Abstract
AIM
To investigate roles of surfactant protein D (SP-D) and relative cytokines in human corneal epithelial(HCE) cells exposed to aspergillus fumigatus (AF) antigens.
METHODS
HCE cells cultured in vitro with AF antigens and sampled at 0, 0.5, 1 hour, 2, 4, 6 and 8 hours. The expression of SP-D mRNA was evaluated by semiquantitative reverse transcription-polymerase chain reaction (RT-PCR).The expression of SP-D protein was shown by ELISA and immunocytochemistry SP methods. The expression of NF-κB and relative downstream cytokines such as TNF-α, IL-1β, IL-8 and IL-10 in supernatant fluid were measured by ELISA.
RESULTS
SP-D mRNA and protein were detected in untreated HCE cells. The expression of SP-D and the relative downstream cytokines rose after being stimulated with AF antigens. SP-D mRNA began to rise at 0.5 hour and the most significantly peak was in 2 hours. The protein of SP-D in supernatant fluid had the same trend with mRNA. Immunocytochemistry of SP-D showed positive expression and gradually increased to 6 hours, and then the expression began to decline. NF-κB was activated after treated by AF antigens and the changes had correlation with SP-D. TNF-α, IL-1β, IL-8 and IL-10 began to rise after given AF antigens 1 hour and were 1.82, 1.43, 1.12 and 1.28 times higher than the untreated HCE cells separately. The expression of TNF-α and IL-1β reached the peak at 2 hours, separately 2.80 and 2.86 times than the untreated. The expression of IL-8 and IL-10 gradually increased with a time-dependent manner.
CONCLUSION
HCE cells exists SP-D and it may play a significant role in pathogenesis of keratomycosis. AF may induce human corneal epithelial cells to express inflammatory cytokines via SP-D and NF-κB pathway. SP-D possibly mediates the recognition to AF mycelium.
Keywords: corneal epithelial cells, aspergillus fumigatus, surfactant protein D, innate immune
INTRODUCTION
In developing countries fungal keratitis (FK) is a kind of very common eye disease. Survey shows that over the past decade in China FK had a rising trend. In some areas of China it had become the primary reason of blind caused by infection[1],[2]. Aspergillus fumigatus is one of the main pathogen of FK[3]. Innate immune molecules of immunity system can recognize fungal antigens. This is the basis of successful defense and removes the fungal infections. In latest studies, Vemuganti et al[4] found that pattem recognition receptors (PRRs) recognized pathogen associated molecular pattern (PAMPs) in the molecular level. It has been discovered that these C-type lectins as PRRs played some important roles in early stage of the fungi inflammation[5]. Surfactant protein D (SP-D) is a kind of collagen-containing C-type (calcium dependent) lectins called collectins, which contribute significantly to surfactant homeostasis and innate immunity. SP-D can interact with most pathogenic fungi, such as Cryptococcus, Aspergillus fumigatus, Candida albicans, Histoplasma capsulatum and so on[6]. SP-D can recognize these pathogenic microorganisms and help to clear them. For the distinctive characteristics of immunology at cornea, we want to know if the corneal epithelial cells recognize the pathogenic fungi via SP-D at cornea area. So we used Aspergillus fumigates(AF) antigens to stimulate the human corneal epithelial(HCE) cells which we cultured in vitro. We studied whether THCEs could express SP-D. We also studied the activation of nuclear factor kappaB (NF-κB) and the expression levels of Th1-type cell cytokines (TNF-α, IL-1β) and Th2-type cell cytokines (IL-8, IL-10).
MATERIALS AND METHODS
Materials
High glucose medium, newborn calf serum and trypsin from American HyClone products; Sabouroud culture from American Sigma company product; AF strains was bought from China General Microbiological Culture Collection Center; THCEs as a gift from Zhongshan University; Trizol Reagent from American Invitrogen products; PCR primers and Marker from Dalian Takara products; SP-D, NF-κB, TNF-α, IL-1β, IL-8 and IL-10 ELISA kits from American R&D products; Immune cell chemistry SP kit and SP-D antibodies from Beijing Biosynthesis company products.
AF spores antigens
AF grew in Sabouroud medium, 28°C, 5 days; physiological saline flushed the fungi surface; collected the fluid; 3000rpm centrifugal 5 minutes after 70% alcohol inactivating 30 minutes, then washed three times by PBS[7]. The above antigens stimulation was saved in -20°C, and these antigens stimulation liquid would be used up in 2 weeks.
HCE cells culture and stimulation
HCE cells (5×106) were seeded into 25cm2 flasks and were cultured in high glucose medium containing 15% FCS. Near 80% confluence, the cells were cultured in serum free DMEM with 0.05% bovine serum albumin for 24 hours. Cells were used for RT-PCR. The experimental groups were cultured with AF spores antigens stimulation liquid at 0.5, 1 hour, 2, 4, 6 and 8 hours. Supernatant fluid was collected in order to detect the expression levels of NF-κB, TNF-α, IL-1β, IL-8 and IL-10. All experimental procedures were performed under normoxic conditions (20% PO2, 5% CO2). At the end of each experiment, the cells on glass cover slides were fixed for 5 minutes in 4% paraformaldehyde and were processed for immunocytochemistry.
RT-PCR
For conventional reverse transcription-polymerase chain reaction (RT-PCR), lung tissue samples (n=6) and THCE cells were crushed in an agate mortar under liquid nitrogen, and then homogenized in 5mL Trizol. Insoluble material was removed by centrifugation (12 000g, 5 minutes, 4°C). Total RNA was isolated by RNA purification. Contamination of the purified RNA by genomic DNA was prevented by performing PCR with the specific primers for SP-D and GAPDH. The primers were synthetized by Sangon from Shanghai. After 5 minutes of heat denaturation at 96°C, the PCR cycle was conducted at 96°C for 60 seconds, 57°C (SP-D) for 60 seconds each, and 72°C for 60 seconds. Thirty-five cycles were performed with each primer pair. The final elongation cycle consisted of 72°C for 4 minutes. Six micro liters PCR was loaded on a 2% agarose gel. After electrophoresis, the amplified products were visualized by fluorescence. Base pair (bp) values were compared with GenBank data. For verification and comparison, human lung tissues carrying the genes for the investigated protein were used as a reference. PCR products were also confirmed by sequencing. To estimate the amount of amplified PCR product, we performed a GAPDH PCR with specific primers for each investigated tissue. For this additional PCR, we used the conditions described.
Primers and Productions sizes.
| Gene | Primer sequence | Product size(bp) |
| SP-D | F:AGGAGCAAAGGGAGAAAGTGGG | 199 |
| R:CAGCTGTGCCTCCGTAAATGG | ||
| GAPDH | F:AGAAGGCTGGGGCTCATTTG | 258 |
| R:AGGGGCCATCCACAGTCTTC |
Immunocytochemistry
HCE cells were cultured on slides for the immunocytochemistry following routine methods. Cells were also seeded on glass cover slides and were incubated. Near confluence, cells were exposed to AF spores antigens stimulation liquid at 0, 0.5, 1 hour, 2, 4, 6 and 8 hours. All experimental procedures were performed under normoxic conditions (20% PO2, 5% CO2). At the end of each experiment, the cells on glass cover slides were fixed for 5 minutes in 4% paraformaldehyde and were processed for immunocytochemistry. The antibody used as the following: rabbit anti–human SP-D antibody (Beijing Boaosen Co., China).
ELISA
We detected the protein concentration of SP-D, NF-κB, TNF-α, IL-1β, IL-8 and IL-10 with ELISA kit. We input the standard product spectrophotometry (A) value and concentration in Excel, forming fitting curve, then we could get the concentrations of generation.
Statistical Analysis
For statistical analysis, data obtained from this experiment were analyzed with SPSS 11.5 using One-way ANOVA. Differences were considered significant for P<0.05.
RESULTS
Expression of SP-D mRNA in HCE Cells
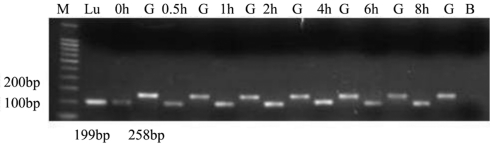
SP-D specific cDNA amplification products (199 bp) were detected in all groups (n=6) for HCE cells (Figure 1); The GAPDH control PCR was positive and of similar amount for all investigated groups, as expected, and it allowed a quantitative correlation of the amplified product (right bands of the agarose gels). Base pair values were equivalent to the expected DNA products compared with GenBank data.
Figure 1. RT-PCR results of SP-D.
Ethidium bromide–stained agarose gels for visualization of PCR amplification products derived from the following groups (n=6 for each group): human lung tissue samples (Lu),untreated THCEs (0h), human GAPDH (G), AF antigens stimulated 0.5 hour groups (0.5h), AF antigens stimulated 1h groups (1h), AF antigens stimulated 2 hours groups (2h), AF antigens stimulated 4 hours groups (4h), AF antigens stimulated 6 hours groups (6h), AF antigens stimulated 8 hours groups (8h). PCR amplification products of the human lung tissue were used for positive control; blank lanes (B) indicate the negative control without template DNA. To estimate quantitative correlation, a GAPDH PCR control was performed for investigated tissue (right lanes). In accordance with the DNA marker (M) and the human lung tissue products, the distinct DNA bands are visible at 199bp for SP-D.
Table 1. SP-D mRNA levels in untreated group and AF antigens stimulated groups.
| Group | Number of samples | SP-D/GAPDH mRNA |
| Untreated | 6 | 0.91±0.01 |
| 0.5h | 6 | 0.93±0.01a |
| 1h | 6 | 1.00±0.01a |
| 2h | 6 | 1.43±0.01a |
| 4h | 6 | 1.31±0.01a |
| 6h | 6 | 0.97±0.11a |
| 8h | 6 | 1.01±0.17a |
The cultured THCE cells were incubated with AF spores antigens stimulation liquid for 0.5h, 1h, 2h, 4h, 6h and 8h. The mRNA expression of SP-D in these cells was evaluated by RT-PCR, with untreated cells as control. We found that the mRNA expression of SP-D was largely increased after treated with AF spores antigens. Results shown are mean±SD of six independent experiments. ameans P<0.01
(mean±SD)
Immunocytochemistry results of SP-D
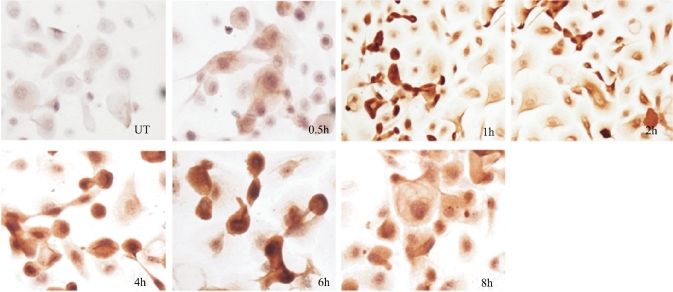
To further investigate the cellular location and stimulation of SP-D protein ex vivo, cultured epithelial cells were incubated with AF spores at different time. Immumihistochemical staining showed that SP-D protein was lowly produced by the normal epithelial cells. Stronger staining throughout the cytoplasm was observed in the cells exposed to AF spores at different time points (Figure 2).
Figure 2. SP-D expression in cultured THCE cells evaluated by immunohistochemical staining.
The cultured THCE cells incubated with AF spores antigens stimulation liquid for 0.5, 1, 2, 4, 6 and 8 hours. Those cells were used for SP-D immunohistochemical staining (200×)
ELISA Analysis
The samples of normal control groups and experimental groups were tested for SP-D, NF-κB, TNF-α, IL-1β, IL-8 and IL-10 using ELISA analysis (Figure 3,4).
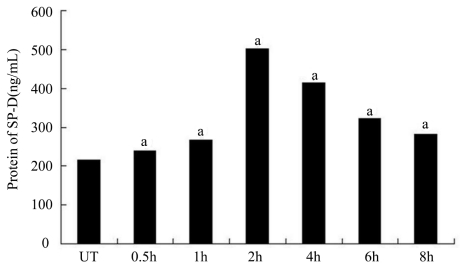
Figure 3. ELISA results of SP-D.
The cultured HCE cells were cultured with AF spores antigens stimulation liquid for 0.5, 1 hour, 2, 4, 6 and 8 hours. Supernatant fluid was collected for Elisa assay. Results shown are mean±SD of six independent experiments. ameans P<0.01
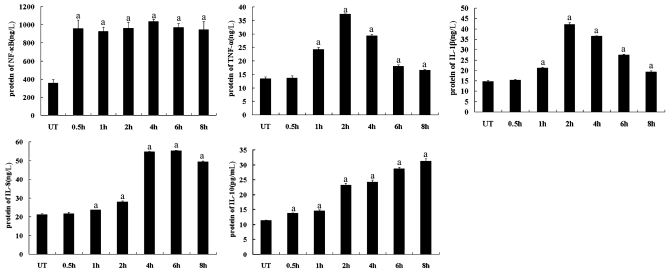
Figure 4. ELISA results of NF-κB, TNF-α, IL-1β, IL-8 and IL-10.
The cultured HCE cells were cultured with AF spores antigens stimulation liquid for 0.5, 1 hour, 2, 4, 6 and 8 hours. Supernatant fluid was collected for ELISA assay. Results shown are mean±SD of six independent experiments. ameans P<0.01
DISCUSSION
Surfactant protein D is a member of the collectin family of C-type lectins that includes a number of molecules which known host defense functions. According to the current notion, collectins bind to carbohydrates expressed on the surfaces of various microorganisms and to specific receptors on phagocytotic cells, thus accelerating microbial clearance. SP-D binds to carbohydrates and lipids in a calcium-dependent manner and probably plays a role in innate host defense against various pathogens like fungal[8]. Thus, SP-D deficiency and dysfunction can lead to identify disorder and reduce susceptibility to pathogenic microorganisms[9]. At the same time the downstream cytokines of SP-D will be released to induce the damage of tissues.
Our results showed that SP-D is present in the human corneal epithelial cells. It was detected on the mRNA level with RT-PCR. In the early stage of ocular surface against fungal infections SP-D mRNA responded to a time-dependent increase, and then it presented a decreasing trend in later stage. That is to say human corneal epithelial cells can recognize AF antigens and lead to the SP-D mRNA change. Immunocytochemistry results also proved that SP-D expressed in the cytoplasm of corneal epithelial cells under normal circumstances and the expression significantly increased after stimulation. With the application of ELISA, SP-D in the supernatant fluid showed that it peaked at 2 hours after stimulation and reduced from 4 hours. These also were consistent with our RT-PCR results. Our experimental results were consistent with Bräuer et al[10] who verified that SP-D was positive in human corneal and conjunctival tissues. They also found SP-D played a protective role in Staphylococcus aureus-based ulceration. Ni et al[11] also reported that SP-D existed in human corneal tissues and played a protective role to resist the Pseudomonas aeruginosa to damage the human corneal epithelial cells.
We adopt AF spores antigens to stimulate THCEs and the results showed that experimental groups THCEs appeared NF-κB activation, and secretion level of inflammatory cytokines TNF-α, IL-1β, IL-8 and IL-10 appeared corresponding change. AF antigens may through SP-D activate NF-κB signal pathway, and then THCEs express downstream related inflammatory cytokines. The expression level of Th1-type cytokines (TNF-α, IL-1β) were raising earlier than Th2-type cytokines (IL-8, IL-10). TNF-α and IL-1β rose in the similar trend with SP-D. But the expressions of NF-κB, IL-8 and IL-10 had the different changing trend with SP-D. Those are because the host firstly through the defense responses produces TNF-α, IL-1β to gather neutrophils, and then through the immune negative adjustment factor such as IL-10 regulates a drift from Th1-type to Th2-type. Th2-type cytokines such as IL-8 increase gradually. Th1 and Th2-type cytokines interacted to form complex cytokine network, adjusted negative feedback effect between Th1-type and Th2-type immune response, maintained the normal immune balance. Usually we thought Th1-type immune reaction of the body enhanced immunity, which was helpful to control infection, and Th2-type can aggravate infection[12]. The study from Rong and Zhou[13] also shows that SP-D can make the production of Th2 cytokines in the spleen, and Th1-type cytokines secretion increased at the same time. Chiba et al[14] reported that SP-D may cut down the inflammatory cytokines such as IL-6 and monocytes chemotaxis protein-1 (KC-1), and it can inhibit synthesis and release of IL-8 to inhibit inflammation.
Our study indicates that the corneal epithelial cells expressed SP-D. AF may induce human corneal epithelial cells to express inflammatory cytokines via SP-D and NF-κB pathway, and the signal pathway can control the expression Th1-type and Th2-type cytokines. SP-D may be another kind of pattern recognition receptors in human corneal epithelial cells to recognize the AF antigens.
Footnotes
Foundation item: National Natural Science Foundation of China (No.81170825)
REFERENCES
- 1.Leal SM, Jr, Cowden S, Hsia YC, Ghannoum MA, Momany M, Pearlman E. Distinct roles for Dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. PLoS Pathog. 2010;6(7):e1000976. doi: 10.1371/journal.ppat.1000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie LX. Advances in basic and clinical corneal research. Zhonghua Yanke Zazhi. 2010;46(10):883–887. [PubMed] [Google Scholar]
- 3.Zhao GQ, Wang X, Hu LT, Lin J, Che CY, Jiang N. Expression of substance P in experimental fungal keratitis. Chinese Journal of Ophthalmology. 2011;47(5):443–450. [PubMed] [Google Scholar]
- 4.Vemuganti GK, Garg P, Gopinathan U, Naduvilath TJ, John RK, Buddi R, Rao GN. Evaluation of agent and host factors in progression of mycotic keratitis: A histologic and microbiologic study of 167 corneal buttons. Ophthalmology. 2002;109(8):1538–1546. doi: 10.1016/s0161-6420(02)01088-6. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Liu WD. Collectins and its roles in the fungal infections. International Journal of Dermatology and Venereology. 2006;11(32):396–398. [Google Scholar]
- 6.Zhang L, Ikegami M, Korfhagen TR, McCormack FX, Yoshida M, Senior RM, Shipley JM, Shapiro SD, Whitsett JA. Neither SP-A nor NH2-terminal domains of SP-A can substitute for SP-D in regulation of alveolar homeostasis. Am J Physiol Lung Cell Mol Physiol. 2006;291(2):L181–190. doi: 10.1152/ajplung.00015.2006. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J, Wu XY, Yu FS. Activation of Toll-like receptors 2 and 4 in Aspergillus fumigatus keratitis. Innate Immun. 2009;15(3):155–168. doi: 10.1177/1753425908101521. [DOI] [PubMed] [Google Scholar]
- 8.Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, Bernal AL, Reid KB, Madan T, Chakraborty T. Surfactant proteins SP-A and SP-D: Structure, function and receptors. Mol Immunol. 2006;43(9):1293–1315. doi: 10.1016/j.molimm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Knudsen L, Wucherpfennig K, Mackay RM, Townsend P, Muhlfeld C, Richter J, Hawgood S, Reid K, Clark H, Ochs M. A recombinant fragment of human surfactant protein D lacking the short collagen-like stalk fails to correct morphological alterations in lungs of SP-D deficient mice. Anat Rec (Hoboken) 2009;292(2):183–189. doi: 10.1002/ar.20830. [DOI] [PubMed] [Google Scholar]
- 10.Bräuer L, Kindler C, Jäger K, Sel S, Nölle B, Pleyer U, Ochs M, Paulsen FP. Detection of surfactant proteins A and D in human tear fluid and the human lacrimal system. Invest Ophthalmol Vis Sci. 2007;48(9):3945–3953. doi: 10.1167/iovs.07-0201. [DOI] [PubMed] [Google Scholar]
- 11.Ni MJ, David JE, Hawgood S, Margot Anders E, Robert AS, Suzanne MJF. Surfactant protein D is present in human tear fluid and the cornea and inhibits epithelial cell invasion by pseudomonas aeruginosa. Infect Immun. 2005;73(4):2147–2156. doi: 10.1128/IAI.73.4.2147-2156.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romani L. Innate and adaptive immunity in Candida albicans infections and saprophytism. J Leukoc Biol. 2000;68(2):l75–l79. [PubMed] [Google Scholar]
- 13.Rong L, Zhou X. Advances in the relationship between C-type lectin-receptors and anti-fungal infection. Zhongguo Ganran Yu Hualiao Zazhi. 2008;8(6):443–446. [Google Scholar]
- 14.Chiba H, Pattanajitvilai S, Mitsuzawa H, Kuroki Y, Evans A, Voelker DR. Pulmonary surfactant proteins A and D recognize lipid ligands on Mycoplasma pneumonia and markedly augment the innate immune response to the organism. Chest. 2003;123(3):426S–a. [PubMed] [Google Scholar]