Abstract
Congenital cataract is a crystallin severe blinding disease and genetic factors in disease development are important. Crystallin growth is under a combination of genes and their products in time and space to complete the coordination role of the guidance. Congenital cataract-related genes, included crystallin protein gene (CRYAA, CRYAB, CRYBA1/A3, CRYBA4, CRYBB1, CRYBB2, CRYBB3, CRYGC, CRYGD, CRYGS), gap junction channel protein gene (GJA1, GJA3, GJA8), membrane protein gene (GJA3, GJA8, MIP, LIM2), cytoskeletal protein gene (BF-SP2), transcription factor genes (HSF4, MAF, PITX3, PAX6), ferritin light chain gene (FTL), fibroblast growth factor (FGF) and so on. Currently, there are about 39 genetic loci isolated to which primary cataracts have been mapped, although the number is constantly increasing and depends to some extent on definition. We summarized the recent advances on epidemiology and genetic locations of congenital cataract in this review.
Keywords: congenital cataract, crystallin protein gene, gap junction channel protein gene, membrane protein gene, cytoskeleton protein, transcription factor genes, ferritin light chain gene, growth factor gene
INTRODUCTION
Paediatric cataract is a major cause of childhood blindness. Several genes have been identified in association with congenital and paediatric cataracts. The aim was to determine the incidence of cataract in a population, the proportion of hereditary cataracts, the mode of inheritance, and the clinical presentation. Congenital cataract is common with visual disability in children, due to abnormal metabolism of embryonic lens transparency results a severe blinding disease. Pathogenesis of congenital cataract is considered complex with 1/4-1/3 family heredity. Congenital cataract can occur in isolation, or as eye syndrome and developmental defects. A multi-system performance can be secondary to systemic metabolic disease[1]-[3]. Lens development depends on a combination of genes and their products in time and space to complete the coordination role of the guidance.
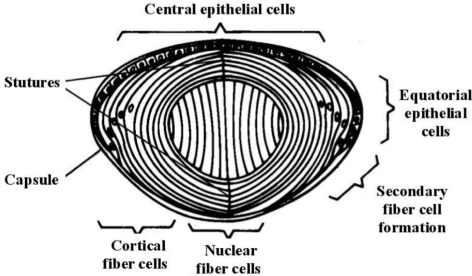
The lens transmits light of wavelengths from 390nm to 1200nm efficiently, extending well above the limit of visual perception (about 720nm). Lens transparency results from appropriate architecture of lens cells and tight packing of their proteins, resulting in a constant refractive index over distances approximating the wavelength of light[1]. Ultrastructurally, the lens comprises an anterior layer of organelle rich cuboidal epithelial cells covering a large fiber cell mass making up the bulk of the lens (Figure 1). Layers of nucleated cortical fiber cells form highly ordered concentric shells around the non-nucleated and essentially organelle-free central fiber cells which make up the lens nucleus. The ends of the more peripheral fiber cells abut on branched anterior and posterior sutures. The cellular architecture and arrangement of the fiber cells and particularly their sutures are critical for light transmission and lens transparency[2]. In addition, the stability and close ordering of lens crystallins, which make up 80-90% of the soluble proteins in the lens, are critical for lens transparency. The high protein content of the lens and especially the lens nucleus, approximately 60% of the wet weight, the highest of any tissue, is particularly important for refraction and focusing of light. Solutions of lens crystallins are highly transparent, and as they are concentrated to levels above 450mg/mL, light scattering actually decreases[1]-[3].
Figure 1. Structure of the mature human lens.
Cell division occurs in the 10 and 2 O'clock positions of the anterior epithelia, and cells move laterally until they invert in the bow region of the lens and begin loosing their organelles to form cortical fiber cells. Nuclear fiber cells are laid down relatively early in development. The ends of the more peripheral fiber cells meet an the sutures, shown here as vertical lines but seen clinically as anterior and posterior Y structures.
Cataracts can be defined by the age at onset: a congenital or infantile cataract presents within the first year of life; a juvenile cataract presents within the first decade of life; a presenile cataract presents before the age of about 45 years, and senile or age-related cataract after that. Between 8.3 and 25 percent of congenital cataracts are believed to be inherited. The lens alone may be involved, accounting for approximately 70% of congenital cataracts[3]. Conversely, lens opacities may be associated with other ocular anomalies such as microphthalmia, aniridia, other anterior chamber developmental anomalies, or retinal degenerations, seen in approximately 15% of cases. Cataracts may also be part of multisystem genetic disorders such as chromosome abnormalities, Lowe syndrome or neurofibromatosis type 2, also accounting for approximately 15% of congenital cataracts. In some cases this distinction can be blurred, e.g. in the developmental abnormality anterior segment mesenchymal dysgenesis resulting from abnormalities in the PITX3 gene, inherited cataracts may be isolated in some family members and associated with additional findings in others. In an attempt to develop a logical classification of congenital cataracts, Merin has proposed a system based on morphological classification. Examples are shown in Figure 2[1].
Figure 2. Congenital cataracts.
A: Slit lamp view of a dense anterior polar cataract. B: Reflex view of posterior subcapsular cataract. C: Dense nuclear cataract. D: Punctate nuclear cataract. E: Reflex view of a lamellar pulverulent cataract with a cortical rider in the upper right. F: Sutural cataract with a pulverulent nuclear lamellar component.
EPIDEMIOLOGY AND ETIOLOGY
Epidemiology
Cataract is the opacification of the eye lens and based upon the age at appearance. Cataract can be classified as congenital, infantile, juvenile, presenile and senile. Pediatric cataracts are responsible for more than 1 million childhood blindness in Asia. In developing countries like India, 7.4-15.3% of childhood blindness is due to cataract. The prevalence of cataract in children has been estimated between 1-15/10,000 children. Hereditary, metabolic and other ocular or systemic disorders and trauma are known factors responsible for cataract in children. In India, half of all childhood cataracts are Idiopathic. Out of 172 children, 88.4% had non-traumatic cataract and 11.6% had traumatic cataracts. Among non-traumatic cataracts, 7.2% were hereditary, 4.6% were due to congenital rubella syndrome, 15.1% were secondary and 73.0% were undetermined. In the group of undetermined cases, during pregnancy 67% of the mother had history of illness, and 22% had taken medications during pregnancy. Inadequate awareness of the causative factors of cataracts within the society has lead to increase of cataracts in children[4]-[6].
Considerable progress has been made in characterizing phenotypes, determining the prevalence and incidence in various population groups, and understanding risk factors for cataract. Cataract surgery research has documented functional improvements following surgery[5]-[7]. Cataract is an independent marker of early mortality, providing a possible system to study the aging process. Promising future work in cataract epidemiology is highlighted. Despite the availability of cataract surgery, cataract is still the leading cause of blindness worldwide. From a public health standpoint, research that can identify ways to delay onset or progression, or achieve the holy grail of prevention of cataract, should remain a leading priority[7].
Foster et al[8] reported that cataract is the most important cause of treatable childhood blindness. There are 200,000 children blind from cataract worldwide, and 20,000 to 40,000 children with developmental bilateral cataract are born each year. Rubella is still an important cause of preventable disease in many countries. In the developing world, there is a need to improve early case detection and referral services and to establish centers with expertise in the assessment, surgical treatment, and long-term management of the child with cataract. Blindness due to cataract presents an enormous problem in India not only in terms of human morbidity but also in terms of economic loss and social burden. The WHO/NPCB (National Programme for Control of Blindness) survey has shown that there is a backlog of over 22 million blind eyes (12 million blind people) in India, and 80.1% of these are blind due to cataract. The annual incidence of cataract blindness is about 3.8 million. The present annual level of performance is in the order of about 1.6-1.9 million cataract operations. To clear the backlog of cataract cases by the year 2000 and to tackle the rising incidence, 5-6 million cataract operations annually will have to be performed as against the present rate of 1.7 million per year[9].
To describe the prevalence and risk factors for cataract in an Australian population aged 40 years and older, McCarty et al[10] have shown that cataract is a public health problem in Australia, which, like other developed countries, is experiencing a demographic shift toward more elderly people in the population. The first step in the design of an appropriate response to the expected increase in the number of cases of cataract is to describe the age-specific prevalence and risk factors of cataract. Many of the risk factors that we identified in the population are potentially modifiable through public health campaigns[11]-[14].
Etiology
Many etiological studies on childhood cataract have been carried out in developed as well as developing countries to determine causative factors. Studies performed in various parts of India show variation in etiological factors affecting childhood cataract. In south India among non-traumatic cataracts, 25% were due to hereditary, 15% were due to congenital rubella syndrome and 51% were idiopathic. Nearly half of non-traumatic cataracts in this population are due to potentially preventable causes like congenital rubella syndrome and autosomal dominant disease.
In the paediatric cataract population examined, approximately half of the patients were diagnosed in the first year of life. More than 18% had a positive family history of cataracts. Of patients with hereditary cataracts 8% presented with unilateral involvement. Identification of the genes that cause paediatric and congenital cataract should help clarify the aetiology of some sporadic and unilateral cataracts. The results showed by Wirth et al[15] that 421 patients with paediatric cataract were identified, which gives an estimated incidence of 2.2 per 10,000 births. Of the 342 affected individuals with a negative family history, 50% were diagnosed during the first year of life, and 56/342 (16%) were associated with a recognised systemic disease or syndrome. Unilateral cataract was identified in 178/342 (52%) of sporadic cases. Seventy-nine children (from 54 nuclear families) had a positive family history. Of these 54 families, 45 were recruited for clinical examination and DNA collection. Ten nuclear families were subsequently found to be related, resulting in four larger pedigrees. Thus, 39 families have been studied. The mode of inheritance was autosomal dominant in 30 families, X linked in four, autosomal recessive in two, and uncertain in three. In total, 178 affected family members were examined; of these 8% presented with unilateral cataracts and 43% were diagnosed within the first year of life[12]-[14].
Cataract is responsible for about 10% blindness among children in India. Etiology of cataract is not well defined especially for childhood cataracts and epidemiological data for Indian population is not available in details. Out of 172 children, 88.4% had non-traumatic cataract and 11.6% had traumatic cataracts. Among non-traumatic cataracts, 7.2% were hereditary, 4.6% were due to congenital rubella syndrome, 15.1% were secondary and 73.0% were undetermined. In the group of undetermined cases, during pregnancy 67% of the mother had history of illness, and 22% had taken medications during pregnancy. Health education of women to childbearing age and school children can decrease incidence of pediatric cataracts[16].
MOLECULAR GENETICS
Cataracts can be isolated or can occur in association with a large number of metabolic diseases and genetic syndromes[17]. Isolated congenital cataracts tend to be highly penetrant Mendelian traits, with autosomal dominant more common than autosomal recessive cataracts. Currently, there are about 39 genetic loci to which isolated or primary cataracts have been mapped, although the number is constantly increasing and depends to some extent on definition (Table 1). Of these, several are associated with additional abnormalities, mostly as part of developmental syndromes. These tend to result from mutations in genes encoding transcriptional activators, and most of these have been identified by sequencing candidate genes in patients with developmental anomalies. A notable exception is the αB-crystallin gene, CRYAB, which is widely expressed in various tissues, especially muscle. Mutations in CRYAB can cause a spectrum of abnormalities ranging from isolated cataracts to mild cataracts associated with myopathy. A second counterexample is the ferritin gene, which causes the hyperferritinemia-cataract syndrome (Table 1)[1],[16],[17].
Table 1. Mapped human cataracts.
| Locus | Chrom | Inh | MIM |
| CCV (Volkmann) | 1p36 | AD | 115665 |
| CTPP | 1p34-p36 | AD | 116600 |
| FOXE3 NM_012186 | 1p32 | AD | 107250, 601094 |
| GJA8 NM_005267 | 1q21-q25, 2p24 | AD,AR | 116200 |
| CCNP | 2p12 | AD | 607304 |
| CRYGC NM_020989 | 2q33-q35 | AD | 123660, 123680, 601286 |
| CRYGD NM_006891 | 2q33−q35 | AD,AR | 115700, 123690 |
| BFSP2 NM_003571 | 3q22.1 | AD | 603212 |
| CRYGS NM_017541 | 3q26.3-qter | AD | 123730 |
| GCNT2 NM_001491 | 6p24-p23 | AR | 110800 |
| EYA1 NM_172060 | 8q13.3 | AD | 601653 |
| CAAR | 9q13-q22 | AR | 212500 |
| PITX3 NM_005029 | 10q25 | AD | 602669 |
| CRYAB NM_001885 | 11q23.3−24.2 | AD | 123590 |
| AQP0 NM_012064 | 12q12−14.1 | AD | 601286 |
| GJA3 NM_021954 | 13q11−13 | AD | 601885 |
| CHX10 NM_182894 | 14q24.3 | AR | 142993 |
| CCSSO | 15q21-q22 | AD | 605728 |
| HSF4 NM_001538 | 16q21 | AD,AR,S | 602438 |
| MAF NM_001031804 | 16q22-q23 | AD | 177074 |
| CTAA2 | 17p13 | AD | 601202 |
| CRYBA3 NM_005208 | 17q11-q12 | AD | 600881 |
| CCA1 (Cerulean - blue dot) | 17q24, 19q13, 19q13.4 | AD,AR | 115660 |
| FTL NM_000146 | 19q13.33 | AD | |
| LIM2 NM_002316 | 19q13.4 | AR | 154045 |
| BFSP1 NM_001195 | 20p11.23-p12.1 | AR | 603307 |
| CPP3 | 20p12-q12 | AD | 605387 |
| CHMP4B NM_176812 | 20q11.22 | AD | 610897 |
| CRYAA NM_000394 | 21q22.3 | AD, AR, Spo radi c | 123580 |
| CRYBB2 NM_00496 | 22q11.2 | AD | 123620 |
| CRYBB1 NM_001887 | 22q11.2-q12.1 | AD, AR | 600929 |
| CRYBB3 NM_004076 | 22q11.23-q12.1 | AR | 123630 |
| CRYBA4 NM_001886 | 22q11.2 | AD | 123631 |
| CXN | Xp22 | XL | 300457 |
| NHS NM_198270 | Xp22.13 | XL | 300457 |
Specific mutations are described above the entry for the gene or locus. The cDNA sequence changes are given in reference to the NCBI sequence identifier in the Locus column. Chrom: chromosomal location, Inh: inheritance pattern, cDNA: changes in the NCBI DNA sequence listed in the Locus column, AA: changes in the protein sequence, MIM: Mendelian Inheritance in Man reference. Specific mutations identified are listed above the gene. AD: autosomal dominant, AR: autosomal recessive, XL: X-linked, S: sporadic. Genes and loci are shown in bold, while individual mutations and their descriptions are shown in small lettering above
Crystallin proteins
Three major classes of ubiquitous crystallins are found in the vertebrate eye lens. In the molecular structure of crystallin proteins, there are α-crystallin protein (40%), β-crystallin protein (35%) and γ-crystallin protein (25%). The ratio of the crystalin protein composition and its spatial sequence in the maintenance of crystallin transparency is very important[18]-[22]. When the gene is mutated, the crystallin proteins are not only the abnormal protein structure and affect it's closely packed, but also to reduce the solubility of crystallin proteins to form opacities.
Alpha-crystallin proteins
Alpha-crystallin protein family has two members: αA-crystallin (CRYAA) and αB-crystallin (CRYAB) gene located in the 21q22.3 gene and located in the CRYAA 1lq22.3-q23.1 CRYAB coding, respectively[18]. αA-crystallin, a small heat shock protein with chaperone-like activity, forms dynamic multimeric complexes. Recently we described the spontaneous generation of a mutant protein (super αA-crystallin) by exon duplication arisen via exon shuffling confirming a classic hypothesis[16]-[18]. Comparison of super αA-crystallin, which is viable in a mouse skeletal muscle cell line, with normal αA-crystallin shows that it has diminished thermostability, increased exposure of hydrophobic patches, a larger complex size and lost its chaperone activity. However, super αA-crystallin subunits exchange as readily between complexes as does normal αA-crystallin. These data indicate that chaperone-like activity may vanish independent of subunit hydrophobicity and exchangeability[19],[20]. Crystallins are separated into two classes: taxon-specific, or enzyme, and ubiquitous. The latter class constitutes the major proteins of vertebrate eye lens and maintains the transparency and refractive index of the lens. Since lens central fiber cells lose their nuclei during development, these crystallins are made and then retained throughout life, making them extremely stable proteins. Mammalian lens crystallins are divided into α, β, and γ families; Beta and γ crystallins are also considered as a super family. Alpha and β families are further divided into acidic and basic groups. Seven protein regions exist in crystallins: four homologous motifs, a connecting peptide, and N- and C-terminal extensions. Alpha crystallins are composed of two gene products: alpha-A and alpha-B, for acidic and basic, respectively. Alpha crystallins can be induced by heat shock and are members of the small heat shock protein (sHSP also known as the HSP20) family. They act as molecular chaperones although they do not renature proteins and release them in the fashion of a true chaperone; instead they hold them in large soluble aggregates. Post-translational modifications decrease the ability to chaperone. These heterogeneous aggregates consist of 30-40 subunits; α-A and α-B subunits have a 3:1 ratio, respectively. Two additional functions of alpha crystallins are an autokinase activity and participation in the intracellular architecture. Alpha-A and α-B gene products are differentially expressed; α-A is preferentially restricted to the lens and α-B is expressed widely in many tissues and organs. Elevated expression of α-B crystallin occurs in many neurological diseases; a missense mutation cosegregated in a family with a desmin-related myopathy[21],[22].
CRYAA (crystallin, αA)
Chromosome: 21; Location: 21q22.3
CRYAB (crystallin, αB)
Chromosome: 11; Location: 11q22.3-q23.1
Beta-crystallin proteins
Beta-crystallins, the most heterogeneous, differ by the presence of the C-terminal extension (present in the basic group, none in the acidic group). Beta-crystallins form aggregates of different sizes and are able to self-associate to form dimers or to form heterodimers with other β-crystallins. This gene, a β acidic group member, encodes two proteins (crystallin, βA3 and crystallin, βA1) from a single mRNA, the latter protein is 17 a shorter than crystallin, βA3 and is generated by use of an alternate translation initiation site. Deletion of exons 3 and 4 causes the autosomal dominant disease zonular cataract with sutural opacities. This gene, a β basic group member, is part of a gene cluster with β-A4, β-B1, and β-B3. A chain-terminating mutation was found to cause type 2 cerulean cataracts. The major β-crystallin structures are as follows.
CRYBA1 (crystallin, βA1)
Chromosome: 17; Location: 17q11.2
CRYBA2 (crystallin, βA2)
Chromosome: 2; Location: 2q34-q36
CRYBB1 (crystallin, βB1)
Chromosome: 22; Location: 22q11.2; 22q12.1
CRYBB3 (crystallin, βB3)
Chromosome: 22; Location: 22q11.23-q12.1
Gama-crystallin proteins
Alpha and β families are further divided into acidic and basic groups. Seven protein regions exist in crystallins: four homologous motifs, a connecting peptide, and N- and C-terminal extensions[21],[22]. γ-crystallins are a homogeneous group of highly symmetrical, monomeric proteins typically lacking connecting peptides and terminal extensions. They are differentially regulated after early development. Four γ-crystallin genes (γ-A through γ-D) and three pseudogenes (γ-E, γ-F, γ-G) are tandemly organized in a genomic segment as a gene cluster. Whether due to aging or mutations in specific genes, γ-crystallins have been involved in cataract formation.
CRYGA (crystallin, γA)
Chromosome: 2; Location: 2q33-q35
CRYGB (crystallin, γB)
Chromosome: 2; Location: 2q33-q35
CRYGC (crystallin, γC)
Chromosome: 2; Location: 2q33-q35
CRYGD (crystallin, γD)
Chromosome: 2; Location: 2q33-q35
CRYGE (crystallin, γE) [Mus musculus]
Chromosome: 1; Location: 1 C2; 1 32.0 cM
CRYGF (crystallin, γG)
Chromosome: 1; Location: 1 C3; 1 32.0 cM
Gap junction proteins
Gap junctions were first characterized by electron microscopy as regionally specialized structures on plasma membranes of contacting adherent cells. These structures were shown to consist of cell-to-cell channels. Proteins, called connexins, purified from fractions of enriched gap junctions from different tissues differ. The connexins are designated by their molecular mass. Another system of nomenclature divides gap junction proteins into 2 categories, α and β, according to sequence similarities at the nucleotide and amino acid levels. For example, CX43 (121014) is designatedα-1 gap junction protein, whereas CX32 (GJB1; 304040) and CX26 are called β-1 and β-2 gap junction proteins, respectively. This nomenclature emphasizes that CX32 and CX26 are more homologous to each other than either of them is to CX43. Gap junction proteins include the following three types: 1)GJB2(Gap junction β2), 26kDa, Connexin 26 (CX26) Gene map locus: 13q11-q12; 2) GJB1(Gap junction β1) 32kDa, Connexin 32 (CX32) Gene map locus: Xq13.1; 3)GJA1(Gap junction α1), 43kDa, connexin 43 (CX43) gene map locus: 20q11.
Using the paired xenopus oocyte assay, Mese et al[23] functionally analyzed 5 CX26 mutations associated with autosomal recessive neurosensory deafness (DFNB1A; 220290). Three of the mutants were unable to form functional channels; the other 2 did electrically couple cells, but their voltage gating properties were different from wild type CX26 channels. The deafness associated with CX26 mutations is caused not only by reduced potassium recirculation in the inner ear, but also by abnormalities in the exchange of other metabolites through the cochlear gap[23]. Elias et al[24] showed that the gap junction subunits CX26 and CX43 (121014) are expressed at the contact points between radial fibers and migrating neurons, and that acute down regulation of CX26 or CX43 impairs the migration of neurons to the cortical plate. Unexpectedly, gap junctions do not mediate neuronal migration by acting in the classical manner to provide an aqueous channel for cell-cell communication. Instead, gap junctions provide dynamic adhesive contacts that interact with the internal cytoskeleton to enable leading process stabilization along radial fibers as well as the subsequent translocation of the nucleus. The gap junction adhesions are necessary for glial-guided neuronal migration[24].
Membrane proteins
Lens is a non-vascular tissue; the vast majority of lens cells rely on glycolysis to meet energy supply. Located in the center of the lens fiber rely on passive diffusion to accept less than adequate nutrition, and by special transport to provide the nutrients and the metabolic products shipped to the lens surface.
A membrane protein is a protein molecule that is attached to, or associated with the membrane of a cell or an organelle More than half of all proteins interact with membranes. Biological membranes consist of a phospholipid bilayer and a variety of proteins that accomplish vital biological functions. 1) Structural proteins are attached to microfilaments in the cytoskeleton which ensures stability of the cell; 2) Cell adhesion molecules allow cells to identify each other and interact. Such proteins are involved in immune response, for example; 3) Membrane enzymes produce a variety of substances essential for cell function; 4) Membrane receptor proteins serve as connection between the cell's internal and external environments; 5) Transport proteins play an important role in the maintenance of concentrations of ions. These transport proteins come in two forms: carrier proteins and channel proteins.
The structures of membrane proteins are stabilized by weak interactions and influenced by additional interactions with the solubilizing environment. The influence of the environment on membrane protein structures is especially significant. Despite the significant functional importance of membrane proteins, the structural biology has been particularly challenging as shown by the low number of membrane protein structures determined. Integral membrane proteins are present in a heterogeneous environment that poses major obstacles for existing structural methodologies.
Aquaporin proteins
Aquaporins are proteins embedded in the cell membrane that regulate the flow of water. Aquaporins are integral membrane proteins from a larger family of major intrinsic proteins (MIP) that form pores in the membrane of biological cells[25]. Aquaporin proteins are made up of six transmembrane α-helices arranged in a right-handed bundle, with the amino and the carboxyl termini located on the cytoplasmic surface of the membrane. The amino and carboxyl halves of the sequence show similarity to each other, in what appears to be a tandem repeat. Some researchers believe that this results from an early evolution event that saw the duplication of the half-size gene. There are also five interhelical loop regions (A-E) that form the extracellular and cytoplasmic vestibules. Loops B and E are hydrophobic loops that contain the highly, although not completely conserved, asparagine–proline–alanine (NPA) motif, which overlap the middle of the lipid bilayer of the membrane forming a 3-D ‘hourglass’ structure where the water flows through. This overlap forms one of the two well-known channel constriction sites in the peptide, the NPA motif and a second and usually narrower constriction known as ‘selectivity filter’ or ar/R selectivity filter. Aquaporins form tetramers in the cell membrane, with each monomer acting as a water channel. The different aquaporins contain differences in their peptide sequence, which allows for the size of the pore in the protein to differ between aquaporins. The resultant size of the pore directly affects what molecules are able to pass through the pore, with small pore sizes only allowing small molecules like water to pass through the pore[26],[27]. Mice homozygous for inactivating mutations in the aquaporin-0 gene develop congenital cataracts[28].
Major intrinsic protein (MIP) is a member of the water-transporting aquaporins as well as the original member of the MIP family of channel proteins. The function of the fiber cell membrane protein encoded by this gene is undetermined, yet this protein is speculated to play a role in intracellular communication. The MIP protein is expressed in the ocular lens and is required for correct lens function.
MIP (Major intrinsic protein)
Chromosome: 12; Location: 12q13
Lens intrinsic membrane protein 2
This gene encodes an eye lens-specific protein (19kDa) found at the junctions of lens fiber cells, where it may contribute to cell junctional organization. It acts as a receptor for calmodulin, and may play an important role in both lens development and cataractogenesis. Mutations in this gene have been associated with cataract formation. Alternatively spliced transcript variants encoding different isoforms have been found for this gene.
LIM2 (Lens intrinsic membrane protein 2)
Chromosome: 19; Location: 19q13.4
Beaded filament structural protein 2
Frame of the lens cells and the cytoplasm by the cytoskeleton in the lens protein interactions determined. Fibrin-like beads (beaded filament structural protein 2, BFSP2) is the only kind of expression only in the eyes of cytoskeletal proteins (cytoskeletal protein) structure.
More than 99% of the vertebrate ocular lens is comprised of terminally differentiated lens fiber cells. Two lens-specific intermediate filament-like proteins, the protein product of this gene (phakinin), and filensin, are expressed only after fiber cell differentiation has begun. Both proteins are found in a structurally unique cytoskeletal element that is referred to as the beaded filament (BF). Mutations in this gene have been associated with juvenile-onset, progressive cataracts and Dowling-Meara epidermolysis bullosa simplex[29].
BFSP2 (Beaded filament structural protein 2)
Chromosome: 3; Location: 3q22.1
Transcription factors
Transcription factor has an important role in embryonic development of eye, mutations in these genes often because eye hypoplasia syndrome, these genes is to study the importance of eye hypoplasia syndrome candidate genes. Antibody-dependent cell-mediated cytotoxicity (ADCC) associated with the occurrence of transcription factor genes, including heat shock transcription factor 4 gene (HSF4), MAF(v-maf musculoaponeurotic fibrosarcoma oncogene homolog), pituitary homeobox gene 3 (PITX3), paired homeobox containing gene 6 (PAX6) and so on.
Heat shock transcription factor 4
Heat shock transcription factors (HSFs) activate heat-shock response genes under conditions of heat or other stresses. HSF4 lacks the carboxyl-terminal hydrophobic repeat which is shared among all vertebrate HSFs and has been suggested to be involved in the negative regulation of DNA binding activity. Two alternatively spliced transcripts encoding distinct isoforms and possessing different transcriptional activity have been described.
HSF4 (Heat shock transcription factor 4)
Chromosome: 16; Location: 16q21
v-maf musculoaponeurotic fibrosarcoma
Much of our knowledge about the underlying mechanisms of cataractogenesis has come from the genetic analysis of affected families: there are contributions from genes coding for transcription factors (such as MAF, PITX3, FoxE3) and structural proteins such as crystallins or connexins[30]. In addition, there are contributions from enzymes affecting sugar pathways (particularly the galactose pathway) and from a quite unexpected area: axon guidance molecules like ephrins and their receptors. Cataractous mouse lenses can be identified easily by visual inspection, and a remarkable number of mutant lines have now been characterized. Generally, most of the mouse mutants show a similar phenotype to their human counterparts; however, there are some remarkable differences. It should be noted that many mutations affect genes that are expressed not only in the lens, but also in tissues and organs outside the eye[31]-[33].
The protein encoded by this gene is a DNA-binding, leucine zipper-containing transcription factor that acts as a homodimer or as a heterodimer. Depending on the binding site and binding partner, the encoded protein can be a transcriptional activator or repressor. This protein plays a role in the regulation of several cellular processes, including embryonic lens fiber cell development, increased T-cell susceptibility to apoptosis, and chondrocyte terminal differentiation. Defects in this gene are a cause of juvenile-onset pulverulent cataract as well as congenital cerulean cataract 4 (CCA4). Two transcript variants encoding different isoforms have been found for this gene[31]-[33].
MAF (v-maf musculoaponeurotic fibrosarcoma)
Chromosome: 16; Location: 16q22-q23
Paired-like homeodomain 3
This gene encodes a member of the RIEG/PITX homeobox family, which is in the bicoid class of homeodomain proteins. Members of this family act as transcription factors. This protein is involved in lens formation during eye development. Mutations of this gene have been associated with anterior segment mesenchymal dysgenesis and congenital cataracts[32],[33].
PITX3 (Paired-like homeodomain 3)
Chromosome: 10; Location: 10q25
Paired box gene 6
This gene encodes paired box gene 6, one of many human homologs of the Drosophila melanogaster gene prd. In addition to the hallmark feature of this gene family, a conserved paired box domain, the encoded protein also contains a homeo box domain. Both domains are known to bind DNA, and function as regulators of gene transcription. This gene is expressed in the developing nervous system, and in developing eyes. Mutations in this gene are known to cause ocular disorders such as aniridia and Peter's anomaly. Alternatively spliced transcript variants encoding either the same or different isoform have been found for this gene. Mutations associated with congenital cataracts form 40, of which only 1 species is caused by mutations in the pathogenesis of the independent occurrence of ADCC (G18W), the mutation interfering with the target gene PAX6 binding, and reduce its transcriptional activation function located in the 1lp13. A novel PAX6 gene mutation was identified in a Chinese aniridia family. This mutation may also contribute to congenital cataracts in these aniridia patients[33]-[35].
PAX6 (Paired box gene 6)
Chromosome: 11; Location: 11p13
Ferritin light chains
Why ferritin concentration can lead to cataracts, it is not clear. Studies suggest that ferritin for cataract may be two factors: first, ferritin light chain and heavy chain in the balance of the population makes the free iron ions and active oxygen content increases, damage to lens function. Second, the cells increase in ferritin levels may lead to the aggregation of lens proteins. This gene encodes the light subunit of the ferritin protein. Ferritin is the major intracellular iron storage protein in prokaryotes and eukaryotes. It is composed of 24 subunits of the heavy and light ferritin chains. Variation in ferritin subunit composition may affect the rates of iron uptake and release in different tissues. A major function of ferritin is the storage of iron in a soluble and nontoxic state. Defects in this light chain ferritin gene are associated with several neurodegenerative diseases and hyperferritinemia-cataract syndrome. This gene has multiple pseudogenes[36].
The mutation observed in FTL in this family highlights the phenotypic heterogeneity of the disorder in relation to the genotype as the identical mutation (32 G>A) has previously been reported in two Italian families with entirely different phenotypes. It is also the first report of hereditary hyperferritinemia-cataract syndrome in a family of Indian origin[37].
FTL (Ferritin light chain)
Chromosome: 19; Location: 19q13.33
Fibroblast growth factors
The protein encoded by this gene is a member of the fibroblast growth factor (FGF) family. FGF family members possess broad mitogenic and cell survival activities, and are involved in a variety of biological processes, including embryonic development, cell growth, morphogenesis, tissue repair, tumor growth and invasion. This protein is a potent epithelial cell-specific growth factor, whose mitogenic activity is predominantly exhibited in keratinocytes but not in fibroblasts and endothelial cells. Studies of mouse and rat homologs of this gene implicated roles in morphogenesis of epithelium, reepithelialization of wounds, hair development and early lung organogenesis[38]. Using linkage analysis, Swaroop et al[39] believed that it may be fibroblast growth factor 7 (FGF7) gene mutation associated with congenital cataract-related.
FGF7 (fibroblast growth factor 7)
Chromosome: 15; Location: 15q21.2
Other proteins
The new genes that cause congenital cataracts are constantly discovered. Studies suggest that there is no more than 12 disease genes identified chromosomal regions associated with congenital cataracts, which include lp32, lp36, 1pter, 2p12, 2p24-pter, 6p24, 8q13.3, 15q21-22, 15q22.3-q23.1, 17p12-13, 17q24, 20p12-q12 and so on[1],[17],[39]-[49].
SUMMARY
The hereditary congenital cataracts provides some insight into those biological systems most important for developing and maintaining lens transparency, or at least those which are most easily disrupted in above overview. Although the normal lens and cataract in clinical molecular biology more complete description is beyond the scope of the review, more detailed reviews are available[48]-[53]. Other important functional systems include cytoskeletal and membrane proteins, especially those limited to or favored in the lens. As suggested by their high expression levels in the lens, the crystallins are the most common group of proteins mutated in inherited congenital cataracts. Growth and differentiation factors also frequently are seen causing congenital cataracts, often in association with other findings in their developmental spectra[54]. Finally, a varied group of proteins can also cause congenital cataracts. Together these studies provide insights into lens biology easily accessible in no other way. They can also be of direct clinical benefit in some families[55]. In addition, while the pathophysiology of congenital and hereditary cataracts differs in fundamental ways from that of age related cataracts, the study of congenital cataracts can provide insights into the mechanisms of lens transparency and to some of the ways in which it can be lost as the lens ages[53]-[56].
Congenital cataract is particularly serious because it has the potential for inhibiting visual development, resulting in permanent blindness. Inherited cataracts represent a major contribution to congenital cataracts, especially in developed countries. While cataract represents a common end stage of mutations in a potentially large number of genes acting through varied mechanisms in practice most inherited cataracts have been associated with a subgroup of genes encoding proteins of particular importance for the maintenance of lens transparency and homeostasis[57]. The increasing availability of more detailed information about these proteins and their functions and is making it possible to understand the pathophysiology of cataracts and the biology of the lens in general.
REFERENCES
- 1.Hejtmancik JF. Congenital cataracts and their molecular genetics. Semin Cell Dev Biol. 2008;19(2):134–149. doi: 10.1016/j.semcdb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuszak JR, Zoltoski RK, Sivertson C. Fibre cell organization in crystalline lenses. Exp Eye Res. 2004;78(3):673–687. doi: 10.1016/j.exer.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Haargaard B, Wohlfahrt J, Fledelius HC, Rosenberg T, Melbye M. A nationwide Danish study of 1027 cases of congenital/infantile cataracts: etiological and clinical classifications. Ophthalmology. 2004;111(12):2292–2298. doi: 10.1016/j.ophtha.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 4.Johar SR, Savalia NK, Vasavada AR, Gupta PD. Epidemiology based etiological study of pediatric cataract in western India. Indian J Med Sci. 2004;58(3):115–121. [PubMed] [Google Scholar]
- 5.Heijl A, Leske MC. Cataract epidemiology. Ophthalmology. 2007;114(1):201. doi: 10.1016/j.ophtha.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Chandrasekaran S, Cumming RG, Rochtchina E, Mitchell P. Associations between elevated intraocular pressure and glaucoma, use of glaucoma medications, and 5-year incident cataract: the Blue Mountains Eye Study. Ophthalmology. 2006;113(3):417–424. doi: 10.1016/j.ophtha.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 7.West S. Epidemiology of cataract: accomplishments over 25 years and future directions. Ophthalmic Epidemiol. 2007;14(4):173–178. doi: 10.1080/09286580701423151. [DOI] [PubMed] [Google Scholar]
- 8.Foster A, Gilbert C, Rahi J. Epidemiology of cataract in childhood: a global perspective. J Cataract Refract Surg. 1997;23(Suppl 1):601–604. doi: 10.1016/s0886-3350(97)80040-5. [DOI] [PubMed] [Google Scholar]
- 9.Vajpayee RB, Joshi S, Saxena R, Gupta SK. Epidemiology of cataract in India: combating plans and strategies. Ophthalmic Res. 1999;31(2):86–92. doi: 10.1159/000055518. [DOI] [PubMed] [Google Scholar]
- 10.McCarty CA, Mukesh BN, Fu CL, Taylor HR. The epidemiology of cataract in Australia. Am J Ophthalmol. 1999;128(4):446–465. doi: 10.1016/s0002-9394(99)00218-4. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal PK, Bowman R, Courtright P. Child eye health tertiary facilities in Africa. J AAPOS. 2010;14(3):263–266. doi: 10.1016/j.jaapos.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Prakalapakorn SG, Rasmussen SA, Lambert SR, Honein MA. National Birth Defects Prevention Study. Assessment of risk factors for infantile cataracts using a case-control study: National Birth Defects Prevention Study, 2000-2004. Ophthalmology. 2010;117(8):1500–1505. doi: 10.1016/j.ophtha.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.You C, Wu X, Zhang Y, Dai Y, Huang Y, Xie L. Visual impairment and delay in presentation for surgery in chinese pediatric patients with cataract. Ophthalmology. 2011;118(1):17–23. doi: 10.1016/j.ophtha.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Wang KJ, Li SS, Yun B, Ma WX, Jiang TG, Zhu SQ. A novel mutation in MIP associated with congenital nuclear cataract in a Chinese family. Mol Vis. 2011;17:70–77. [PMC free article] [PubMed] [Google Scholar]
- 15.Wirth MG, Russell-Eggitt IM, Craig JE, Elder JE, Mackey DA. Aetiology of congenital and paediatric cataract in an Australian population. Br J Ophthalmol. 2002;86(7):782–786. doi: 10.1136/bjo.86.7.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johar SR, Savalia NK, Vasavada AR, Gupta PD. Epidemiology based etiological study of pediatric cataract in western India. Indian J Med Sci. 2004;58(3):115–121. [PubMed] [Google Scholar]
- 17.Hejtmancik JF, Kaiser-Kupfer MI, Piatigorsky J. Molecular biology and inherited disorders of the eye lens. In: The Metabolic and Molecular Basis of Inherited Disease. 8th ed. New York: McGraw Hill; 2001. pp. 6033–6062. [Google Scholar]
- 18.Yang G, Xiong C, Li S, Wang Y, Zhao J. A recurrent mutation in CRYGD is associated with autosomal dominant congenital coralliform cataract in two unrelated Chinese families. Mol Vis. 2011;17:1085–1089. [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar M, Agarwal T, Khokhar S, Kumar M, Kaur P, Roy TS, Dada R. Mutation screening and genotype phenotype correlation of α-crystallin, γ-crystallin and GJA8 gene in congenital cataract. Mol Vis. 2011;17:693–707. [PMC free article] [PubMed] [Google Scholar]
- 20.Mothobi ME, Guo S, Liu Y, Chen Q, Yussuf AS, Zhu X, Fang Z. Mutation analysis of congenital cataract in a Basotho family identified a new missense allele in CRYBB2. Mol Vis. 2009;15:1470–1475. [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Q, Ma J, Yan M, Mothobi ME, Liu Y, Zheng F. A novel mutation in CRYAB associated with autosomal dominant congenital nuclear cataract in a Chinese family. Mol Vis. 2009;15:1359–1365. [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen L, Yao W, Eiberg H, Kjaer KW, Baggesen K, Hejtmancik JF, Rosenberg T. Genetic heterogeneity in microcornea-cataract: five novel mutations in CRYAA, CRYGD, and GJA8. Invest Ophthalmol Vis Sci. 2007;48(9):3937–3944. doi: 10.1167/iovs.07-0013. [DOI] [PubMed] [Google Scholar]
- 23.Mese G, Londin E, Mui R, Brink PR, White TW. Altered gating properties of functional Cx26 mutants associated with recessive non-syndromic hearing loss. Hum Genet. 2004;115:191–199. doi: 10.1007/s00439-004-1142-6. [DOI] [PubMed] [Google Scholar]
- 24.Elias LAB, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448(3):901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- 25.Agre P. The aquaporin water channels. Proc Am Thorac Soc. 2006;3(1):5–13. doi: 10.1513/pats.200510-109JH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonen T, Walz T. The structure of aquaporins. Q Rev Biophys. 2006;39(4):361–396. doi: 10.1017/S0033583506004458. [DOI] [PubMed] [Google Scholar]
- 27.Chepelinsky AB. Structural function of MIP/aquaporin 0 in the eye lens; genetic defects lead to congenital inherited cataracts. Handb Exp Pharmacol. 2009;190(IV):265–297. doi: 10.1007/978-3-540-79885-9_14. [DOI] [PubMed] [Google Scholar]
- 28.Okamura T, Miyoshi I, Takahashi K, Mototani Y, Ishigaki S, Kon Y, Kasai N. Bilateral congenital cataracts result from a gain-of-function mutation in the gene for aquaporin-0 in mice. Genomics. 2003;81(4):361–368. doi: 10.1016/s0888-7543(03)00029-6. [DOI] [PubMed] [Google Scholar]
- 29.Ma X, Li FF, Wang SZ, Gao C, Zhang M, Zhu SQ. A new mutation in BFSP2 (G1091A) causes autosomal dominant congenital lamellar cataracts. Mol Vis. 2008;14:1906–1911. [PMC free article] [PubMed] [Google Scholar]
- 30.Churchill A, Graw J. Clinical and experimental advances in congenital and paediatric cataracts. Philos Trans R Soc Lond B Biol Sci. 2011;366(1568):1234–1249. doi: 10.1098/rstb.2010.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berry V, Francis PJ, Prescott Q, Waseem NH, Moore AT, Bhattacharya SS. A novel 1-bp deletion in PITX3 causing congenital posterior polar cataract. Mol Vis. 2011;17:1249–1253. [PMC free article] [PubMed] [Google Scholar]
- 32.Churchill A, Graw J. Clinical and experimental advances in congenital and paediatric cataracts. Philos Trans R Soc Lond B Biol Sci. 2011;366(1568):1234–1249. doi: 10.1098/rstb.2010.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang KJ, Zhu SQ, Cheng J. Progress in pathogenic genes and their functions of congenital cataract. Zhonghua Yan Ke Za Zhi. 2010;46(3):280–284. [PubMed] [Google Scholar]
- 34.Cai F, Zhu J, Chen W, Ke T, Wang F, Tu X, Zhang Y, Jin R, Wu X. A novel PAX6 mutation in a large Chinese family with aniridia and congenital cataract. Mol Vis. 2010;16:1141–1145. [PMC free article] [PubMed] [Google Scholar]
- 35.Song S, Liu Y, Guo S, Zhang L, Zhang X, Wang S, Lu A, Li L. A novel PAX6 gene mutation in a Chinese family with aniridia. Mol Vis. 2005;11:335–337. [PubMed] [Google Scholar]
- 36.Wussuki-Lior O, Abu-Horowitz A, Netzer I, Almer Z, Morad Y, Goldich Y, Yahalom V, Pras E, Pras E. Hematologic biomarkers in childhood cataracts. Mol Vis. 2011;17:1011–1015. [PMC free article] [PubMed] [Google Scholar]
- 37.Vanita V, Hejtmancik JF, Hennies HC, Guleria K, Nürnberg P, Singh D, Sperling K, Singh JR. Sutural cataract associated with a mutation in the ferritin light chain gene (FTL) in a family of Indian origin. Mol Vis. 2006;12:93–99. [PubMed] [Google Scholar]
- 38.Xiao Y, Zhao B, Gao Z, Pan Q. Overaccumulation of transforming growth factor-β1 and basic fibroblast growth factor in lens epithelial cells of congenital cataract. Can J Ophthalmol. 2009;44(2):189–192. doi: 10.3129/i09-006. [DOI] [PubMed] [Google Scholar]
- 39.Swaroop A, Xu JZ, Pawar H, Jackson A, Skolnick C, Agarwal N. A conserved retina-specific gene encodes a basic motif/leucine zipper domain. Proc Natl Acad Sci USA. 1992;89(1):266–2670. doi: 10.1073/pnas.89.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanita V, Singh JR, Singh D, Varon R, Sperling K. Novel mutation in the gamma-S crystallin gene causing autosomal dominant cataract. Mol Vis. 2009;15:476–481. [PMC free article] [PubMed] [Google Scholar]
- 41.Gao L, Qin W, Cui H, Feng G, Liu P, Gao W, Ma L, Li P, He L, Fu S. A novel locus of coralliform cataract mapped to chromosome 2p24-pter. J Hum Genet. 2005;50(6):305–310. doi: 10.1007/s10038-005-0251-y. [DOI] [PubMed] [Google Scholar]
- 42.Pras E, Raz J, Yahalom V, Frydman M, Garzozi HJ, Pras E, Hejtmancik JF. A nonsense mutation in the glucosaminyl (N-acetyl) transferase 2 gene (GCNT2): association with autosomal recessive congenital cataracts. Invest Ophthalmol Vis Sci. 2004;45(6):1940–1945. doi: 10.1167/iovs.03-1117. [DOI] [PubMed] [Google Scholar]
- 43.Hilal L, Nandrot E, Belmekki M, Chefchaouni M, El Bacha S, Benazzouz B, Hajaji Y, Gribouval O, Dufier J, Abitbol M, Berraho A. Evidence of clinical and genetic heterogeneity in autosomal dominant congenital cerulean cataracts. Ophthalmic Genet. 2002;23(4):199–208. doi: 10.1076/opge.23.4.199.13881. [DOI] [PubMed] [Google Scholar]
- 44.Khaliq S, Hameed A, Ismail M, Anwar K, Mehdi SQ. A novel locus for autosomal dominant nuclear cataract mapped to chromosome 2p12 in a Pakistani family. Invest Ophthalmol Vis Sci. 2002;43(7):2083–2087. [PubMed] [Google Scholar]
- 45.Vanita, Singh JR, Sarhadi VK, Singh D, Reis A, Rueschendorf F, Becker-Follmann J, Jung M, Sperling K. A novel form of “central pouchlike” cataract, with sutural opacities, maps to chromosome 15q21-22. Am J Hum Genet. 2001;68(2):509–514. doi: 10.1086/318189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamada K, Tomita HA, Kanazawa S, Mera A, Amemiya T, Niikawa N. Genetically distinct autosomal dominant posterior polar cataract in a four-generation Japanese family. Am J Ophthalmol. 2000;129(2):159–165. doi: 10.1016/s0002-9394(99)00313-x. [DOI] [PubMed] [Google Scholar]
- 47.Ionides AC, Berry V, Mackay DS, Moore AT, Bhattacharya SS, Shiels A. A locus for autosomal dominant posterior polar cataract on chromosome 1p. Hum Mol Genet. 1997;6(1):47–51. doi: 10.1093/hmg/6.1.47. [DOI] [PubMed] [Google Scholar]
- 48.Kramer P, Yount J, Mitchell T, LaMorticella D, Carrero-Valenzuela R, Lovrien E, Maumenee I, Litt M. A second gene for cerulean cataracts maps to the beta crystallin region on chromosome 22. Genomics. 1996;35(3):539–542. doi: 10.1006/geno.1996.0395. [DOI] [PubMed] [Google Scholar]
- 49.Padma T, Ayyagari R, Murty JS, Basti S, Fletcher T, Rao GN, Kaiser-Kupfer M, Hejtmancik JF. Autosomal dominant zonular cataract with sutural opacities localized to chromosome 17q11-12. Am J Hum Genet. 1995;57(4):840–845. [PMC free article] [PubMed] [Google Scholar]
- 50.Stambolian D, Ai Y, Sidjanin D, Nesburn K, Sathe G, Rosenberg M, Bergsma DJ. Cloning of the galactokinase cDNA and identification of mutations in two families with cataracts. Nat Genet. 1995;10(3):307–312. doi: 10.1038/ng0795-307. [DOI] [PubMed] [Google Scholar]
- 51.Eiberg H, Lund AM, Warburg M, Rosenberg T. Assignment of congenital cataract Volkmann type (CCV) to chromosome 1p36. Hum Genet. 1995;96(1):33–38. doi: 10.1007/BF00214183. [DOI] [PubMed] [Google Scholar]
- 52.McKay JD, Patterson B, Craig JE, Russell-Eggitt IM, Wirth MG, Burdon KP, Hewitt AW, Cohn AC, Kerdraon Y, Mackey DA. The telomere of human chromosome 1p contains at least two independent autosomal dominant congenital cataract genes. Br J Ophthalmol. 2005;89(7):831–834. doi: 10.1136/bjo.2004.058495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hejtmancik JF, Piatigorsky J. Lens Proteins and their Molecular Biology. In: Alpert DM, Jakobiec FA, Azar DT, Gragoudas ES, editors. Principles and Practice of Ophthalmology. Philadelphia: W.B.Saunders Co.; 2000. pp. 1409–1428. [Google Scholar]
- 54.Hejtmancik JF, Datiles M. Congenital and Inherited Cataracts. In: Tasman W, Jaeger EA, editors. Duane's Clinical Ophthalmology. Philadelphia: Lippincott Willliams and Wilkins; 2001. pp. 1–22. [Google Scholar]
- 55.Reches A, Yaron Y, Burdon K, Crystal-Shalit O, Kidron D, Malcov M, Tepper R. Prenatal detection of congenital bilateral cataract leading to the diagnosis of Nance-Horan syndrome in the extended family. Prenat Diagn. 2007;27:662–664. doi: 10.1002/pd.1734. [DOI] [PubMed] [Google Scholar]
- 56.Finzi S, Li Y, Mitchell TN, Farr A, Maumenee IH, Sallum JM, Sundin O. Posterior polar cataract: genetic analysis of a large family. Ophthalmic Genet. 2005;26(3):125–130. doi: 10.1080/13816810500229124. [DOI] [PubMed] [Google Scholar]
- 57.Medina-Martinez O, Jamrich M. Foxe view of lens development and disease. Development. 2007;134(8):1455–1463. doi: 10.1242/dev.000117. [DOI] [PubMed] [Google Scholar]