pathogen-free 7 to 8 weeks old male BALB/c mice weighing 20 to 25 g were obtained from Shanghai SLAC Laboratory Animal Co., Ltd and were kept in our animal facility under specific pathogen-free conditions. All animal experiments were done in accordance with the Guideline for the Care and Use of Laboratory Animals on the Chinese Medical Academy and the Soochow University Animal Care Committee, and with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Animals were kept in groups of 5 and fed regular lab chow and water ad libitum. A 12-hour day and night cycle was maintained.
Methods
Alkali-induced corneal injury model
Alkali-induced corneal injury model was prepared for target genes and proteins detection and CRNV areas analysis. Mice about 7 to 8 weeks old were divided into 2 groups. Each group contained 10 mice. Among them, one group was treated with neutralizing goat anti-mouse SDF-1α (CL9189AP) mAbs, and another group was treated with 0.2% sodium hyaluronate as control. Corneal injury was induced by placing a 2 mm2 filter disc saturated with 1N NaOH onto the left eye of the mouse for 40 seconds as previously described[9],[10]. In neovascularization enumeration or gene detection experiments, the alkali-treated eyes received 5 µl of anti-mouse SDF-1 mAbs dissolved in 0.2% sodium hyaluronate at a concentration of 5 µg/ml, or 5 µl of 0.2% sodium hyaluronate as vehicle twice a day for 7 days immediately after the alkali injury. At the indicated time intervals, mice were sacrificed, and whole eyes were removed. The eyes were snap-frozen in O.C.T compound for histological analysis, or the corneas were removed and placed immediately into RNALate (Qiagen, German), and kept at -86°C until total RNA extraction was performed. Each experiment was repeated at least three times.
Biomicroscopic examination
Eyes were examined with a slit lamp from Haag Streit (BQ 900®, Swiss made), and results were photographed on day 14. In brief, under anesthesia, photographs of the corneas were obtained using a digital camera (Nikon, Tokyo, Japan) linked to the slit lamp.
Enumeration of corneal neovascularization
The fixed cryosections (8 µm thick) were stained using anti-CD31 mAb. The numbers and sizes of the CRNV were determined as described previously[9] by an examiner with no knowledge of the experimental procedures. Briefly, images were captured with a digital camera and imported into Adobe Photoshop. Then, the numbers of neovascular tubes per mm2, and the proportions of CRNV in the hot spots were determined using NIH Image analysis software version 1.62 (National Institutes of Health, Bethesda, MD). Most sections were taken from the central region of the cornea. The numbers and areas of corneal neovascularization were evaluated on at least two sections from each eye. The relative neovascular area was compared between treated group and control group. We used Student's t test to statistically analyze the difference between treated and control group.
Flow cytometrical analysis of SDF-1 and CXCR4 expression in injured corneas
Relative cells were isolated from corneas according to the procedure described previously with some modifications[9]. Briefly, at 2-4 days after the alkali injury, corneas were removed, teased away with scissors, and were incubated at 37°C for 40 minutes with constant shaking in the presence of 0.5 mg/ml collagenase type D (Roche Diagnostics, Mannheim, Germany). Cell suspensions were then passed over a nylon filter with 100-µm pore size. The resultant cells were further stained with goat anti-mouse SDF-1α mAbs and Rabbit anti-mouse CXCR4 Ab respectively following by staining with PE-labeled rabbit anti-goat IgG Ab or FITC-labeled rat anti-rabbit IgG Ab. Fluorescence intensities were determined with the help of FACS Calibur (Becton Dickinson), together with the samples stained with non-immunized rabbit IgG or rat IgG as an isotype control separately.
Semi-quantitative reverse transcription (RT)-polymerase chain reaction (PCR)
Total RNAs were extracted from the corneas with the use of RNeasy Mini Kit (Qiagen, German). The resultant RNA preparations were further treated with ribonuclease-free deoxyribonuclease (DNase) I (Life Technologies Inc., Gaithersburg, MD) to remove genomic DNA. 2µg of total RNAs were reverse-transcribed at 42°C for 1 hour in 20µL of reaction mixture containing mouse Moloney leukemia virus reverse transcriptase and hexanucleotide random primers (Qiagen). Serially two-fold diluted cDNA was amplified for GAPDH to estimate the amount of transcribed cDNA. Then, equal amounts of cDNA products were amplified for the target genes using the primers under the following conditions; denaturation at 94°C for 2 minutes, followed by the optimal cycles of 30 sec at 94°C, 35 sec at 56-58°C, 35 sec at 72°C, and a final 10 minutes extension step at 72°C. Primers and PCR conditions used were shown in Table 1. The amplified PCR products were fractionated on a 1.5% agarose gel and visualized by ethidium bromide staining. The band intensities were measured and their ratios to GAPDH were determined with the aid of NIH Image Analysis software.
Table 1. Specific sets of primers and conditions of PCR.
| Primers | Nucleotide sequence(5′→3′)sense/anti-sense | Annealing Temperature(°C) | PCR Cycles |
| VEGF | 5′-AGCCGAGCTCATGGACGGGT-3′ | 56 | 35 |
| 5′-GCACGCACTCCAGGGCTTCA-3′ | |||
| C-Kit | 5′-TCGCAGCTGGCGCGATGG-3′ | 57 | 35 |
| 5′-AGTGCCGCTTCTGCCTGCTC-3′ | |||
| CXCR4 | 5′-TTTGCCGACGTCAGCCAGGG-3′ | 58 | 37 |
| 5′-GGATG ACGATGCCGGGCAGG-3′ | |||
| SDF-1α | 5′-TGCCCCTGCCGGTTCTTCGAG-3′ | 57 | 35 |
| 5′-CTGTTGTTGTTCTTCAGCCGT-3′ | |||
| GAPDH | 5′-ACCACAGTCCATGCCATCAC-3′ | 58 | 25 |
| 5′-TCCACCACCCTGTTGCTGTA-3′ |
Statistical Analysis
The means and standard error of the mean (SEM) were calculated for all parameters determined in the study. Values were processed for statistical analyses (Student's t test) with statistic software SPSS15.0. A value of P < 0.05 was considered statistically significant.
RESULTS
Intracorneal Expression of SDF-1α and CXCR4 between Normal Corneas and Alkali-burned Corneas
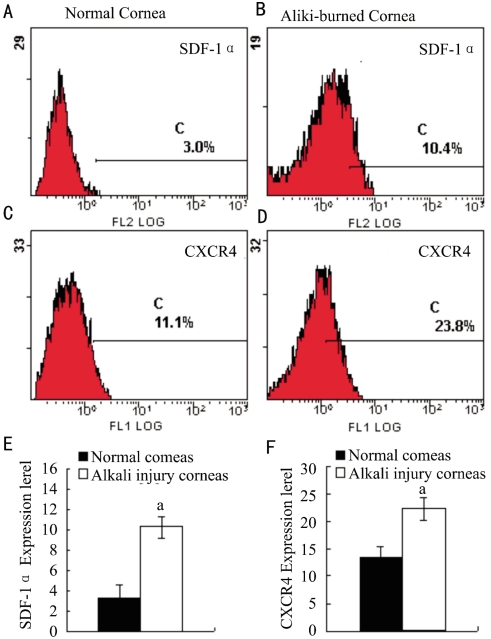
We have previously examined target genes expression including SDF-1α and CXCR4 in mice corneas[11]. In this study, we further examined the protein expression of SDF-1α and CXCR4 in corneas after alkali-induced corneal injury by FCS. We found that the protein expression of SDF-1α and CXCR4 was detectable in alkali-induced corneas and normal corneas compared to IgG isotype negative control, their expression was markedly increased at day 4 after alkali injury (P<0.05, Figure 1). The enhanced intracorneal SDF-1α and CXCR4 protein expression suggests the possible involvement of the SDF-1α-CXCR4 interactions in alkali-induced CRNV.
Figure 1. SDF-1α and CXCR4 protein expression in normal and alkali injured corneas.
Corneal tissues were pooled 4 days after injury from alkali-burned mice or untreated mice. Each test of tissues was immunostained with anti-SDF-1α and CXCR4 antibody respectively. Ab host originated isotype IgG as negative control. The intracorneal SDF-1α and CXCR4 protein expression were tested by FCS. The representative results from five to eight animals are shown above. Each value represents mean±SEM (n = 5-8 animals). The data was analyzed by t test. aP<0.05 vs control.
Effects of Neutralizing Anti-mouse SDF-1α mAbs on Alkali Injury Induced Experimental CRNV
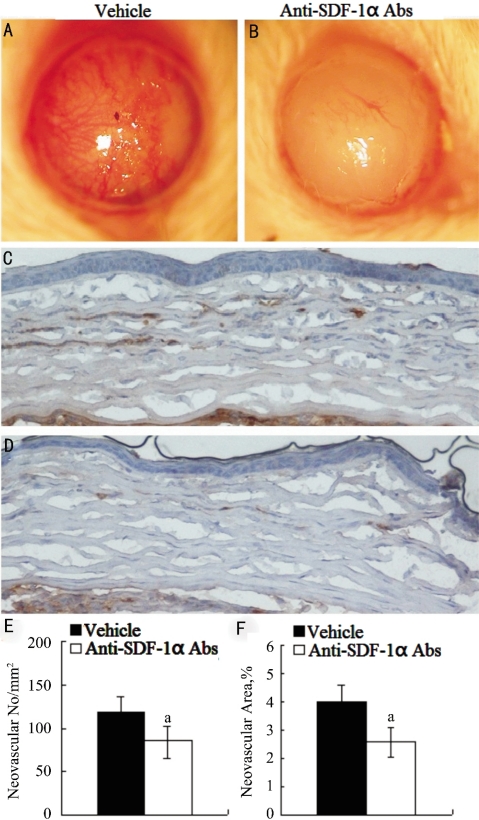
We stepped to explore the effects of neutralizing anti-mouse SDF-1α mAbs on alkali-induced CRNV. CRNV was macroscopically evident in BALB/c mice 2 weeks after the injury. All of the corneas showed stromal opacification and none of them exhibited perforation. Alkali induced CRNV in neutralizing anti-SDF-1α mAb treated mice was markedly decreased compared to control mice (Figure 2A, B). Immunohistochemical analysis using anti-CD31 antibodies revealed similar tendencies in anti-SDF-1α mAbs or vehicle treated BALB/c mice even at microscopical levels (Figure 2C-F). These results would indicate the involvement of SDF-1α-CXCR4 axis in alkali-induced CRNV.
Figure 2. Alkali injury-induced CRNV in anti-SDF-1α mAbs treated and vehicle treated BALB/c mice.
A, B: Macroscopic appearance of eyes from vehicle and anti-SDF-1α mAbs treated BALB/c mice 2 weeks after alkali injury; C, D: Tissues were immunostained with anti-CD31 antibody, and representative results are shown here. Original magnifications×400; E: CRNV numbers per square millimeter in whole section; F: Percentage CRNV areas in hot spots were determined from the corneas obtained from vehicle and anti-SDF-1α mAbs treated BABL/c mice 2 weeks after injury. Each value represents mean±SEM (n=5-8 animals). aP<0.05 vs vehicle-treated mice.
Reduced mRNA Expression of C-kit and VEGF in the Wound Corneas in Neutralizing Anti-mouse SDF-1α mAbs treated mice
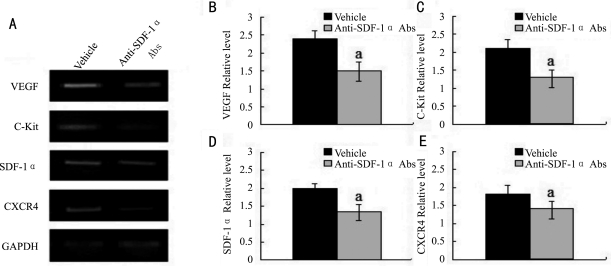
We previously revealed that the VEGF and C-Kit expressing cells are located in the corneal matrix after alkali injury[10],[11]. In order to further delineate the roles of SDF-1α-CXCR4 axis in CRNV, we next examined the effects of neutralizing anti-mouse SDF-1α mAbs on the expression of C-kit and VEGF. By RT-PCR we observed the mRNA expression of CXCR4 and SDF-1α were reduced in SDF-1α mAbs treated mice compared to control mice. C-kit and VEGF in corneas of neutralizing anti-mouse SDF-1α mAbstreated mice was reduced compared to vehicle treated mice (Figure 3, P<0.05). These observations would indicate that neutralizing anti-mouse SDF-1α mAbs can block the function of SDF-1α-CXCR4 interaction to stimulate VEGF expression and C-kit+ cells infiltration.
Figure 3. RT-PCR analysis of gene expression in the injured corneas of vehicle and anti-SDF-1α mAbs treated BALB/c mice.
A: Representative result from three independent experiments of RT-PCR; B-E: Ratios of indicated genes to GAPDH of alkali-injured vehicle (black bar) and anti-SDF-1α mAbs treated BALB/c mice (grey bar) determined by RT-PCR at the indicated time intervals after alkali injury. All values represent mean±SEM (n=5-8 animals). aP<0.05 vs vehicle-treated mice.
DISCUSSION
SDF-1α is a member of CXC- chemokine family which involves in the migration of bone marrow progenitor cells[12] and previous reports indicated that Bone marrow-derived endothelial progenitor cells (BM) migration can promote the occurrence of choroidal neovascularization[13]. Therefore, in our study, we speculated that SDF-1α may promote CRNV by recruiting stem cells migration to injured corneas. We have detected the expression of SDF-1α and CXCR4 in the corneal stroma with or without alkali injury. To further observe the role of CXCR4/SDF-1α axis in alkali induced CRNV, we next topically administrated neutralizing anti-SDF-1α mAbs after alkali injury. The results showed that the intracorneal C-Kit mRNA expression in neutralizing anti-SDF-1α mAbs treated group was markedly reduced compare to vehicle treated mice. There is evidence that BM source of C-Kit-positive cells can differentiate into vascular endothelial cells and promote angiogenesis[14]. Therefore, our results revealed that anti-SDF-1α neutralizing antibody could halt C-Kit-positive cells infiltration into alkali injured corneas, thereby reducing the alkali-induced CRNV.
In our experiment, we also found that the VEGF gene expression in SDF-1α antibody treated group decreased significantly. There is evident that VEGF expression is related to SDF-1α/CXCR4 signaling pathway. On one hand, VEGF can chemotaxis CXCR4-positive bone marrow-derived hematopoietic stem cells to sites of inflammation through its receptor-1 and up-regulate CXCR4 expression on these cells[15]. Namely, VEGF involves in SDF-1/CXCR4 signal regulated angiogenesis[16]. On the other hand, CXCR4/SDF-1α axis can induce Akt kinase phosphorylation, and ultimately enhance the expression of VEGF both of mRNA and protein, thereby promoting VEGF-mediated angiogenesis[17]. Recent report reveals that SDF-1α can affect angiogenesis by acting on types of cells other than monocytes/macrophages. It is generally believed endothelial cells derived from endothelial progenitor cells (EPCs) play critical roles in the corneal neovascularization. The recruitment pathway for EPCs is dependent on the chemokine SDF-1α and its receptor CXCR4 on the progenitor cell. Reported data indicate myeloid lineage cells can also serve as endothelial progenitor cells and contribute to neovascularization and SDF-1α can enhance and accelerate the differentiation of myeloid cells to endothelial cells[18]. More over, Kijowski and colleagues[19] observed SDF-1α augments the lymphoid cell lines VEGF production. Thus, the differentiate potential of CXCR4+ bone marrow derived EPCs to the endothelial progenitor cells would further facilitate the CRNV. Published data suggest the CXCR4 expression and function such as tube formation and migration to SDF-1α in human retinal microvascular endothelial cells[20]. More over, stimulation of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) increases the expression of CXCR4 on endothelial cells, suggesting VEGF and bFGF enhanced CXCR4-dependent neovascularization[21]. Our experiments showed that SDF-1α antibody intervene in the early phase after alkali injury could decrease intracorneal VEGF mRNA expression.
In summary, we can conclude that SDF-1α neutralizing antibody can reduce the occurrence of CRNV. SDF-1α neutralizing antibody treatment exhibited impaired experimental CRNV through down regulated VEGF and C-Kit expression These results would provide a theoretical basis for the feasibility of using SDF-1α neutralizing antibody to inhibit the occurrence of ocular neovascularization.
Footnotes
Foundation items: Supported by National Natural Science Foundation in China (NSFC No. 30771978 and No 30972712); Qing-Lan Project of Education Bureau of Jiangsu Province, and Supported by Jiangsu Province's Key Provincial Talents Program, China (No. RC2011104)
REFERENCES
- 1.Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJ, Richter E, Sakurai E, Newcomb MT, Kleinman ME, Caldwell RB, Lin Q, Ogura Y, Orecchia A, Samuelson DA, Agnew DW, St Leger J, Green WR, Mahasreshti PJ, Curiel DT, Kwan D, Marsh H, Ikeda S, Leiper LJ, Collinson JM, Bogdanovich S, Khurana TS, Shibuya M, Baldwin ME, Ferrara N, Gerber HP, De Falco S, Witta J, Baffi JZ, Raisler BJ, Ambati J. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang S, Ma J. Ocular neovascularization: Implication of endogenous angiogenic inhibitors and potential therapy. Prog Retin Eye Res. 2007;26:1–37. doi: 10.1016/j.preteyeres.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pablos JL, Amara A, Bouloc A, Santiago B, Caruz A, Galindo M, Delaunay T, Virelizier JL, Arenzana-Seisdedos F. Stromal-cell derived factor is expressed by dendritic cells and endothelium in human skin. Am J Pathol. 1999;155:1577–1586. doi: 10.1016/S0002-9440(10)65474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sengupta N, Caballero S, Mames RN, Butler JM, Scott EW, Grant MB. The role of adult bone marrow-derived stem cells in choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:4908–4913. doi: 10.1167/iovs.03-0342. [DOI] [PubMed] [Google Scholar]
- 7.Murdoch C, Monk PN, Finn A. CXC chemokine receptor expression on human endothelial cells. Cytokine. 1999;11:704–712. doi: 10.1006/cyto.1998.0465. [DOI] [PubMed] [Google Scholar]
- 8.Sengupta N, Caballero S, Mames RN, Butler JM, Scott EW, Grant MB. Paracrine modulation of CXCR4 by IGF-1 and VEGF: implications for choroidal neovascularization. Invest Ophthalmol Vis Sci. 2010;51:2697–2704. doi: 10.1167/iovs.09-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu P, Li L, Kuno K, Wu Y, Baba T, Li YY, Zhang X, Mukaida N. Protective roles of the fractalkine/CX3CL1-CX3CR1 axis against alkali-induced corneal neovascularization through enhanced anti-angiogenic factor expression. J Immunol. 2008;108:4283–4291. doi: 10.4049/jimmunol.180.6.4283. [DOI] [PubMed] [Google Scholar]
- 10.Lu P, Li L, Mukaida N, Zhang X. Alkali-induced corneal neovascularization is independent of CXCR2-mediated neutrophil infiltration. Cornea. 2007;26:199–206. doi: 10.1097/01.ico.0000248385.16896.34. [DOI] [PubMed] [Google Scholar]
- 11.Liu G, Lu P, Li L, Jin H, He X, Mukaida N, Zhang X. Critical role of SDF-1α-induced progenitor cell recruitment and macrophage VEGF production in the experimental corneal neovascularization. Molecular Vision. 2011;17:2129–2138. [PMC free article] [PubMed] [Google Scholar]
- 12.Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi H, Yanagi Y, Tamaki Y, Muranaka K, Usui T, Sata M. Contribution of bone-marrow-derived cells to choroidal neovascularization. Biochem Biophys Res Commun. 2004;320:372–375. doi: 10.1016/j.bbrc.2004.05.177. [DOI] [PubMed] [Google Scholar]
- 14.Ohki Y, Heissig B, Sato Y, Akiyama H, Zhu Z, Hicklin DJ, Shimada K, Ogawa H, Daida H, Hattori K, Ohsaka A. Granulocyte colony-stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. FASEB J. 2005;19:2005–2007. doi: 10.1096/fj.04-3496fje. [DOI] [PubMed] [Google Scholar]
- 15.Sawano A, Iwai S, Sakurai Y, Ito M, Shitara K, Nakahata T, Shibuya M. Vascular endothelial growth factor receptor-1 (Flt-1) is a novel cell surface marker for the lineage of monocyte-macrophages in human. Blood. 2001;97:785–791. doi: 10.1182/blood.v97.3.785. [DOI] [PubMed] [Google Scholar]
- 16.Zagzag D, Lukyanov Y, Lan L, Ali MA, Esencay M, Mendez O, Yee H, Voura EB, Newcomb EW. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab Invest. 2006;86:1221–1232. doi: 10.1038/labinvest.3700482. [DOI] [PubMed] [Google Scholar]
- 17.Liang Z, Brooks J, Willard M, Liang K, Yoon Y, Kang S, Shim H. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem Biophys Res Commun. 2007;359:716–722. doi: 10.1016/j.bbrc.2007.05.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stellos K, Langer H, Daub K, Schoenberger T, Gauss A, Geisler T, Bigalke B, Mueller I, Schumm M, Schaefer I, Seizer P, Kraemer BF, Siegel-Axel D, May AE, Lindemann S, Gawaz M. Platelet-derived stromal cell-derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation. 2008;117:206–215. doi: 10.1161/CIRCULATIONAHA.107.714691. [DOI] [PubMed] [Google Scholar]
- 19.Kijowski J, Baj-Krzyworzeka M, Majka M, Reca R, Marquez LA, Christofidou-Solomidou M, Janowska-Wieczorek A, Ratajczak MZ. The SDF-1-CXCR4 axis stimulates VEGF secretion and activates integrins but does not affect proliferation and survival in lymphohematopoietic cells. Stem Cells. 2001;19:453–466. doi: 10.1634/stemcells.19-5-453. [DOI] [PubMed] [Google Scholar]
- 20.Sameermahmood Z, Balasubramanyam M, Saravanan T, Rema M. Curcumin modulates SDF-1alpha/CXCR4-induced migration of human retinal endothelial cells (HRECs) Invest Ophthalmol Vis Sci. 2008;49:3305–3311. doi: 10.1167/iovs.07-0456. [DOI] [PubMed] [Google Scholar]
- 21.Salcedo R, Zhang X, Young HA, Michael N, Wasserman K, Ma WH, Martins-Green M, Murphy WJ, Oppenheim JJ. Angiogenic effects of prostaglandin E2 are mediated by up-regulation of CXCR4 on human microvascular endothelial cells. Blood. 2003;102:1966–1977. doi: 10.1182/blood-2002-11-3400. [DOI] [PubMed] [Google Scholar]