Abstract
AIM
To evaluate the long-term results and complications of ahmed glaucoma valve (AGV) implantation in refractory glaucoma.
METHODS
A retrospective review of 13 patients (13 eyes) with refractory glaucoma who underwent AGV implantation and had a minimum follow-up of 18 months was performed. All patients underwent a complete ophthalmologic examination and intraocular pressure (IOP) measurement before surgery and at 1 month, 3 months, 6 months, 1 year after surgery and yearly afterwards. Complications and the number of antiglaucoma medications needed were recorded.
RESULTS
Mean age was 27.3±16.0 years. All eyes (100%) had at least one prior incisional surgery. Mean follow-up was 61.3±30.8 months. IOP was reduced from a mean of 35.0 ±7.0mmHg to 18.2±7.9mmHg at 12 months and to 17.0±4.1mmHg at 96 months (P<0.05) with a lower number of medications from baseline, 76.9% patients required additional procedures to achieve the success criteria set by previously published series. The most common complications were encapculated cyst formation in eight eyes (61.5 %) and tube exposure in four eyes (30.8%).
CONCLUSION
Encapsulated cyst formation was the most common complication which hindered succesful IOP control after AGV implant insertion for refractory glaucoma. Despite cyst excision with anti-fibrotic agents, successful IOP reduction was not achieved in 76.9% of the patients without antiglaucoma medication.
Keywords: Ahmed glaucoma valve, encapsulated cyst, intraocular pressure, refractory glaucoma
INTRODUCTION
Various surgical approaches have been proposed for refractory glaucomas such as trabeculectomy with adjunctive anti-fibrotic agents, cyclodestructive procedures, and glaucoma drainage devices[1]. Glaucoma drainage devices drain aqueous humor from the anterior chamber through a tube into a bleb overlying its plate that is located at the equatorial subconjunctival space. The aqueous filters through the bleb and is reabsorbed into the ocular and systemic circulation[2],[3]. Different kinds of glaucoma drainage devices have been used for three decades as an alternative to standard perilimbal filtering surgery or cyclodestructive prosedures in refractory glaucomas[4]-[6]. Indications include eyes with refractory glaucoma, such as neovascular glaucoma, uveitic glaucoma, glaucoma in aphakia and pseudophakia, glaucoma associated with trauma, vitreoretinal disorders, penetrating keratoplasty and eyes that have failed previous filtration surgery[7],[8]. In refractory glaucomas, prior glaucoma surgery seems to increase the risk of failure of AGV implantation by more than three-fold[1],[7]. The reported success rates with AGV implants are between 60% and 82% at 2 years[1]-[4], and 49% at five years of follow-up[1], with an 10% per year failure rate. However, long-term follow-up data are limited.
A number of complications involving the tube have been reported following insertion; such as tube blockage, retraction, exposure, malposition as well as more serious complications like hypotony, choroidal effusion, choroidal hemorrage, cataract, retinal detachment and diplopia[7],[9].
The purpose of this study was to evaluate the long-term efficacy and safety of AGV implantation in complicated glaucomas.
MATERIALS AND METHODS
Materials
A retrospective chart review of patients who underwent implantation of ahmed glaucoma valve (AGV) at the Department of Ophthalmology, Dokuz Eylül University, between January 2001 and January 2008 was performed. Patients who underwent AGV implantation for refractory glaucoma were evaluated and 20 eyes of 20 patients with a follow-up longer than 18 months were included. Seven patients were excluded due to irregular follow-up. Inclusion criteria were elevated intraocular pressure(IOP) not responsive to conventional medical and surgical therapy or significant conjunctival scarring or inflammation precluding trabeculectomy.
Demographic data, included age, sex and preoperative data such as age at the time of the surgery, eye laterality, glaucoma diagnosis, prior ocular surgery, history of intraocular surgeries and laser procedures, best-corrected Snellen visual acuity (BCVA), IOP, antiglaucoma medications and type of implants. Major postoperative complications were noted and the occurrence of encapsulated cysts and their management were assessed.
Postoperative data included BCVA, IOP levels, number of medications used, surgical complications, additional surgeries performed, and duration of follow-up. Preoperative and postoperative IOPs were measured by Goldmann applanation tonometry when applicable and/or Tono-Pen in eyes using Goldmann tonometry was not possible. Success was defined as IOP lower than 21 mmHg and higher than 5 mmHg and at least 30% of IOP reduction with or without glaucoma medications, without additional glaucoma surgery, and a visual acuity (VA) of hand motion at least.
Methods
The surgical procedure consisted of an 1-stage polypropylene AGV (models S-2 or S-3 according to the age of the patient) implantation using a standardized surgical technique by three surgeons. After administration of regional or general anesthesia, a fornix-based conjunctival flap was fashioned in the superotemporal quadrant. After the excision of redundant Tenon's, a large Weck-cell sponge soaked in a 0.4-mg/mL solution of MMC was placed on the episclera, under the conjunctiva and Tenon's capsule at the site where the implant plate was to be placed, for a contact time of 5 minutes. The application of the sponge was followed by irrigation with 30 mL of balanced salt solution (BSS). The anterior edge of the plate was then secured with 9-0 nylon sutures to the sclera at least 8 mm away from the limbus. The tube tip was cut obliquely to protect the tube lumen from the iris. A 23-gauge needle tract was used to enter the anterior chamber through the limbus. The tube in the anterior chamber was positioned anterior to the iris and away from the corneal endothelium. Viscoelastic was injected to maintain the anterior chamber before tube insertion. The conjunctiva and Tenon capsule were reapproximated to the limbus with 8-0 polyglactin sutures (Vicryl; Ethicon, Inc, Somerville, New Jersey, USA).
During follow up, antiglaucoma medications were introduced when the IOP was higher than 21 mm Hg. For the purpose of comparing our results with previously published series, we defined successful IOP control as an IOP between 5mmHg and 21 mmHg and a minimum of 30% IOP reduction. Eyes requiring further glaucoma surgery (including needling, encapsulated cyst excision, cyclophotocoagulation or removal of the implant) or eyes which lost light perception were classified as failures.
The postoperative regimen included topical antibiotics, cycloplegics and steroids for approximately 1 month. Antiglaucoma medication was added when required to improve IOP reduction. Patients were examined the next day and 1 week, 2 weeks, 1 month, 3, 6 months, 1 year, 2, 3 , 4, 6 and 8 years after surgery.
Statistical Analysis
Statistical analysis of preoperative and postoperative data were performed using Wilcoxon test for two-related samples. Differences with P values <0.05 were considered statistically significant.
RESULTS
Thirteen eyes (13 patients) were included in the study. Eleven eyes received model S-2 and two eyes received model S-3 AGV implants. Patient demographics were summarized in Table 1. The mean age was 27.3±16.0 years (range:13-71 years). Mean preoperative IOP was 35.0±7.0mmHg (26-45mmHg).
Table 1. Patient demographics.
| No.of eyes (patients) | % | |
| Race | ||
| Caucasion | 13 | 100 |
| Gender | ||
| Female | 8 | 61 |
| Male | 5 | 39 |
| Eye | ||
| Right | 7 | 54 |
| Left | 6 | 56 |
| Glaucoma subtype | ||
| Secondary glaucoma | 8 | 62 |
| Aphakic glaucoma | 2 | 15 |
| Congenital glaucoma | 2 | 15 |
| Primary open-angle glaucoma | 1 | 8 |
| Previous ocular surgery | ||
| Trabeculectomy | 5 | 39 |
| Penetrating keratoplasty | 2 | 15 |
| Cataract extraction ± ACIOL | 4 | 30 |
| Retinal detachment repair | 2 | 15 |
| Corneal repair | 1 | 8 |
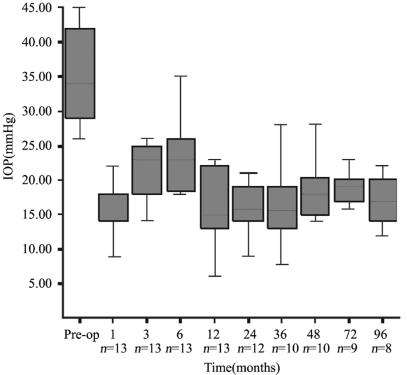
All eyes had at least one prior intraocular surgery. Six of them (46.2%) had at least one prior glaucoma surgery before insertion of the AGV. Mean number of total previous surgeries was 1.4±0.8 (range: 1-4). Mean number of previous glaucoma surgeries was 1.8±1.1 (range: 1-4). Seven eyes (53.8%) had no prior glaucoma surgery. Of these 7 eyes, two eyes (15.4%) were aphakic, and two eyes (15.4%) had pars plana vitrectomy, one eye (7.7%) had prior penetrating keratoplasty and one eye (7.7%) had corneal repair and anterior segment reconstruction surgery. The patients were followed for 61.3±30.8 months (18-96 months). There were twelve patients at 18 months, and eight patients at 96 months of follow-up. Mean number of preoperative antiglaucoma medication was 2.9±0.9 (1-4). Before surgery, mean IOP was 35.2 mmHg (±7.4 mmHg) with an average of 2.9 (±0.9) antiglaucoma medications. The postoperative mean IOP was 18.2 mmHg at 12 months with a mean of 1.8 (±0.9) medications. Mean IOP was 17.0mmHg (± 4.1mmHg) with 2.2 (±0.5) medications at 96 months after AGV implantation (P<0.05, Wilcoxon test). Figure 1 displays the course of mean IOP during the study.
Figure 1. Graph showing the mean IOP(mmHg±Standard deviation) before surgery (preoperative) and during follow-up.
All patients had complicated glaucoma and/or multiple surgeries before surgery, and in 46.1% of eyes, IOPs could be controlled with at least one antiglaucoma medication at the first month after surgery. Beta-blockers were the first choice and followed by combination antiglaucomatous therapy thereafter.
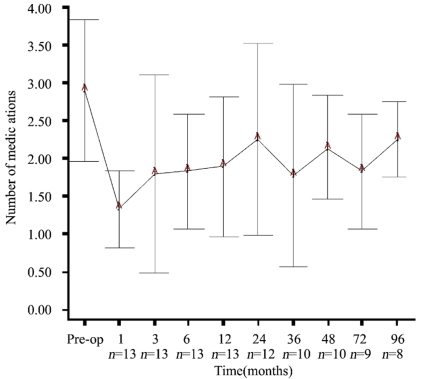
The average (±SD) number of antiglaucoma medications decreased from 2.8 (±0.9) medications at baseline to 1.3 (±0.5) at first month, 1.8 (±1.3) at three months, 1.8 (±0.7) at six months, however none of the decreases were stastically significant (P>0.05, Wilcoxon test). Also, the average (±SD) number of antiglaucoma medications at first, second, third, fourth, sixth and eighth year were similar to baseline average (P>0.05, Wilcoxon test). Figure 2 displays the change in the number of medications during the study.
Figure 2. Graph showing the mean number of medications (±standard deviation) before surgery (preoperative) and during follow-up.
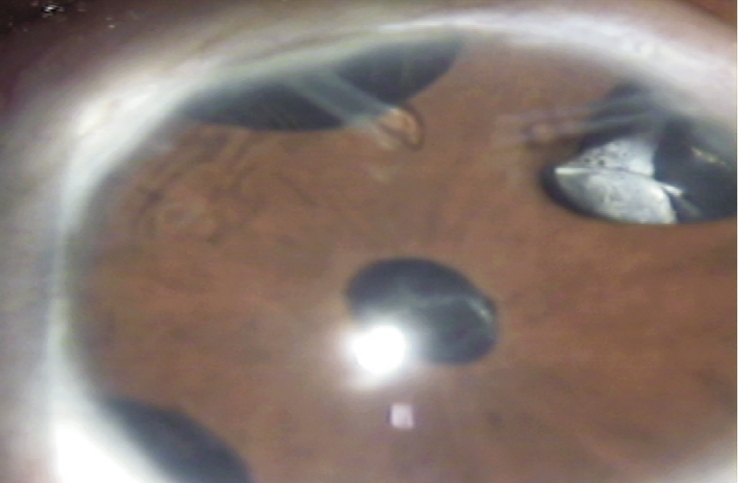
Complications were observed in 10 eyes of 13 patients (76.9%). Four eyes (30.8%) had more than one complication. The most common complications were encapculated cyst formation in eight eyes (61.5%) and tube exposure in four eyes (30.8%) (Figure 3). Three eyes (23.1%) developed encapsulated blebs despite 5-fluorouracil (5-FU) injections with needling. The mean time from surgery to encapsulation was 8.2±2.7 months (5-14 months). Three eyes (23.1%) underwent more than one encapsulated cyst excision. They subsequently had additional surgeries due to recurrent encapsulation with high IOP (two eyes underwent second AGV implantation (Figure 4) and two eyes underwent contact diode laser (transscleral cyclophotocoagulation). Recurrent encapsulation ocurred at a mean of 20.1±6.9 months after excision (11-30 months). Three eyes (23%) with corneal grafts experienced graft failure during the follow-up and underwent repeat keratoplasty. Transient hypotony, defined as an IOP less than 5mmHg, occurred in two eyes (15.4%). Mild to moderate anterior chamber hyphema was seen in two eyes (15.4%). Bullous choroidal detachment occurred in one eye (7.7%), in the early postoperative period, and resolved spontaneously.
Figure 3. Photograph depicting tube exposure.
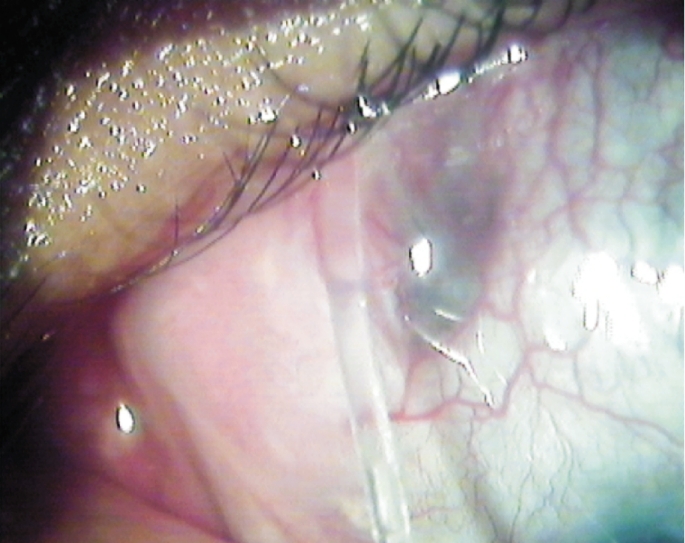
Figure 4. Picture of a patient with congenital glaucoma with two valve implants.
Visual acuity remained stable in most of the eyes during follow-up. Mean visual acuity at baseline was 1.33 (±0.57) logMAR units and at the last follow-up was 1.34 (±0.60) logMAR units (P=1.00, Wilcoxon test).
DISCUSSION
AGV is a shunt device with a restriction flow mechanism that is used in refractory glaucoma either as a primary surgical option or after failure of conventional filtration procedures. The overall success rate varies among different types of glaucomas, ranging from 63 % to 100 % at one year of follow-up in different case series with different success criteria and various lengths of follow-up[1]. The major cause of failure was excessive fibrosis and encapsulated cyst formation which were consistent with the other studies[9],[10]. The incidence of bleb encapsulation has been estimated to be between 40 % and 80 % with the AGV implantation depending on the severity of glaucoma and number of prior surgeries[6],[8]. Encapculated cyst formation was seen in 62% of patients in our study which is in accordance with literaure. Numerous surgical procedures including different types of implants with various sizes and materials with or without anti-fibrotic agents like MMC have been utilized in order to decrease the risk of encapsulated cyst formation. Three eyes of our patients developed encapsulated blebs despite 5-fluorouracil (5-FU) injections with needling. Cyst excision and MMC application, needling and 5-FU injection, implantation of a second drainage device and contact diode laser cyclophotocuagulation are other surgical options in the management of encapsulated cysts and failure following AGV[2],[4],[10]-[11]. Two eyes in our group underwent second AGV implantation and two eyes underwent contact diode laser (transscleral cyclophotocoagulation).
In our study, the AGV implant was able to decrease the mean (SD) preoperative IOP from 35.0 (7.0) mm Hg to 18.2 (7.9) mmHg at 12 months and 17.0 (4.1) mmHg at 96 months postoperatively. Long term IOP control was provided with additional drug combinations. Because, all eyes had at least one prior intraocular surgery. Six of them (46.2 %) had at least one prior glaucoma surgery before insertion of the AGV. One of the possible reasons is that we have a much higher percentage of cases who had secondary glaucoma (intraocular surgery, travmatic glaucoma, alcali burn). Secondary glaucoma was the most common preoperative diagnosis (62%) in our study.
Implant endplate size and its biomaterial have been considered to play a role in the final effect of glaucoma drainage devices on IOP control. Brasil et al[2] demonstrated similar efficacy between silicon (model FP-7) and polypropylene (model S-2) AGVs in controlling IOP in patients with refractory glaucoma. Ishida et al[11] showed probabilities of success of 94.2% at 12 months and 82.4% at 24 months for the silicone plate group and 83.2% at 12 months and 56.7% at 24 months for the polypropylene plate group. Tenon's cysts that required needling or surgical excision were observed in 4.5% underwent silicone device implantation and in 18.2% that underwent the polypropylene Ahmed glaucoma valve implantation. Unfortunately we were unable to obtain silicone AGV implants at the time of the current study. The poyproylene material may be another reason fort he high risk of our failures. Lai et al[12] reported that 16 of 65 eyes (24.6%) undergoing AGV implantation developed an encapsulated cyst as a postoperative complication. Overall, the cumulative probability of success with the AGV implant was 38.5% at mean 48 months of follow-up. In 2007, Souza et al[1] reported that approximately 50% of single-plate AGV implantations in refractory glaucoma were successful after five years of follow-up. Bayraktar et al[13] reported complete or partial success in 21 of the 25 eyes ( 84%) at the end of mean 8.5 months follow-up.
Capsule excision after failed Molteno surgery was examined by Valimaki et al[10]. They reported a 52% success rate with revision alone (intraocular pressure less than 22 mm Hg), whereas 42 % required a second tube shunt for intraocular pressure control. Smith et al[14] defined complete or partial success in sixteen of the 19 patients (84.2%) that were undergone second AGV surgeries, in all but two of these cases, topical antiglaucoma medication was necessary, and three patients were considered complete failures at 12 months and final follow-up. The mean drop in IOP at 12 months and final follow-up was 8 mmHg (43%) and 7.9 mmHg (42%), respectively. The mean number of glaucoma medications used postoperatively (2.4 at 12 months and 2.6 at final follow-up) was significantly less than preoperatively (4.1).
In a multicentre, prospective, non-masked, paralel comparative study reported by Susana[4], 92 eyes with neovascular glaucoma were included. After randomisation, 45 eyes (48.9%) were given Ahmed valve insertion with partial intraoperative Tenon's capsule resection (PTRC) with adjunctive MMC and 47 eyes (51.1%) without PTRC. The success rate at the 1 year follow-up in PTRC eyes was 70.4% and for non-PTRC eyes 77.7%, both with and without any additional medication for lowering IOP. There was no statistical difference between the two group.
We performed partial intraoperative encapsulated cysts resection in eight eyes, and additional tube placement in two eyes. Three eyes underwent more than one encapsulated cyst excision. Total excision of the thick fibrous capsule and closure of the overlying conjunctiva was technically challenging due to excessive scarring and the fibrous capsule often reformed. Additional tube shunts can be placed in areas without prior surgical procedures. Our study, consistent with the study of Shah et al[6], showed that placing an additional tube shunt was more effective than revision in controlling IOP. Corneal decompensation after AGV implant occured in two eyes with previous penetrating keratoplasty in our study and due to tube-corneal touch from anterior tube migration in one eye of our patients. Also occasional corneal touch due to pressure on the eye-ball at different times of the day such as during sleep is one of the postulated reasons about corneal decompensation ie eyes with tube implants.
In failures after AGV implantation cyclocryotherapy and contact diode laser cyclophotocuagulation are considered as a last resort option despite serious risks. In our study, two eyes underwent contact diode laser (transscleral cyclophotocoagulation). No major complication was observed in any of our patients after contact diode laser. Cyclocryotherapy as a means of ciliary body ablation to lower intraocular pressure has had variable success rates ranging from 29% to 76% and is associated with a high incidence of vision loss and phthisis bulbi[15]-[17]. Contact diode laser cyclophotocuagulation has success rates ranging from 52% to 66%[18],[19].
In the current study, 62% of the patients developed encapsulated cysts. Our management methodology consisted of needling and 5-FU injection first; followed by cyst excision and MMC application and finally second device implantation in two eyes. Three eyes underwent more than one encapsulated cyst excision+MMC. Successful IOP reduction was still not achieved in 77% without antiglaucoma medication. The low success rate in our study could be attributed to the fact that all patients had undergone at least one prior incisional surgery and hence had tendency for excessive scarring. Also small sample size is a major limitation of our study making it difficult to compare with other published reports. However high incidence of encapsulated cysts and their tendency to recur despite the use of antifibrotic agents besides complications like tube exposure due to shortage of overlying conjunctiva have in time caused us to perform AGV implantation less and prefer diode laser surgery in refractory glaucoma.
In conclusion, refractory glaucoma is an extremely difficult situation to manage. Even with the variety of tube shunts available today, encapsulated cysts occur in 40-80 %. Valve type, material, plate size do not seem to have much effect on failure rates even with additional procedures like cyst excision and anti-fibrotic agents.
REFERENCES
- 1.Souza C, Tran DH, Loman J, Law SK, Coleman AL, Caprioli J. Long-term outcomes of Ahmed glaucoma valve implantation in refractory glaucomas. Am J Ophthalmol. 2007;144(6):893–900. doi: 10.1016/j.ajo.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 2.Brasil MV, Rockwood EJ, Smith SD. Comparison of silicone and polypropylene Ahmed Glaucoma Valve implants. J Glaucoma. 2007;16(1):36–41. doi: 10.1097/01.ijg.0000243477.82779.31. [DOI] [PubMed] [Google Scholar]
- 3.Syed HM, Law SK, Nam SH, Li G, Caprioli J, Coleman A. Baerveldt-350 implant versus Ahmed valve for refractory glaucoma: a case-controlled comparison. J Glaucoma. 2004;13(1):38–45. doi: 10.1097/00061198-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Susanna R., Jr Latin American Glaucoma Society Investigators. Partial Tenon's capsule resection with adjunctive mitomycin C in Ahmed glaucoma valve implant surgery. Br J Ophthalmol. 2003;87(8):994–998. doi: 10.1136/bjo.87.8.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgoyne JK, WuDunn D, Lakhani V, Cantor LB. Outcomes of sequential tube shunts in complicated glaucoma. Ophthalmology. 2000;107(2):309–314. doi: 10.1016/s0161-6420(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 6.Shah AA, WuDunn D, Cantor LB. Shunt revision versus additional tube shunt implantation after failed tube shunt surgery in refractory glaucoma. Am J Ophthalmol. 2000;129(4):455–460. doi: 10.1016/s0002-9394(99)00410-9. [DOI] [PubMed] [Google Scholar]
- 7.Assaad MH, Baerveldt G, Rockwood EJ. Glaucoma drainage devices: pros and cons. Curr Opin Ophthalmol. 1999;10(2):147–153. doi: 10.1097/00055735-199904000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz KS, Lee RK, Gedde SJ. Glaucoma drainage implants: a critical comparison of types. Curr Opin Ophthalmol. 2006;17(2):181–189. doi: 10.1097/01.icu.0000193080.55240.7e. [DOI] [PubMed] [Google Scholar]
- 9.Eibschitz-Tsimhoni M, Schertzer RM, Musch DC, Moroi SE. Incidence and management of encapsulated cysts following Ahmed glaucoma valve insertion. J Glaucoma. 2005;14(4):276–279. doi: 10.1097/01.ijg.0000169391.94555.c1. [DOI] [PubMed] [Google Scholar]
- 10.Valimaki J, Tuulonen A, Airaksinen PJ. Capsule excision after failed Molteno surgery. Ophthalmic Surg Lasers. 1997;28(5):382–386. [PubMed] [Google Scholar]
- 11.Ishida K, Netland PA, Costa VP, Shiroma L, Khan B, Ahmed II. Comparison of polypropylene and silicone Ahmed Glaucoma Valves. Ophthalmology. 2006;113(8):1320–1326. doi: 10.1016/j.ophtha.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 12.Lai JS, Poon AS, Chua JK, Tham CC, Leung AT, Lam DS. Efficacy and safety of the Ahmed glaucoma valve implant in Chinese eyes with complicated glaucoma. Br J Ophthalmol. 2000;84(7):718–721. doi: 10.1136/bjo.84.7.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayraktar Ş, Altan T, Özçelik F, Yılmaz ÖF. Results of Ahmed Glaucoma Valve implantation in refractory glaucoma. T Oft Gaz. 2003;33(2):204–209. [Google Scholar]
- 14.Smith M, Buys YM, Trope GE. Second Ahmed valve insertion in the same eye. J Glaucoma. 2009;18(4):336–340. doi: 10.1097/IJG.0b013e318182edfb. [DOI] [PubMed] [Google Scholar]
- 15.Benson MT, Nelson ME. Cyclocryotherapy: a review of cases over a 10 year period. Br J Ophthalmol. 1990;74(2):103–105. doi: 10.1136/bjo.74.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caprioli J, Strang S, Spaeth G, Poryzees E. Cyclocryotherapy in the treatment of advanced glaucoma. Ophthalmology. 1985;92(7):947–954. doi: 10.1016/s0161-6420(85)33951-9. [DOI] [PubMed] [Google Scholar]
- 17.Bilge AH, Yıldırım E. Laser in the treatment of glaucoma. T Klin J Ophthalmol. 1992;1(1):56–62. [Google Scholar]
- 18.Kosoko O, Gaasterland DE, Pollack IP, Enger CL. Long-term outcome of initial ciliary ablation with contact diode laser transscleral cyclophotocoagulation for severe glaucoma. The Diode Laser Ciliary Ablation Study Group. Ophthalmology. 1996;103(8):1294–1302. doi: 10.1016/s0161-6420(96)30508-3. [DOI] [PubMed] [Google Scholar]
- 19.Bloom PA, Tsai JC, Sharma K, Miller MH, Rice NS, Hitchings RA, Khaw PT. “Cyclodiode”. Transscleral diode laser cyclophotocoagulation in the treatment of advanced refractory glaucoma. Ophthalmology. 1997;104(9):1508–1520. doi: 10.1016/s0161-6420(97)30109-2. [DOI] [PubMed] [Google Scholar]