Abstract
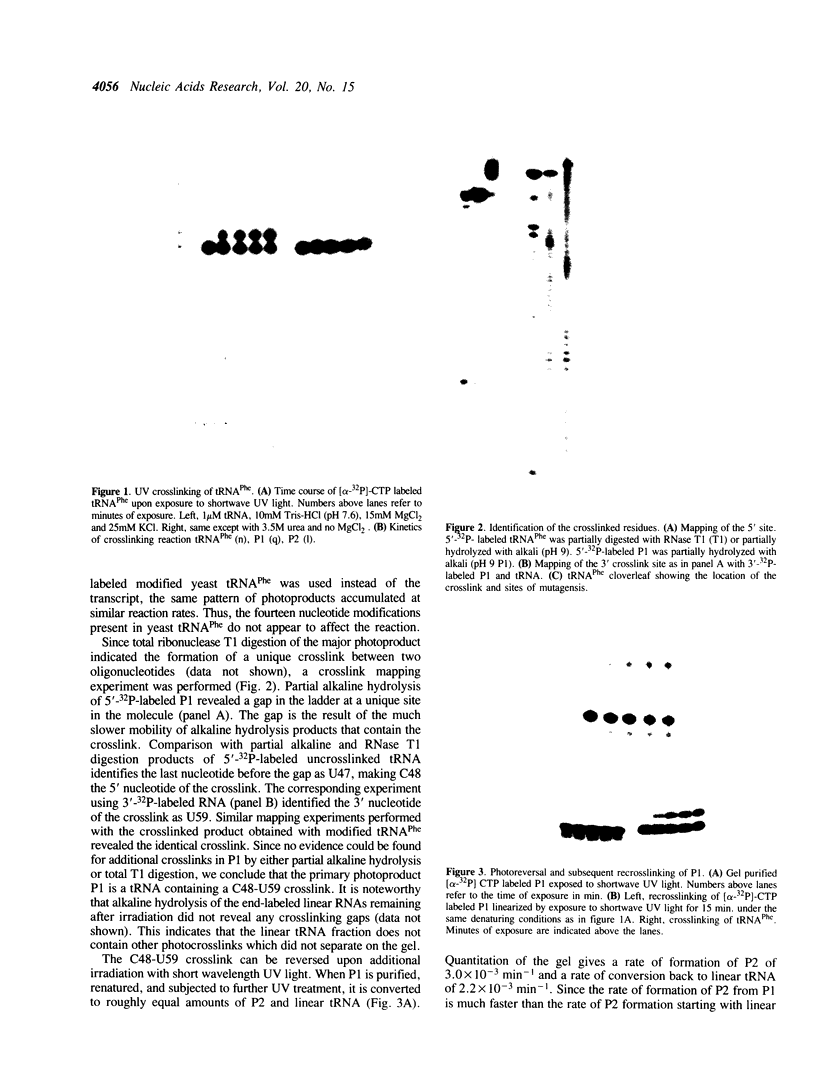
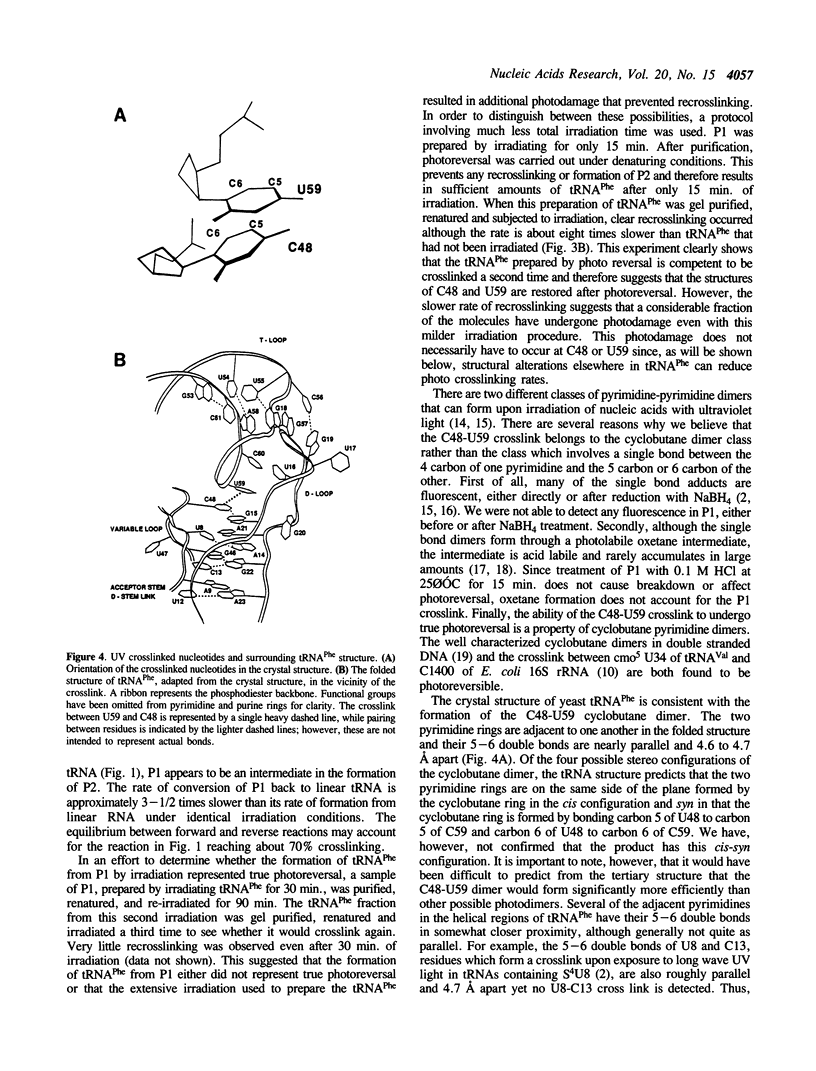
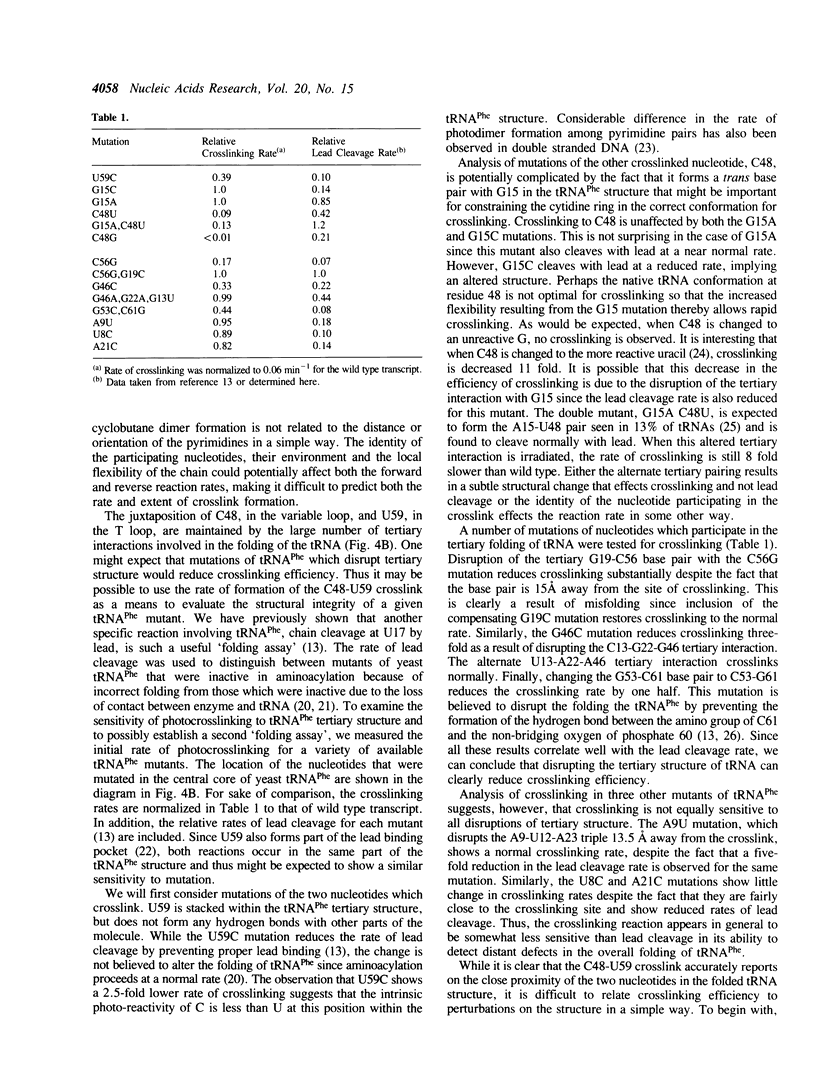
The irradiation of native or unmodified yeast tRNA(Phe) by short wavelength UV light (260 nM) results in an intramolecular crosslink that has been mapped to occur between C48 in the variable loop and U59 in the T loop. Photo-reversibility of the crosslink and the absence of fluorescent photo adducts suggest that the crosslink product is a cytidine-uridine cyclobutane dimer. This is consistent with the relative geometries of C48 and U59 in the crystal structure of yeast tRNA(Phe). Evaluation of the crosslinking efficiency of the mutants of tRNA(Phe) indicates that the reaction depends on the correct tertiary structure of the RNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atmadja J., Brimacombe R., Blöcker H., Frank R. Investigation of the tertiary folding of Escherichia coli 16S RNA by in situ intra-RNA cross-linking within 30S ribosomal subunits. Nucleic Acids Res. 1985 Oct 11;13(19):6919–6936. doi: 10.1093/nar/13.19.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behlen L. S., Sampson J. R., DiRenzo A. B., Uhlenbeck O. C. Lead-catalyzed cleavage of yeast tRNAPhe mutants. Biochemistry. 1990 Mar 13;29(10):2515–2523. doi: 10.1021/bi00462a013. [DOI] [PubMed] [Google Scholar]
- Bergstrom D. E., Leonard N. J. Photoreaction of 4-thiouracil with cytosine. Relation to photoreactions in Escherichia coli transfer ribonucleic acids. Biochemistry. 1972 Jan 4;11(1):1–9. doi: 10.1021/bi00751a001. [DOI] [PubMed] [Google Scholar]
- Branch A. D., Benenfeld B. J., Robertson H. D. Ultraviolet light-induced crosslinking reveals a unique region of local tertiary structure in potato spindle tuber viroid and HeLa 5S RNA. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6590–6594. doi: 10.1073/pnas.82.19.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brash D. E., Haseltine W. A. UV-induced mutation hotspots occur at DNA damage hotspots. Nature. 1982 Jul 8;298(5870):189–192. doi: 10.1038/298189a0. [DOI] [PubMed] [Google Scholar]
- Brown R. S., Dewan J. C., Klug A. Crystallographic and biochemical investigation of the lead(II)-catalyzed hydrolysis of yeast phenylalanine tRNA. Biochemistry. 1985 Aug 27;24(18):4785–4801. doi: 10.1021/bi00339a012. [DOI] [PubMed] [Google Scholar]
- Downs W. D., Cech T. R. An ultraviolet-inducible adenosine-adenosine cross-link reflects the catalytic structure of the Tetrahymena ribozyme. Biochemistry. 1990 Jun 12;29(23):5605–5613. doi: 10.1021/bi00475a027. [DOI] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Favre A., Yaniv M. Introduction of an intramolecular flourescent pobe in E. coli tRNA(Val)(1). FEBS Lett. 1971 Oct 1;17(2):236–240. doi: 10.1016/0014-5793(71)80154-0. [DOI] [PubMed] [Google Scholar]
- Favre A., Yaniv M., Michelson A. M. The photochemistry of 4-thiouridine in Escherichia coli t-RNA Vał1. Biochem Biophys Res Commun. 1969 Oct 8;37(2):266–271. doi: 10.1016/0006-291x(69)90729-3. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A. Ultraviolet light repair and mutagenesis revisited. Cell. 1983 May;33(1):13–17. doi: 10.1016/0092-8674(83)90329-x. [DOI] [PubMed] [Google Scholar]
- Hauswirth W., Wang S. Y. Excited state processes and solution conformation of dipyrimidine adducts. Photochem Photobiol. 1977 Feb;25(2):161–166. doi: 10.1111/j.1751-1097.1977.tb06892.x. [DOI] [PubMed] [Google Scholar]
- Hauswirth W., Wang S. Y. Pyrimidine adduct fluorescence in UV irradiated nucleic acids. Biochem Biophys Res Commun. 1973 Apr 2;51(3):819–826. doi: 10.1016/0006-291x(73)91388-0. [DOI] [PubMed] [Google Scholar]
- Ofengand J., Liou R. Evidence for pyrimidine-pyrimidine cyclobutane dimer formation in the covalent cross-linking between transfer ribonucleic acid and 16S ribonucleic acid at the ribosomal P site. Biochemistry. 1980 Oct 14;19(21):4814–4822. doi: 10.1021/bi00562a016. [DOI] [PubMed] [Google Scholar]
- Pan T., Uhlenbeck O. C. In vitro selection of RNAs that undergo autolytic cleavage with Pb2+. Biochemistry. 1992 Apr 28;31(16):3887–3895. doi: 10.1021/bi00131a001. [DOI] [PubMed] [Google Scholar]
- Prince J. B., Taylor B. H., Thurlow D. L., Ofengand J., Zimmermann R. A. Covalent crosslinking of tRNA1Val to 16S RNA at the ribosomal P site: identification of crosslinked residues. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5450–5454. doi: 10.1073/pnas.79.18.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romby P., Carbon P., Westhof E., Ehresmann C., Ebel J. P., Ehresmann B., Giegé R. Importance of conserved residues for the conformation of the T-loop in tRNAs. J Biomol Struct Dyn. 1987 Dec;5(3):669–687. doi: 10.1080/07391102.1987.10506419. [DOI] [PubMed] [Google Scholar]
- Sampson J. R., Behlen L. S., DiRenzo A. B., Uhlenbeck O. C. Recognition of yeast tRNA(Phe) by its cognate yeast phenylalanyl-tRNA synthetase: an analysis of specificity. Biochemistry. 1992 May 5;31(17):4161–4167. doi: 10.1021/bi00132a002. [DOI] [PubMed] [Google Scholar]
- Sampson J. R., DiRenzo A. B., Behlen L. S., Uhlenbeck O. C. Role of the tertiary nucleotides in the interaction of yeast phenylalanine tRNA with its cognate synthetase. Biochemistry. 1990 Mar 13;29(10):2523–2532. doi: 10.1021/bi00462a014. [DOI] [PubMed] [Google Scholar]
- Sampson J. R., Uhlenbeck O. C. Biochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1033–1037. doi: 10.1073/pnas.85.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Weber J., Blank J., Zeidler R. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1989;17 (Suppl):r1–172. doi: 10.1093/nar/17.suppl.r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG S. Y. Reversible behaviour of the ultra-violet irradiated deoxyribonucleic acid and its apurinic acid. Nature. 1960 Dec 3;188:844–846. doi: 10.1038/188844a0. [DOI] [PubMed] [Google Scholar]
- Wang S. Y. Thymine phototrimer. J Am Chem Soc. 1971 Jun 2;93(11):2768–2771. doi: 10.1021/ja00740a030. [DOI] [PubMed] [Google Scholar]
- Wollenzien P., Expert-Bezançon A., Favre A. Sites of contact of mRNA with 16S rRNA and 23S rRNA in the Escherichia coli ribosome. Biochemistry. 1991 Feb 19;30(7):1788–1795. doi: 10.1021/bi00221a009. [DOI] [PubMed] [Google Scholar]
- Yaniv M., Favre A., Barrell B. G. Structure of transfer RNA. Evidence for interaction between two non-adjacent nucleotide residues in tRNA from Escherichia coli. Nature. 1969 Sep 27;223(5213):1331–1333. doi: 10.1038/2231331a0. [DOI] [PubMed] [Google Scholar]
- Zwieb C., Schüler D. Low resolution three-dimensional models of the 7SL RNA of the signal recognition particle, based on an intramolecular cross-link introduced by mild irradiation with ultraviolet light. Biochem Cell Biol. 1989 Aug;67(8):434–442. doi: 10.1139/o89-069. [DOI] [PubMed] [Google Scholar]