Abstract
AIM
To analyze osteopontin (OPN) expression in vitreous and proliferative retinal membranes of patients with proliferative vitreous retinopathy (PVR).
METHODS
A total of 54 vitreous fluid samples were obtained between 2009 and 2010, which contained 45 with PVR (group A) and 9 without PVR (group B). Enzyme-linked immunosorbent assay was applied to quantify the OPN concentrations in vitreous fluid. Four samples of proliferative retinal membrane were also obtained at the time of vitrectomy, and their contents of OPN were measured by Real-time RT-PCR.
RESULTS
The OPN levels in the vitreous fluid were 778.48±62.06ng/mL in group A and 452.99±32.52ng/mL in group B. The vitreous OPN levels in group A were significantly higher than those in group B and to rise by time in the early stages of PVR. The average OPN levels in the proliferative retinal membranes (F=0.14) were also higher than those in the retinal pigment cells (F=0) using Real-time RT-PCR.
CONCLUSION
The high vitreous and proliferative retinal membrane OPN levels in PVR suggest that OPN might promote the development of PVR. The vitreous OPN concentrations are rising by the time in the early phases of PVR.
Keywords: osteopontin, proliferative vitreous retinopathy, vitreous, proliferative retinal membranes
INTRODUCTION
OPN has been detected in a variety of human body fluids such as blood, urine, and milk, which are suggested to reflect the pathobiology of human diseases, and to induce the development of the proliferative diseases. Due to the transferring, coherence and accrementition of the retinal pigment epithelium (RPE) and glia cell in pathological condition, PVR, one of the proliferative diseases, can form a kind of the cell-membrane and induce advanced tractional retinal-detachment. But the concentration of OPN in vitreous fluids of the patients with PVR has not been reported. This study aims to analyze the difference of OPN levels in vitreous and in proliferative retinal membranes between patients with PVR and healthy people using enzyme-linked immunospecific assay and Real-time quantitative RT-PCR, and to find the association between OPN and the development of PVR.
MATERIALS AND METHODS
Materials
Forty-five vitreous fluid samples (group A) were obtained from patients with PVR, whose average age was 53.6 years old, from Jun. 2009 to Jun. 2010, including 24 males and 21 females. The other 9 vitreous fluid samples (group B) were obtained from healthy people's eyes (corneal transplant donors) as normal control, whose average age was 47.1 years old. There was no difference in age and gender between group A and group B. Four samples of proliferative retinal membranes (three samples of PVR D1; one sample of PVR D2), which adhere to the surface of retina, were also obtained at the time of vitreous-dissection surgery of group A. The diagnosis, according to the direct and indirect ophthalmofundoscope, was in accordance with the international denominator of PVR reported in 1983. Both 3 samples of huge hole (PVR B) and the other 42 samples of hole-resource detachment of retina (thirty-two samples of PVR C3; seven samples of PVR D1; three samples of PVR D2 ) were to be given modern clogging vitreous- dissection surgery without any primary ocular operation, eye trauma, diabetes, high blood pressure and the detachment of retina by traction due to the occlusion of retina vein (Table 1). Informed consent was obtained from all patients. All studies conformed to the tenets of the Declaration of Helsinki and approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanjing Medical University. Human osteopontin immunoassay was purchased from R&D Company of USA, using machine of tipe ∑550 (USA) to measure. Ultrapure water, 10*pcr buffer, ion of Mag, sybr (50x), and Taq enzyme were purchased from Invitrogen Company of USA. The dNTPs of TaKaRa Company, the RPE of ATCC Company of USA and the IQ5 fluorescent quantitation equipment of Bio-rad Company were also used in this study.
Table 1. Patients information.
| PVR level | Samples(n) | Disease course(d) | Ranking |
Hyperplasiamembrane(n) | ||
| 1 | 2 | 3 | ||||
| B | 3 | 11.67 | / | / | / | 0 |
| C | 32 | 52.50 | 0 | 0 | 32 | 0 |
| D | 10 | 69.50 | 7 | 3 | 0 | 4 |
According to the international denominator of PVR reported by Robert Machemer in 1983
Methods
The saccus conjunctiva were disinfected by 0.5g/L Iodophors, then they were washed by stroke-physiological saline solution, finally the remaining fluid were absorbed completely from the saccus conjunctivae. About 0.2mL of the vitreous fluids samples were obtained by absorbing at orbiculus ciliaris before cavum vitreum dabbling, and the proliferative retinal membranes in some PVR patients were stripped by intraocular forceps. Both of the two type samples were stored by 0.5mL Eppendorf pipes disinfected at -70°C until to be used.
Measurement of OPN levels in vitreous fluid
The samples should be stored at ordinary temperature about 10 minutes after being taken out of the freezer. The standard provided in ELISA kit was diluted in extensor by dilution according to the demonstration. 100µL of Assay Diluent RD1-6 was added to each well. Then, 50µL of standard, control, or sample was added per well to incubate for 2 hours at room temperature covering with the adhensive strip provided. To add 200µL of OPN conjugate to every well after 4 times washing, and incubate for 2 hours at room temperature again. 200µL of substrate solution was added to each well of the 96-well microtiter plates which was incubated for 30 minutes protecting from light after 4 times washing. Color development was stopped by the addition of 50µL of stop solution. A biorad plate reader was used to quantify the signal at 450nm in 30 minutes. Reproducibility of the data was comfirmed by testing each sample at least three times.
Assaying the OPN levels in proliferative retinal membranes
Real-time RT-PCR quantified mRNAs. Total RNA was isolated from pools of proliferative retinal membrane. Then the RNA contained in the aqueous solution was purified using RNeasy Mini Kit (invitrogen, USA), reverse transcribed, and subjected to real-time polymerase chain reaction (PCR) using sequence-specific primers as follows: 5'-TACCCTGATGCTACAGACGAGG-3' (forward); 5'-CTGACTATCAATCACATCGGAATG-3' (reverse)[1]. PCR was performed by using the Taq Man PCR Master Mix and the IQ5 fluorescent quantitation equipment (Applied BIO-rad company) using the following parameters: 95°C for 3 minutes, 94°C for 30 seconds, 60°C for 45 seconds and 72°C for 30 seconds, followed by 50 cycles and 72°C for 10 minutes. The expression level of each mRNA was normalized to GAPDH, and each state of development was compared against the mRNA of RPE using the 2−ΔΔCT method.
Statistical Analysis
The results were expressed as the mean±SE. Difference were evaluated by Student's t test using SPSS 11.5 for Windows. There was a magnificent difference when P<0.05.
RESULTS
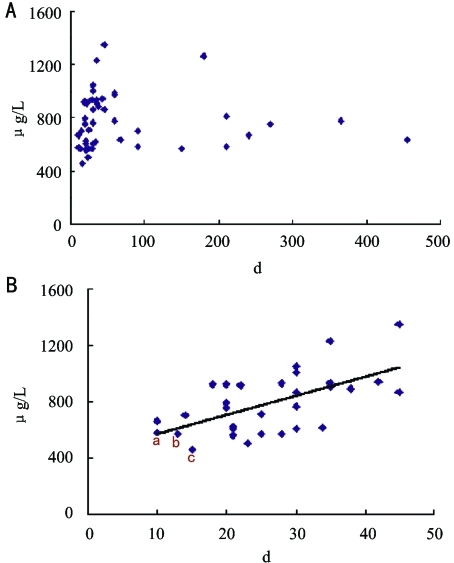
The OPN levels in vitreous fluid were 778.48±62.06ng/mL in PVR (group A) and 452.99±32.52ng/mL in group B. The vitreous OPN levels of group A, which were rising by time in the early stages of the PVR, were significantly higher than those of group B (P<0.05). Linear regressions between the OPN levels and the course of disease before 1.5 months showed positive correlation in group A. The average OPN levels in the proliferative retinal membranes (average F=0.14) were also higher than those in the retinal pigment cells (F=0) using Real-time RT-PCR (Figures 1A, B).
Figure 1. The time-concentration scatterplot of OPN about group A.
A: 45 samples; B: Autopathy to 1.5 months (31 samples)
DISCUSSION
Proliferative-related protein OPN, which can be synthesized and secreted by a variety of cells, is a negative production of phosphate and sugar protein with molecular weight 44 KD, containing about 300 amino acids. It is found that OPN engaged in a variety of cells, can repair damage in many diseases, having functions such as promoting cell, adhesion and proliferation division, and so on. PVR is a proliferative disease of eyes, the pathological mechanisms are the movement and hyperplasy of retinal pigment cells (RPE), with the help of retinal glial cells and other factors. It could effectively block the impact of vascular system to the intraocular environment because of the blood-retinal barrier in eye, so that the measured concentration of OPN in vitreous obtained at the time of vitreous-dissection surgery reflected objectively the cell secretion level of OPN in PVR, and the association of OPN and PVR could be identified. In this study, the OPN concentration in PVR was much higher than that of group B (778.48±62.06ng/mL vs 452.99±32.52ng/mL). It showed that increased concentration of OPN might have a very close association with the formation of PVR.
OPN plays a physiological role mainly relying on its combination of integrin receptor and CD44 receptor[2],[3]. It had been reported that the adhesion between integrin-mediated RPE cells and extracellular matrix protein would cause the formation of PVR. Yang et al[4] also confirmed that the synthetic or biological-extracted soluble small-molecules-peptide containing the same sequence as OPN (RGD sequence, Arg-Gly-Asp) could competitively bind to the intergrin receptor on the surface of cells and cause inhibition of adhesion, proliferation, migration and differentiation of cell. These extracts with the RGD sequence could also inhibit the development of tractional detachment of retina caused by RPE-mediated proliferation of cell in vivo. All of these assumptions were in accordance with the results of our study that the increasing concentration of OPN in PVR might promote proliferation.
It had been found during the analysis that the distribution of scatterplot was irregular and most of the distribution localized within two months when we focused on the relation of the disease-time and concentration of OPN (Figure 1A). There was even a linearity-increasing tendency in the scatterplot from zero to one and half months (Figure 1B), suggesting that the OPN concentration of vitreous might increase by time at the primary stage of disease and reached a higher level, eventually forming a ridge, between one to two months to promote the development of PVR in patients of PVR. There were still a few plots in scatterplot figure after two months, and the contents of OPN decreased to a relative steady level and kept for a longer time. It might be concerned with the externalization-reduction of PVR when the vitreoretinal hyperplasy reached a higher level. More samples should be collected for analysis. The three plots (a, b, c Figure 1) were cases of PVR B with short time course of disease, which OPN concentration was 531.26±167.29ng/mL (average course of disease was 11.67 days). Its concentration, being similar with the concentration of group B, was different from that of the samples of PVR C3 or above, which were 796.14±63.58ng/mL (average course of disease was 56.55 days). It suggested that the second weekend of the course of disease was a sensitive period of increasing of OPN concentration and promoting of the development of PVR, and the course of disease from second weekend to one month was a critical period for the development of PVR. If the assumption was correct, it would provide us a new valuable message about the prophylactic treatment of PVR that it might reduce the incidence rate of PVR when an adequacy and adequacy treatment was given at the priming stage or initial phase of development of PVR.
High expression of OPN was found in many other systemic diseases such as tumor[5] in primary studies. Therefore, four samples of proliferative retinal membrane were collected at the time of the vitrectomy surgery to test by Real-time RT-PCR. The Real-time RT-PCR showed the expression of OPN in proliferative retinal membranes but not RPE, indicating that the forming of proliferative retinal membrane might be promoted by high level OPN. In animal, OPN reside mostly in ganglion cell layer of retina[6] but few in the other layer of retina, and can't be detected normally because of no or few OPN in healthy RPE. It's possible that the hyperplasia-related protein OPN was excreted by irritated retinal cells in pathological condition, then influenced the normal RPE cells and colloid cells, finally proliferative retinal membranes formed by the high level OPN. The OPN, excreted by irritated retina cells existed in proliferative retinal membranes and reached a high level, was also excreted into vitreous fluid, the external environment of proliferative retinal membranes, and having a synergistic action with other related factors. Our conclusion was similar to their presumption.
To sum up that OPN levels were high in the vitreous fluid and proliferative retinal membrane of PVR and were rising by time at the early stages of PVR provided a foundation to flow on investigating the association of the OPN and PVR. There was another report about the cellular epithelia migration promoted by OPN resulting from vascular endothelial growth factor (VEGF) in other systematic diseases[7]. The joint action of VEGF and OPN that inducing visualization, enhancing the growth and transfusion of tumor, irritating the movement of endothelial cell and forming cavitation and the effectiveness of VEGF promoted by OPN was identified. VEGF level was also found high in vitreous fluids of PVR in some other studies[8], and promoted the formation of proliferative retinal membrane. But the forming of PVR, which might effected many bioactivity paths, is a very complicated process, it involves many cellular factors such as PDGF, TGF-ß2, MMPs et al[9]. Therefore, PVR was considered as a mostly encountered complication in rhegmatogenous detachment of retina (RRD), and after reattachment of retina surgery, which was reduced in some degree by vitrectomy, but its incidence after surgery also reached 10%. Currently, the movement and hyperplasy of cell was considered generally as the mechanisms of development of PVR, but its basic mechanisms of proliferation was still not clear. One of the shortages of our study is that the sample size is a bit smaller, more related cases of PVR should be collected to analyze the association between secretion of PVR, the course of diseases and the related cell factor.
Footnotes
Foundation item: National Nature Science Foundation of China (No. 30973257and 81070743); Research Found of Jiangsu Health Department, China (No. H200908)
REFERENCES
- 1.Kikuchi K, Tanaka A, Miyakawa H, Kawashima Y, Kawaguchi N, Matsushita M, Gershwin ME. Eta-1/osteopontin genetic polymorphism and primary biliary cirrhosis. Hepatol Res. 2003;26(2):87–90. doi: 10.1016/s1386-6346(03)00012-3. [DOI] [PubMed] [Google Scholar]
- 2.Casals G, Ordi J, Creus M, Fabreques F, Casamitjana R, Quinto L, Campo E, Balasch J. Osteopontin and αvβ3 integrin expression in the endometrium of infertile and fertile women. Reprod Biomed Online. 2008;16(6):808–816. doi: 10.1016/s1472-6483(10)60146-0. [DOI] [PubMed] [Google Scholar]
- 3.Maria CGM, Matthew P, Debbie DW, De SH, Howard SF. In vivo osteopontin-induced macrophage accumulation is dependent on CD44 expression. Cell Immunol. 2008;254(1):56–62. doi: 10.1016/j.cellimm.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang CH, Huang TF, Liu KR, Chen MS, Hung PT. Inhibition of retinal pigment epithelial cell-induced tractional retinal detachment by disintegrins, a group of Arg-Gly-Asp-containing peptides from viper venom. Invest Ophthalmol Vis Sci. 1996;37(5):843–854. [PubMed] [Google Scholar]
- 5.Kao CL, Chiou SH, Ho DMT, Chen YJ, Liu RS, Lo CW, Tsai FT, Lin CH, Ku HH, Yu SM, Wong TT. Elevation of plasma and cerebrospinal fluid osteopontin levels in patients with atypical teratoid/rhabdoid tumor. Am J Clin Pathol. 2005;123(2):297–304. doi: 10.1309/0ftkbkvnk4t5p1l1. [DOI] [PubMed] [Google Scholar]
- 6.Ju WK, Kim KY, Cha JH. Ganglion cells of the rat retinal show osteopotin-like immunoreactivity. Brain Res. 2000;852(1):217–220. doi: 10.1016/s0006-8993(99)02140-x. [DOI] [PubMed] [Google Scholar]
- 7.Shijubo N, Uede T, Kon S, Nagata M, Abe S. Vascular endothelial growth factor and osteopontin in tumor biology. Crit Rev Oncog. 2000;11:135–146. [PubMed] [Google Scholar]
- 8.Dieudonné SC, La Heij EC, Diederen RMH, Kessels AGH, Liem ATA, Kijlstra A, Hendrikse F. Balance of vascular endothelial growth factor and pigment epithelial growth factor prior to development of proliferative vitreoretinopathy. Ophthalmic Res. 2007;39(3):148–154. doi: 10.1159/000103234. [DOI] [PubMed] [Google Scholar]
- 9.Kita T, Hata Y, Kano K, Miura M, Nakao S, Noda Y, Shimokawa H, Ishibashi T. Transforming growth factor-beta2 and connective tissue growth factor in proliferative vitreoretinal diseases: possible involvement of hyalocytes and therapeutic potential of Rho kinase inhibitor. Diabetes. 2007;56(1):231–238. doi: 10.2337/db06-0581. [DOI] [PubMed] [Google Scholar]