Abstract
AIM
To investigate auto-cortex of crystalline lens induced iris neovascularization (INV).
METHODS
Thirty-six eyes of 36 guinea-pigs were included and divided into three groups randomly in this cohort study. Group A: the right lens nucleus was extracted and the remaining cortical lens material was aspirated thoroughly. Group B: the lens was removed and 30µL precipitated lens cortex was injected into the anterior chamber again. Group C: aspirated the lens cortex of the left eyes and inject them into the right anterior chambers about 10µL. Clinical changes were followed by slit-lamp examination and photograph. The eye balls were enucleated at the day of 2, 4, 7, 11, 13, 17 after operation. HE was used to detect the pathological changes.
RESULTS
Group A: INV had not been observed until the end of empirical study. The stromal layer contained thick wall vessels, without expansion. Group B: All eyes developed INV. Postoperative (po) 7 days; the eyes developed intense and extensive INV. The vessels of iris expanded remarkably and neovascularization was observed erupting from it's lateral wall and stretching towards the anterior surface. Po11 days, INV regressed gradually after lens cortex had been absorbed. Group C: Po four (4) days, new blood vessels liking red line were presented on the anterior surface of the iris and they were not obvious.
CONCLUSION
Anterior chamber inside lens coriaceous can induce iris new blood vessels.
Keywords: iris, new vessels, crystallins
INTRODUCTION
As a common ophthalmological disorder, iris neovascularization (INV) usually accompanies many kinds of retinal diseases. Diabetic retinopathy can cause capillary nonperfusion, which initiates new vessels in iris. According to statistics, 43% of new vessels in iris are associated with diabetic retinopathy. Central retinal vein occlusion, another retinal vascular disease commonly seen in the clinic, is the second largest blinding retinal vascular disease, next only to diabetic retinopathy. Ischemic CRVO brings about new vessels in retinal and iris, especially the anterior segment. INV could grow into anterior chamber angle, induce neovascular glaucoma and damage patient's vision. In addition, INV also exist in retinal detachment and ocular tumors[1], etc. Some cataract patients develop INA following the surgical removal of crystalline lens in current practice. However, whether the lens cortex is associated with the occurrence of INA remains unknown. In this study, we stimulated the residual of lens cortex on Guinea pigs in the process of cataract surgery, which successfully induced the INV.
MATERIALS AND METHODS
Materials
The use and care of laboratory animals complied with the institutional Animal Research Committee at Sun Yat-sen University. Adult Guinea pigs weighing 300±20g (n=36) were provided by Sun Yat-sen Laboratory Animal Center and housed in Zhongshan Ophthalmic Center Department of Laboratory Animal Medicine. The animals were randomly assigned to right cataract extraction surgery (group A, n=12), right cataract extraction surgery preceding the back-injection of cortex residual into anterior chamber (group B, n=12), and the injection of lens cortex extracted from left eye into right anterior chamber (group C, n=12). The eyeballs receiving no treatment were used as controls.
Methods
Establishment of animal model
Conjunctival sacs were rinsed with ofloxacin q.i.d. from preoperative day 3. Ketamine hydrochloride (5%, Gutian Pharmaceutical Industry Co., Ltd, Fujian, China) and chlorpromazine hydrochloride (2.5%, Hefeng Pharmacy Co., Ltd, Shanghai, China) were mixed at the ratio of 1: 1 (v/v) and the mixture solution was intramuscularly injected into the hindlimbs at the dosage of 2 mL/kg. Compound tropicamide (Xingqi Pharmaceutical Industry Co., Ltd, Shenyang, China) was given to dilate pupils and tetracaine eyedrops (Affiliated Ophthalmic Center, Sun Yat-sen University, Guangzhou, China) were used for the topical anesthesia. Eyelashes were scissored and eyeballs were rinsed with mercuric chloride and normal saline preoperatively.
For group A receiving cataract extraction surgery, anterior chamber puncture was performed at the anterior margin of corneal rim, followed by the injection of viscoelastics. Can opener capsulotomy was performed and the corneal incision was expanded bilaterally by using a pair of corneal scissors toward 3 o'clock position. The lens nucleus was removed and the corneal incision was closed with 10-0 silk sutures (Alcon Laboratories Inc, U.S.A) in an interrupted manner. The residual cortex was extracted by using a syringe. Tobramycin ointment was applied at the completion of surgery. For group B, the additional back-injection of extracted lens cortex precipitates (approximately 30 µL) was given to the anterior chamber following the cataract removal. For group C, following the extraction of the lens nucleus and cortex of the left eye, the residual lens cortex precipitates (approximately 10 µL) were injected into the anterior chamber of the right eye. The bilateral eyes were daily reviewed and topically treated with ofloxacin eyedrops (Santen Pharmaceutical Co., Ltd., Japan), q.i.d.[2] Slit-lamp examination (Topcon SL-7F, Japan) was performed on postoperative day 2, 4, 7, 11, 13, and 17, in addition to the microscopic photography.
Histopathological examinations
Guinea pigs were sacrificed on postoperative day 4, 7, 11, and 17. The eyeballs were harvested from each group (n=3) and the contralateral eyeballs were used as normal controls. The specimens were fixed in 10% neutral formaldehyde (Guangzhou Chemical Reagent Factory, China) for 48 hours, followed by the sagittal dissection from 6 o'clock through 12 o'clock. The tissues were dehydrated in upgraded ethanol, embedded in paraffin, and serially sectioned in 4 micron for the subsequent hematoxylin-eosin staining and light microscopy.
RESULTS
Slit-lamp Observations
The animals of group A on postoperative day 2 exhibited transparent cornea, slightly dilated but less round pupils, without any marked residual lens cortex, vascular dilation, congestion and neovascularization of iris, or aqueous flare (Figure 1A), in addition to minimal pigmentation of posterior capsular surface. On postoperative day 7, the cornea was not edematous, the aqueous humour was clear, the turbid zone of the posterior capsule was expanded and less transparent, and the iris vasculature did not show any congestion, dilation, circuity, or neovascularization (Figure1B). On postoperative day 11, the cornea remained transparent, the aqueous humour stayed clear, the anterior capsule became mildly grayish and turbid (Figure 1C), and the iris presented the localized atrophy, in the absence of neovascularization.
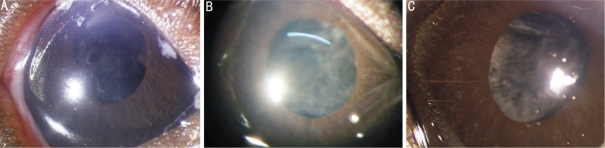
Figure 1. No iris neovascularization in goup A.
A: Po 2d of group A, the cornea and aqueous humor were clear. There is not lens cortex in the anterior chamber; B: Po 7d of group A, the posterior capsule of lens was not very transparent. INV was not observed and the vessels of iris did not expand; C: Po 11d of group A, the corneas and aqueous humor kept clear. The posterior capsule of lens appeared to be opacity slightly and there was no INV in the iris
The animals of group B on postoperative day 2 exhibited transparent corneal central zones and the absence of aqueous flare. Some lens cortexes were scattered in either small blocks or masses in the anterior chamber. On postoperative day 4, more scattered lens cortexes were visible in the anterior chamber and the anterior iris surface in close contact with the lens cortex developed sparse neovascularization, whereas mild aqueous flare was present in individual eyes, without any hypopyon or corneal edema otherwise (Figure 2A). On postoperative day 7, more small lined neovascularizations emerged on the anterior iris surface, which were more evident around the pupil margin without vascular dilation, congestion or hypertrophy, or aqueous flare (Figure 2B). On postoperative day 11, some lens cortex residuals were visible in the anterior chamber, whereas some of the INVs disappeared. On postoperative day 11, minimal lens cortex masses were still in the anterior chamber and the INV maintained the regression. On postoperative day 17, no marked lens cortex was visible in the anterior chamber and almost all the INVs disappeared. The anterior iris surface presented the localized atrophy and complete regression of neovascularization, in addition to the constriction of the previously dilated vessels and the mildly turbid posterior lens capsule.
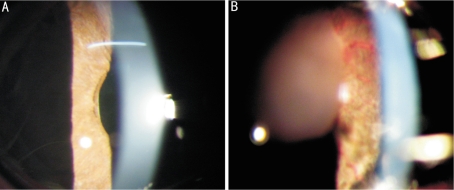
Figure 2. Extensive iris neovascularization induced by auto-cortex of crystalline lens in group B.
A: Po 7d of group B, the cornea and the aqueous humor were clear. Tiny redline-like blood vessels (white arrow) were observed in the iris; B: Po 7d of group B, there were larger pieces of cortex (black arrow) in the anterior chamber. Intense and extensive grossus vessels and thread-like small vessels were observed on the surface of iris. The dense iris neovascularization (white arrow) were located on the anterior surface of iris
The animals of group C exhibited on postoperative day 2 some lens cortexes in the anterior chamber, without any corneal edema or aqueous flare. On postoperative day 4, lens cortex masses were floating in the anterior chamber and some neovascularizations emerged on the iris adjacent to the pupil (Figure 3A). On postoperative day 7, some of the lens cortexes were absorbed and the anterior iris surface in close contact with the lens cortex developed small, scattered and lined neovascularizations without evident vascular hypertrophy or dilation, which were less and sparse compared to those in group B (Figure 3B). The INV began to regress from postoperative day 11 and almost disappeared on postoperative day 17 when the lens cortex was completely absorbed. In the course of the experiment, the corneas remained generally transparent and aqueous flare emerged on postoperative day 4, mild in severity and transient in duration, which became dissolved on postoperative day 7.
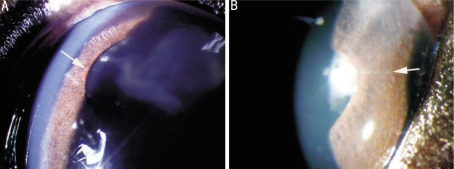
Figure 3. Scattered iris neovascularization in group C.
A: Po 4d of group C, there were pieces of lens cortex in the anterior chamber, a few of iris neovascularization (white arrow) occurred on the papillary edge; B: Po 7d of group C, there were some scattered tiny redline-like neovascularization (white arrow) on the anterior surface of iris.
Histopathological Results
Group A: The superficial layer of iris was plenty of pigment cells and there were not neovascularization. The matrix layer of iris was comprised of pigment cells and connective tissues, and the pigment cells in the matrix were less than them in the superficial layer. There were some thick-walled blood vessels in the matrix layer and they weren't dilated. Post-pigment layer of which the posterior edge looked like a lace was composed of intensive pigment cells. Group B: Some homogeneous, red-staining protein-like substances were observed on the anterior surface of iris. The blood vessels in the matrix layer were dilated, especially close to the surface layer were obviously, neovascularization was observed erupting from it's lateral wall and stretching towards the anterior surface (Figure 4A). Group C: Scattered thin-walled neovascularization and a few of protein-like substances were observed on the anterior surface of iris. The blood vessels in the matrix layer didn't dilate obviously (Figure 4B). There weren't apparent inflammatory cells on the surface of and in the iris in all groups.
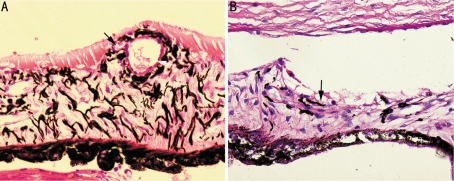
Figure 4. Iris neovascularization in HE stain.
A: Po 7d of group B, dilated vessels (white arrow) were observed in the iris, and tiny iris neovascularization erupted from its wall and stretched towards the surface of iris (black arrow); B: Po 7d of group C, scattered neovascularization (black arrow) were observed on the anterior surface of iris
DISCUSSION
Multiple INV animal models were previously reported. Zhou et al[3] used to block all the retinal veins around the optic disk by using a 514 nm-wavelength argon laser (spot diameter =100-200µm) on monkey eyes for 7-10 days. Excessive neovascularizations were present on the iris surface, and circuitous, radial and circular neovascular loops were visible on early iris fluorescein angiography, with the rapid leakage of fluorescein. On postoperative day 14, the neovascularization was widely spread on the iris surface. Shabo et al[4] medicated monkeys with systemic and intralens crystal bovine insulin for a course of 3 weeks. Neovascularization emerged on the iris surface and stromal layer 1-2 weeks later, in the latter of which the neovascularization did not regress following the regression of iris surface neovascularization. The mechanism was presumed to be the type IV hypersensitivity reaction of the anterior ocular segment in response to the intralens injection following the sensitization. Both the slit lamp examination and microscopic study confirmed the presence of neovascularization on the iris surface 1-2 months following the retinal detachment from the vitrectomy with lensectomy and subretinal injection into cat eyes. The pathogenesis of INV was attributed to the intraocular hypoxia from vitrectomy and retinal detachment; in addition to the contribution of the absence of lens barrier[5]. Our pilot study showed the possibility of the close contact of lens cortex with the retinal tissue to induce the epiretinal neovascularization[6]. However, the induction of INV by autogenous lens cortex was never reported.
Cataract is the primary cause of blindness in China, which is correctable otherwise[7]. Early treatment consists mainly of extracapsular cataract extraction (ECCE) surgery, susceptible to corneal astigmatism, malposition of artificial lens implant and lens cortex residual due to the poor stability of anterior chamber, greatly compromising the postoperative recovery of visual acuity. However, phacoemulsification overcomes the drawbacks of ECCE and is superior in the rapid visual recovery, due to the small surgical incision and the allowance of foldable artificial lens. The progress and improvement of surgical techniques and instruments have greatly increased the operative rate and visual rehabilitation rate[8]. Either procedure cannot completely eliminate the lens cortex residual. In the cases of hypermature cataract and lens capsular rupture from ocular trauma, lens cortex overflowed into the anterior chamber, resulting in the hypersensitive granulomatous reaction[9],[10]. Whether the lens cortex residual was able to directly induce the INV remained unknown.
Guinea pigs were used as laboratory animals for the observation of INV due to the larger cornea and the abundant iris pigments. The lens cortex residual that was not completely absorbed induced iris rubeosis or neovascularization, which was absent in the case of complete absorption of lens cortex, indicating the close relationship of the lens cortex residual in anterior chamber with the onset of INV. In the case of either lens cortex residual or intracameral lens cortex injection, the amount of lens cortex in the anterior chamber was correlated to the number and congestion extent of INV in a significantly proportional manner. The INV usually began to emerge on postoperative day 4 and reached its peak on postoperative day 7, significantly earlier than the emergence of inflammation-induced INV. Additionally, no marked aqueous flare was observed, whereas individual animals developed aqueous flare, which was mild and transient. No granulomatous inflammation was evident on pathological examination, suggesting that the INV was not secondary to the hypersensitive inflammation of lens. The corneal central zones maintained not edematous or hypertrophic, excluding the effects of the increased intraocular pressure on the INV. In summary, INV was likely to be directly induced by lens cortex.
Both clinical and animal studies on the development and progression of INV showed that the dilation of iris minor arterial circle and stromal vessels preceded the iris rubeosis, manifesting as vascular extrusion, circuity and discontinuity and the INV emerged around the pupil and expanded on the anterior iris surface, in addition to the proliferation of fibroconnective tissues[11]-[13]. Such neovascularizations manifested as small, curved and irregular red lines, different from the radially arranged normal iris vessels, which did not exhibit any blood column due to the thicker vascular walls[14], consistent with our findings. Early pathological findings showed vascular dilation, extrusion and circuity of iris and significantly hypertrophic and congested stromal vessels on microscopy, in addition to the emergence of neovascularizations on the anterior iris surface. Such neovascularizations were more evident around the pupils, probably due to the abundance of capillaries[15].
The induction of INV by lens cortex involved complex signalling pathways, including other cells and factors, such as VEGF, angiogenesis inhibitor, and extracellular matrix, in addition to vascular endothelial cells, which was yet to be investigated.
Footnotes
Foundation items: Supported by National Natural Science Foundation of China (No.39870801); Innovative Drug and Technological Development Program of Guangzhou Municipality, China (No.2006Z3-E4091) and Guangdong Provincial Medical Science and Technology Research Foundation, China (No.B2006118)
REFERENCES
- 1.Umeda N, Ozaki H, Hayashi H, Kondo H, Uchida H, Oshima K. Non-paralleled increase of hepatocyte growth factor and vascular endothelial growth factor in the eyes with angiogenic and nonangiogenic fibroproliferation. Ophthalmic Res. 2002;34:43–47. doi: 10.1159/000048324. [DOI] [PubMed] [Google Scholar]
- 2.Zhou XL, Li YP, Zhang WX, Li ZR. Epithelial-smooth muscle-like cell differentiation of lens epithelium after micro-trauma of mouse lens. Chin Ophthalmic Res. 2007;25:721–724. [Google Scholar]
- 3.Zhou HY, Zhang F, Gao LQ, Yan W, Xiong Y. Photodynamic therapy of experimental INA using hematoporphyrin monomethyl ether. Chin Ophthalmic Res. 2005;23:617–620. [Google Scholar]
- 4.Shabo AL, Maxwell DS, Shintaku P, Kreiger AE, Straatasma BR. Experimental immunogenic rubeosis iridis. Invest Ophthalmol Vis Sci. 1977;16:343–352. [PubMed] [Google Scholar]
- 5.Stefansson E, Landers MB, III, Wolbarsht ML, Klintworth GK. Neovascularization of the iris: an experimental model in cats. Invest Ophthalmol Vis Sci. 1984;25:361–364. [PubMed] [Google Scholar]
- 6.Li YP, Zhou XL, Liang D, Zhang WX. Auto-cortex of crystalline lens-induced neovascular epiretinal membrane national. Chin Ocul Fundus Dis. 2008;24:118–121. [Google Scholar]
- 7.Wei M, Lei CT, Chen H, Fan YC. Analysis of the situation of visual disability of Sichuan Province. Intl J Ophthalmol. 2007;7:1653–1654. [Google Scholar]
- 8.Zhao JL. The progress in the prevention of blindness in China. Chin J Ophthalmol. 2005;41:697–701. [PubMed] [Google Scholar]
- 9.Sigle KJ, Nasisse MP. Long-term complications after phacoemulsification for cataract removal in dogs: 172 cases (1995-2002) J Am Vet Med Assoc. 2006;228:74–79. doi: 10.2460/javma.228.1.74. [DOI] [PubMed] [Google Scholar]
- 10.Ekundo W, Augustin AJ. Differences in the composition of inflammatory cell infiltrate in lens-induced uveitis under therapy with allopurinol or steroids. Eur J Ophthalmol. 2001;11:264–268. doi: 10.1177/112067210101100309. [DOI] [PubMed] [Google Scholar]
- 11.Aques M, Girmens JF, Riviere E. Dilation of the minor arterial circle of the iris preceding rubeosis iridis during retinal vein occlusion. J Ophthalmol. 2004;138:1083–1086. doi: 10.1016/j.ajo.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 12.Adamis AP, Shima DT, Tolentino MJ, Gragoudas ES, Ferrara N, Folkman J, D'Amore PA, Miller JW. Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated INA in a nonhuman primate. Arch Ophthalmol. 1996;114:66–71. doi: 10.1001/archopht.1996.01100130062010. [DOI] [PubMed] [Google Scholar]
- 13.Husain D, Miller JW, Kenney AG, Michaud N, Flotte TJ, Gragoudas ES. Photodynamic therapy and digital cengiography of experimental INA using liposomal benzoporphyrin derivathe. Ophthalmology. 1997;104:1242–1250. doi: 10.1016/s0161-6420(97)30151-1. [DOI] [PubMed] [Google Scholar]
- 14.Packer AJ, Tse DT, Gu XQ, Hayreh SS. Hematoporphyrin photoradiation therapy for INA. A preliminary report. Arch Ophthalmol. 1984;102:1193–1197. doi: 10.1001/archopht.1984.01040030971028. [DOI] [PubMed] [Google Scholar]
- 15.Gartner S, Henkind P. Neovascularization of the iris (rubeosis iridis) Surv Ophthalmol. 1978;22:291–312. doi: 10.1016/0039-6257(78)90175-3. [DOI] [PubMed] [Google Scholar]