Abstract
AIM
To evaluate αB-crystallin malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione peroxidase (GPx) changes in X-ray irradiated rat lens.
METHODS
Eight-week-old Sprague-Dawley male rats received X-ray irradiation to the head with rest of the body protected. The exposure dose ranged from 2 to 25 Grays (Gy). The cataract status were examined by slit lamp and rated with “four-grade systems” post-irradiation. The lens MDA level, and the activities of SOD and GPx were measured in a short-term experiment post-irradiation, and αB-crystallin protein levels were quantified.
RESULTS
The lenses of normal control and the X-ray irradiated groups with the dose up to 10 Gy remained transparent throughout the experiment. The lens first appeared tiny scatters, and even lamellar opacities in the posterior capsule 45 days post-irradiation with the dose of 15 Gy, and progressed slowly to the advance stage of cataract; while, for the higher dose (25 Gy), the opacity of lens appeared much earlier, and progressed more rapidly to mature stage of cataract within 1 month. At the end of the observation (90 days post-irradiation), almost all lenses became complete opacity with the higher dose (25 Gy). The degree of lens opacity was rated accordingly. The lens MDA level was increased, and SOD and GPx activities were decreased with a dose-dependent manner post-irradiation. The αB-crystallin protein level was decreased dose-dependently at the end point of observation.
CONCLUSION
Oxidative events and αB-crystallin may play important roles in the pathogenesis of cataract in X-ray irradiated rat lens.
Keywords: αB-crystallin malondialdehyde, superoxide dismutase, glutathione peroxidase, X-ray irradiation, cataract
INTRODUCTION
Cataract, the opacification of the lens of the eye, is the most common cause of blindness worldwide[1], and its prevalence in developing countries is much more than that in the developed ones. In developing countries, 50-90% of all blindness is caused by cataracts[1]. At present the most effective treatment of cataract is the surgical extirpation of the lens, but surgery associated complications are always an issue[2],[3]. Pharmaceutical therapy for cataract has so far not been successful. Hence, it is important to explore the mechanisms of the cataract and look into the pharmacological approaches for the treatment of this disorder. The pathogenesis of cataract is known to be influenced by a number of factors including oxidative stress. Oxidative stress has been identified as one of the major causes of age-related diseases including cataract. And oxidative damage decreases antioxidant capacity or decreased antioxidant capacity resulting in oxidative damage. Lens antioxidant status and lipid peroxidation products have been implicated in human cataract[4]. The antioxidant enzymes, catalase (CAT), GPx, and SOD, are some of the antioxidant enzymes that protect the organisms from oxidative damage[5]. Free radical induced lipid peroxidation, such as MDA, is one of the basic mechanisms of lens opacity. The lens proteins are subjected to extensive oxidative modifications. α-crystallin (αA- and αB-crystallin) is a major protein component of the mammalian eye lens. As a member of the small heat-shock protein family, αB-crystallin possesses chaperone-like function. The α-crystallins and especially αB-crystallin is also found outside the lens having an extensive tissue distribution. An important finding in the past decade has been the association of increased levels of αB-crystallin with various neurological diseases, and mutations in αB-crystallin can cause cataract and myopathy. Lens epithelial cells derived from αB-crystallin knockout mice demonstrated hyper-proliferation and genomic instability, indicating that the widely distributed molecular chaperone αB-crystallin might play an important role in maintaining genomic integrity. Cataract is an unavoidable complication in patients with tumors if radiotherapy includes the orbit in the treated volume, even with very low doses of radiation. It was also reported that X-rays irradiation was associated with a hazard ratio of cataract among radiologic technologists with respect to occupational and nonoccupational exposures[6],[7]. But the exact mechanisms for X-ray irradiated cataract are not fully understood. Based on the knowledge of the oxidative stress and the important role of αB-crystallin on the pathogenesis of the cataract, the present study was to determine the level of free radicals (MDA), the activities of antioxidants (SOD and GPx), and the changes of αB-crystallin in order to correlate them to the X-ray induced cataract.
MATERIALS AND METHODS
Materials
Malondialdehyde (MDA) Detection Kit (A003-1), Superoxide Dismutase (SOD) Detection Kit (A001-1) and Glutathione Peroxidase (GSH-PX) Detection Kit (A005-1) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Rabbit anti-αB-crystallin antibody (sc-22744) and rabbit anti-actin antibody (sc-7210) were from Santa Cruz Biotechnology (Gene Company Ltd., Shanghai, China). Seventy-two eight-week-old Sprague-Dawley male rats were included in the study (Slaccas, Shanghai Institutes for Biological Sciences, Shanghai, China). The animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All animals were maintained on a 12-hour alternating light/dark cycle, and allowed to eat and drink ad libitum.
Methods
The rats were divided into 2 groups: normal control and X-irradiated groups with different doses from 2 to 25 Gy. For X-ray irradiation, the rats were anesthetized with intra-peritoneal pentobarbital sodium (20g/L, 50mg/kg BW). The pupils were dilated with 5g/L tropicamide (Wuxi Shanhe Group, Jiangsu, China). X-ray irradiation was performed to the rat head with a medical electron accelerator (Mid-energy Primus Linear Accelerator, MD-2, Siemens, Germany). The rats were placed from the source. Five rats at a time were placed on a circular rotating base with heads to the center and the remainder of the body including the tail covered with 3mm of lead. Thus, only the heads were exposed to the X-ray radiation. In a dose-response experiment, animals were exposed to 3 different doses of X-ray (2, 5, and 10 Gy) and kept for 11 days after exposure, with 10 animals in each group. In a long-term experiment, animals were exposed to the doses of 5, 15 and 25 Gy and kept for 90 days after exposure, with 8 animals in each group. In both experiments, the exposure time was always 15 minutes and the different doses of X-ray where set by varying the irradiance in the exposure plane. Age-matched control was maintained at the same conditions but without irradiation treatment. The rats were anesthetized and the pupils were dilated with tropicamide. Before and after irradiation from 1 to 90 days, the anterior segments were examined and photographed through the slit lamp with an anterior segment digital imaging system (YZ5F. Slit lamp; 66 Vision Tech. Co., Ltd. Suzhou, China and YTFX-QB1A Imaging System, Xiamen, China). The degree of lens opacity was rated from 0 (completely clear) to 4 (complete opacity of a mature cataract) by two persons without knowledge of the irradiated or control status of the donor rats. None of the rats in either the control or the head-irradiated group showed any signs of distress, reduced physical activity, or reduced food intake during the study period from the beginning to the conclusion of the experiment 90 days following the irradiation. There was a general hair loss from the head that was obvious two weeks after irradiation with re-growth of hair over the subsequent three to four weeks.
MDA, SOD and GPx detection
The rats were killed by an overdose of pentobarbital sodium (intra-peritoneal), followed by cervical dislocation. The eyes were enucleated and the lens from experimental and control rats were isolated and frozen at -80°C. Before analysis, the lenses (8 single lens from 8 rats selected randomly per group) were homogenized in ice-cold radioimmune precipitation assay (RIPA) buffer supplemented with a protease inhibitor PMSF (Shenergy Bicolor Bioscience Technology Company, Shanghai, China) and sonicated at 0.5Hz for 40 seconds (50-watt sonicator, Sonics & Materials, Danbury, CT, USA). The lysate was centrifuged at 20 000g for 15 minutes at 4°C. The supernatants were collected independently and frozen at -80°C. Protein concentrations were determined by Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA). The lens MDA levels, SOD and GPx activities were measured with the kits according to the manufacturer's instructions.
αB-crystallin analysis
The lens lysate was prepared as mentioned above. Equal amounts of protein were resolved in SDS-polyacrylamide gels and transferred electrophoretically onto a nitrocellulose membrane (Bio-Rad). Membranes were blocked for 30 minutes using 50mL/L non-fat milk. The membranes after blocking were incubated overnight with anti-αB-crystallin antibody (1:500), or anti-β-actin antibody (1:500). After washing with TBST, the membranes were incubated for 1 hour with horseradish peroxidase conjugated anti-rabbit antiserum in TBST and 50mL/L non-fat milk. The membranes were washed three times with TBST, and proteins were visualized by enhanced chemiluminescence. The optical density of each band was determined using Quantity One software (Bio-Rad). The densitometric values for the protein of αB-crystallin were normalized for protein loading using β-actin and the resulting values were compared statistically using Student's t test.
Statistical Analysis
Data are expressed as mean±SEM. The statistical analysis was carried out using Student's t test. A P<0.05 was considered statistically significant.
RESULTS
Lens opacity
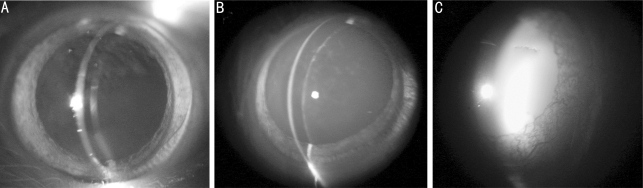
The lenses remained clear in both normal control and low dose irradiated rats up to 10 Gy during the observation period (data not shown). The lenses appeared diffused, dotted and even lamellar opacities in the posterior capsule of the irradiated lens with the dose of 15 Gy, 45 days post-irradiation, and slowly progressed to more advance stage of cataract (Figure 1). For the higher dose (25 Gy), the opacity of the lenses appeared much earlier (30 days post-irradiation) than the lower dose (15 Gy) (data not shown), and it progressed more rapidly to mature stage within one month. At the end point of observation (90 days post-irradiation), almost all the lenses with high dose (25 Gy) appeared completely opacity (7 of 8 lenses, Table 1). The degree of lens opacity in irradiated rat lens was rated from 0 (completely clear) to 4 (complete opacity of a mature cataract) with slit lamp examination by the two viewers, who were blinded as to the animal's previous treatment (Table 1). The distributions of the rats from grade 0 to grade 4 groups were 75% (grade 0) and 25% (grade 1) with low dose of X-ray (15 Gy); and 0, 12.5%, 37.5%, 12.5% and 37.5% with higher dose (25 Gy), 45 days post-irradiation. The opacity of the irradiated lenses progressed from the incipient stage. The distributions of rats were 62.5% (grade 2) and 37.5% (grade 3) with low dose (15 Gy); and 12.5% (grade 3) and 87.5% (grade 4) with higher dose (25 Gy) at the end point of observation (90 days post-irradiation), which indicated much more rapid progression of cataract with a higher dose than the low dose, consistent with the results observed by slit lamp (Table 2).
Figure 1. Representatives photos examined by slit lamp 45 days post-irradiation with different dosage of X-rays treatment in normal control (A), 15 Gy (B), and 25 Gy (C) groups.
Table 1. Distribution of rats irradiated with the dosage of 15 Gy.
| Post-irradiation | 0 | 1 | 2 | 3 | 4 | Total | |
| 45 days | Number | 6 | 2 | 0 | 0 | 0 | 8 |
| Frequency (%) | 75 | 25 | 0 | 0 | 0 | 100 | |
| Cumulative total | 6 | 8 | - | - | - | - | |
| Cumulative frequency (%) | 75 | 100 | - | - | - | - | |
| 60 days | Number | 2 | 5 | 1 | 0 | 0 | 8 |
| Frequency (%) | 25 | 62.5 | 12.5 | 0 | 0 | 100 | |
| Cumulative total | 2 | 7 | 8 | - | - | - | |
| Cumulative frequency (%) | 25 | 87.5 | 100 | - | - | - | |
| 90 days | Number | 0 | 0 | 5 | 3 | 0 | 8 |
| Frequency (%) | 0 | 0 | 62.5 | 37.5 | 0 | 100 | |
| Cumulative total | 0 | 0 | 5 | 8 | - | - | |
| Cumulative frequency (%) | 0 | 0 | 62.5 | 100 | - | - |
Table 2. Distribution of rats irradiated with the dosage of 25 Gy.
| Post-irradiation | Grade | 0 | 1 | 2 | 3 | 4 | Total |
| 45 days | Number | 0 | 1 | 3 | 1 | 3 | 8 |
| Frequency (%) | 0 | 12.5 | 37.5 | 12.5 | 37.5 | 100 | |
| Cumulative total | 0 | 1 | 4 | 5 | 8 | - | |
| Cumulative frequency (%) | 0 | 12.5 | 50 | 62.5 | 100 | - | |
| 60 days | Number | 0 | 1 | 1 | 1 | 5 | 8 |
| Frequency (%) | 0 | 12.5 | 12.5 | 12.5 | 62.5 | 100 | |
| Cumulative total | 0 | 1 | 2 | 3 | 8 | - | |
| Cumulative frequency (%) | 0 | 12.5 | 25 | 37.5 | 100 | - | |
| 90 days | Number | 0 | 0 | 0 | 1 | 7 | 8 |
| Frequency (%) | 0 | 0 | 0 | 12.5 | 87.5 | 100 | |
| Cumulative total | 0 | 0 | 0 | 1 | 8 | - | |
| Cumulative frequency (%) | 0 | 0 | 0 | 12.5 | 100 | - |
MDA levels, SOD and GPx activities in short-term experiment post-irradiation
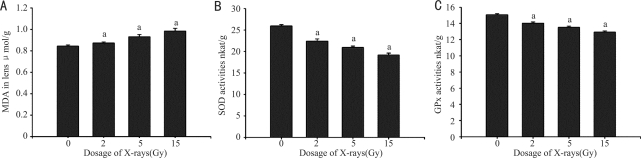
The changes of MDA levels, SOD and GPx activities in the lens reflect the oxidative stress after irradiation. The changes of above factors were measured in a short-term experiment (11 days post-irradiation, Figure 2). When compared with normal control, the MDA levels increased in X-ray irradiated rat lens with a dose-dependent fashion (Figure 2A), it was increased by 3.6%, 10.4% and 16.8%, respectively (P<0.05), in the rat lens irradiated with the dose of 2, 5 and 10 Gy. While the activities of SOD and GPx decreased dose-dependently in X-ray irradiated rat lens (Figure 2B and C), the SOD activity was decreased by 13.6%, 19.4% and 26.1%, respectively (P<0.05); and the GPx activity was decreased by 6.9%, 10.3% and 14.3%, respectively (P<0.05), in the rat lens irradiated with the dose of 2, 5 and 10 Gy.
Figure 2. MDA, SOD and GPx changes post-irradiation. n=10, aP< 0.05 vs normal control.
αB-crystallin expression
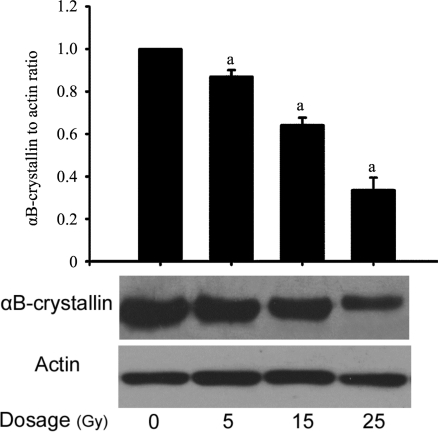
The change of αB-crystallin protein in rat lens was detected after a long-term irradiation (at the end point of observation). The expression of αB-crystallin was down-regulated in the irradiated lens 90 days post-irradiation (Figure 3). Compared with the normal control, the expressions of αB-crystallin were decreased by 12.9%, 22.8% and 30.5%, respectively (P<0.05), in the rat lens irradiated with the dose of 5, 15 and 25 Gy. The decrease of αB-crystallin protein expression also followed a dose-dependent fashion.
Figure 3. αB-crystallin expression in lenses treated with different dosages of X-rays 90 days post-irradiation. n=8, aP< 0.05 vs normal control.
DISCUSSION
In the last 20 years, cataract surgery has progressed from extracapsular cataract extraction to small incisional phacoemulsification. Long-term success rates have been found to generally decrease with the developmental age of the cataract reported in dogs[8]. The exact mechanism of cataract formation has not been clearly defined[1].
Cataract is the opacity of the lens that leads to progressive and painless vision loss. The ocular lens is one of the most radiosensitive tissues and the lens epithelium is considered to be the initiation site for the development of radiation-induced cataract. Ionizing radiation, such as X- and γ-rays and ultraviolet lights, is known to be cataractogenic factor for rat lenses[9]. Since damaging effects of ionizing radiation on living cells are predominantly due to reactive oxygen species (ROS) generated by the decomposition of water and/or Fenton reaction[9], the theory of oxidative damage for cataract development is of interest[9]. The important anti-oxidative enzymes are SOD, GPx and catalase (CAT)[5]. One of the indices of oxidative damage is MDA formation as an end product of lipid peroxidatioin. One report showed that total-cranium irradiation of rats significantly increased the MDA level, and also significantly decreased SOD and GPx activity, emphasizing the generation of increased oxidative stress[10]. Bardak et al found that one week after exposure, SOD and GPx activities in the rat lenses were lower in the UVB group than those in the controls, and the MDA level, an index of cellular damage by free radicals, was higher than that in the controls, suggesting that the depletion of important intracellular antioxidant stores by UV-irradiation in the lenses of the animals might have been the main cause of lens opacification. The changes of the oxidative stress parameters (MDA, SOD and GPx) were also detected in our short-term experiment (Figure 2), and consistent with the previous reports. We found that MDA level was significantly increased in a dose-dependent manner; and the activities of SOD and GPx were dose-dependently decreased after x-irradiation. These results in combination with ours showed that the oxidative stress palys important roles in the pathogenesis of cataract in X-ray irradiated rat lens. And over-expression of SOD in intact lenses could prevent cataract formation induced by oxidative stress[11], which provided a new way to prevent the formation of cataract.
Merriam and Focht reported that radiation induced cataract occurred a period over 3 months at the doses of 2 Gy given single and 5.5 Gy given fractioned. Others reported that total-cranium irradiation of 5 Gy in a single dose enhanced cataract formation 10 days after irradiation[10]. Our results showed that X-ray induced cataract first appeared 45 days post-irradiation with a dose of 15 Gy, and this occurred much earlier with a higher dose of 25 Gy (Table 1, Figure 1). The different irradiation resources and doses used may explain this difference.
Previous studies have demonstrated that α-crystallin, a major protein of the lens, plays a role in maintaining lens transparency, and functions as a molecular chaperone. Both αA- and αB-crystallin are necessary for proper fiber cell formation, and that the absence of α-crystallin can lead to cataract formation. The αB-crystallin is ubiquitously expressed; mutations in the αB-crystallin gene are associated with a broad variety of neurological, cardiac and muscular disorders, indicating its importance throughout the body. The R120G mutation in αB-crystallin gene can cause desmin-related myopathy, cardiomyopathy and cataract[12]. This mutation is a dominant negative mutation. And in vitro, experiments using a recombinant R120G αB-crystallin showed that not only did this mutation cause a decrease in chaperone protection, but with some target proteins it also became an “antichaperone” promoting aggregation. In our experiment, the protein level of αB-crystallin post-irradiation was decreased significantly with a dose-dependent fashion at the time of conclusion (Figure 3), suggesting the loss of its protection as a chaperone with the progression of the cataract.
In conclusion, the results showed that the oxidative stress and the decreased chaperone function of αB-crystallin may contribute to the pathogenesis of X-ray induced cataract. This study also indicated that supplement of the antioxidants as well as αB-crystallin introduction to the lens may have inhibitory effects on formation of X-ray induced cataract.
Footnotes
Foundation item: Scientific Research Foundation for Returned Scholars, the Second Hospital Affiliated to Soochow University (No. SDFEY-2007-10); National Natural Science Foundation of China (No. 81000383); Research Fund for the Doctoral Program of Higher Education of China (No. 20100072120051); Program of Tongji University (No. 1500219024; No. 2010QH04 and No. 2010YF02).
REFERENCES
- 1.Kyselova Z, Stefek M, Bauer V. Pharmacological prevention of diabetic cataract. J Diabetes Complications. 2004;18(2):129–140. doi: 10.1016/S1056-8727(03)00009-6. [DOI] [PubMed] [Google Scholar]
- 2.Toda J, Kato S, Oshika T, Sugita G. Posterior capsule opacification after combined cataract surgery and vitrectomy. J Cataract Refract Surg. 2007;33(1):104–107. doi: 10.1016/j.jcrs.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Bockelbrink A, Roll S, Ruether K, Rasch A, Greiner W, Willich SN. Cataract surgery and the development or progression of age-related macular degeneration: a systematic review. Surv Ophthalmol. 2008;53(4):359–367. doi: 10.1016/j.survophthal.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Ganea E, Harding JJ. Glutathione-related enzymes and the eye. Curr Eye Res. 2006;31(1):1–11. doi: 10.1080/02713680500477347. [DOI] [PubMed] [Google Scholar]
- 5.Chandrasena LG, Chackrewarthy S, Perera PT, de Silva D. Erythrocyte antioxidant enzymes in patients with cataract. Ann Clin Lab Sci. 2006;36(2):201–204. [PubMed] [Google Scholar]
- 6.Chodick G, Bekiroglu N, Hauptmann M, Alexander BH, Freedman DM, Doody MM, Cheung LC, Simon SL, Weinstock RM, Bouville A, Sigurdson AJ. Risk of cataract after exposure to low doses of ionizing radiation: a 20-year prospective cohort study among US radiologic technologists. Am J Epidemiol. 2008;168(6):620–631. doi: 10.1093/aje/kwn171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robman L, Taylor H. External factors in the development of cataract. Eye. 2005;19(10):1074–1082. doi: 10.1038/sj.eye.6701964. [DOI] [PubMed] [Google Scholar]
- 8.Sigle KJ, Nasisse MP. Long-term complications after phacoemulsification for cataract removal in dogs: 172 cases (1995-2002) J Am Vet Med Assoc. 2006;228(1):74–79. doi: 10.2460/javma.228.1.74. [DOI] [PubMed] [Google Scholar]
- 9.Ertekin MV, Kocer I, Karslioglu I, Taysi S, Gepdiremen A, Sezen O, Balci E, Bakan N. Effects of oral Ginkgo biloba supplementation on cataract formation and oxidative stress occurring in lenses of rats exposed to total cranium radiotherapy. Jpn J Ophthalmol. 2004;48(5):499–502. doi: 10.1007/s10384-004-0101-z. [DOI] [PubMed] [Google Scholar]
- 10.Karslioglu I, Ertekin MV, Taysi S, Kocer I, Sezen O, Gepdiremen A, Koc M, Bakan N. Radioprotective effects of melatonin on radiation-induced cataract. J Radiat Res (Tokyo) 2005;46(2):277–282. doi: 10.1269/jrr.46.277. [DOI] [PubMed] [Google Scholar]
- 11.Lin D, Barnett M, Grauer L, Robben J, Jewell A, Takemoto L, Takemoto DJ. Expression of superoxide dismutase in whole lens prevents cataract formation. Mol Vis. 2005;11:853–858. [PubMed] [Google Scholar]
- 12.Rajasekaran NS, Connell P, Christians ES, Yan LJ, Taylor RP, Orosz A, Zhang XQ, Stevenson TJ, Peshock RM, Leopold JA, Barry WH, Loscalzo J, Odelberg SJ, Benjamin IJ. Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell. 2007;130(3):427–439. doi: 10.1016/j.cell.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]