Abstract
AIM
To establish a repeatable rat model of Fusarium solani keratitis (F. solani keratitis) that mimicked fungal keratitis in humans.
METHODS
Wistar rats' corneas were scratched on the superficial stroma after scraping the unilateral corneal epithelia. Then, the corneal surface was inoculated with different inoculum dose of F. solani spore suspension. Doses ranged from 106 to 109 colony-forming unit per milliliter (CFU/mL). The treated corneas were covered by contact lenses that were made of Parafilm M membrane. Negative controls were inoculated with sterile phosphate-buffered saline (PBS). For statistical analysis, corneas were evaluated daily on a 12-point scale to check the state of corneal inflammation. Furthermore, the pathological characteristics of this model were investigated.
RESULTS
The rat model of F. solani keratitis was established by the combination methods of corneal trauma and parafilm-made contact lens and inoculation of fungus spore suspension. 106 and 107CFU/mL of F. solani induced mild corneal infection, while 108CFU/mL of F. solani was sufficient to induce moderate infection that was consistent with human keratomycosis. Dose of 109CFU/mL of F. solani was excessive and led to perforated corneas.
CONCLUSION
The rat model of F. solani keratitis, established by the combinational methods of corneal trauma, parafilm-made contact lens and the appropriate dose of inoculum, that imitates the developing processes of F. solani keratitis in human beings and provides a repeatable method of creating a rat model.
Keywords: fungal keratitis, Fusarium solani, corneal trauma, contact lens, rat
INTRODUCTION
Ocular fungal infections and ophthalmic mycoses are the main causes of blindness in humans. The incidence of fungal keratitis has increased dramatically over the past several decades worldwide, especially in developing countries, such as India, Ghana and China. This increase in fungal infections may be related to the abuse of corticosteroids and antibiotics, as well as the increase in use of contact lenses[1]-[3].
Ocular fungi include yeasts and filamentous fungi. Yeasts are opportunistic fungal infections that can rapidly cause ocular infections and yeast keratitis if the patient is taking immunosuppressive agents. Unlike yeasts, filamentous fungi usually do not cause keratitis unless corneal trauma was also present. A specific clinic survey in China indicated that the most prominent species of fungi that resulted in fungal keratitis was Fusarium solani (F. solani) in humans. Currently, there is no standard treatment of fungal keratitis because the pathogenesis of fungal keratitis is largely unknown. The common antifungal drugs have minimal effect on this disease.
To fully elucidate the problems caused by F. solani to the human eyes and understand the pathogenic nature of the disease, appropriate experimental animal models of fungal keratitis are required to further the basic investigation of keratomycosis. Several methods have been reported to establish similar experimental disease models. However, these methods are not easy to reproduce and there is considerable interference of the immune system of the experimental animals. Additionally, some methods applied unnatural methods to establish corneal disease, while others required advanced clinical practical skills for the optical surgery.
To overcome the disadvantages of the previous models, we have designed a novel method that combines the corneal trauma with the use of a parafilm-made contact lens to establish the rat model of F. solani keratitis. The immune system of the treated animals was not disturbed throughout the experimental procedures. The aim of this work is to provide an appropriate research model, which is reproducible and comparable to the developmental study of fungal keratitis in humans.
MATERIALS AND METHODS
Fungal culture
The stain for F. solani was bought from China General Microbiological Culture Collection Center (CGMCC 3.5840), which grows on potato dextrose agar (PDA). 37g of PDA powder was suspended in one liter of ddH2O and then was sterilized at 121°C for 20 minutes. Afterwards, it was poured into culture bottles to become solid PDA medium for fungus culture. The fungus was cultured at 24°C for 5 to 7 days. It was harvested in sterile phosphate-buffered saline (PBS) and diluted with sterile PBS to reach the final concentrations of 106, 107, 108 and 109 colony-forming units per milliliter (CFU/mL).
Parafilm-made contact lens
The average radius of corneal diameter of Wistar rats was 3.03mm. Parafilm M membrane was applied to create a contact lens with a diameter of 3.13mm, to ensure complete coverage of corneal surface. The parafilm made contact lens was sterilized in 75% alcohol solution and then washed with ddH2O for 3 times before using.
Animals
Adult Wistar rats (from the Animal Culturing Center of Fudan University, Shanghai) were bought at the weight of 180-220g. All animals were treated in accordance with the ARVO statements of the Use for Animals in Vision and Ophthalmology Research. Experimental rats were randomly selected to undergo all treatments and all rats were screened with a slitlamp biomicroscope to exclude any existing corneal diseases.
Eight rats were randomly placed into one of 4 groups. Each group received a different inoculum dose of F. solani. Group A, B, C and D received 106, 107, 108 and109 CFU/mL, respectively. An additional 8 rats served as a negative control group. During the first three days, one eye of the rats was treated with Ofloxacin Eye Drops. On the fourth day, rats were anesthetized intraperitoneally with 3% pentobarbital (45mg/kg) and 1% dicaine was placed on the corneal surface. The entire corneal epithelium was removed with sterile 25G needle under microscopic guidance. The Bowman's membrane and corneal stroma was scratched with a hypodermic needle to create a superficial wound, and then a 5-µL inoculum of F. solani was put on the corneal surface. Afterwards, the hand made contact lens covered the wound to exclude the effects of tearing. Finally, the eyelids were sutured with 4/0 polyester suture material. Control rats were mock inoculated with the carrier (sterile PBS). After 1 day of inoculation, the eyelids were reopened and the contact lenses were removed.
Visual scoring
To evaluate the severity of F-solani-induced keratomycosis, experimental eyes were examined at different time points, ranging from 1 to 14 days under a slitlamp biomicroscope. A modified visual scoring system for murine fungal keratitis was used in the present study i.e. the area of corneal opacity, density of corneal opacity and shape of corneal ulcer were independently evaluated. Score of all three parameters were added to provide a total score that ranged from 0 to 12 (revised from Wu et al[4]). A normal cornea, with no opacity, containing all of the epithelia, was given a score of 0 for each parameter, and thus had a total score of 0.
Pathogenic study
Two infected or mock-infected rats were randomly selected from each group to characterize the pathogen. The corneal surfaces of these rats were cleaned with sterile ddH2O and the necrotic tissue was harvested from the edge and the bottom of the corneal ulcer. This tissue was then cultured on solid PDA medium. In parallel, some of necrotic cornea was stained with Gram stain and 10% potassium hydroxide (10% KOH) to identify the micro organisms and thus mixed infections with bacteria were excluded.
Histology
Rats were sacrificed with a lethal dose of 3% pentobarbital at different time points after inoculation. The cornea was removed and fixed in formalin. The paraffin embedded samples were sectioned at a thickness of 4μm for histological study. The deparaffinized slices were stained with hematoxylin eosin (HE) and periodic acid schiff (PAS) and examined under a light microscope.
RESULTS
Inoculation dosage
Eight eyes were infected with F. solani at different concentrations to test the association between the quantity and the virulence of F. solani. Visual opacity was determined according to the scoring system in Table 1. When the eyes were infected with F. solani at the concentration of 106CFU/mL, four out of eight treated corneas developed keratitis, although the infections were more prevalent in corneas infected with concentrations greater than that of 107CFU/mL group. The majority of the treated corneas developed visual opacity at the concentration of 108 and 109CFU/mL (Table 2). Zero of the eight mock-inoculated corneas showed visual opacity. In all, it was concluded that the appropriate concentration of inoculum should be higher than that of 108CFU/mL group, which could induce stable experimental keratomycosis in Wistar rats.
Table 1. Visual scoring system for rat's fungal keratitis.
| Score | 0 | 1 | 2 | 3 | 4 |
| Area of corneal opacity | No opacity | 1%-25% | 26%-50% | 51%-75% | 76%-100% |
| Density of corneal opacity | Clear cornea | Slight cloudiness, outline of pupil and iris discernable | Cloudy, but outline of pupil and iris visible faintly | Cloudy, opacity not uniform | Cloudy, opacity uniform |
| Shape of corneal ulcer | Normal cornea | Edema | Edema, corneal ulcer with indistinct shapes | Corneal ulcer with clear shapes or descemetocele | Corneal perforation |
Table 2. Inoculation dosage on the effect of visual opacity.
| Inoculation Dosage (CFU/mL) | Mock | 106 | 107 | 108 | 109 |
| Visual opacity (%) | 0 | 50 | 80 | 100 | 100 |
Visual examination of keratomycosis
After the corneal inoculation with varying doses of F. solani, the animals were visually evaluated under a slitlamp biomicroscope for corneal involvement. All mock-inoculated corneas developed mild irregularity on the surface and swelling after 1 day of inoculation, but regained normal appearance within 2 days and no deterioration was seen through the remainder of the study in these eyes.
Corneas infected with 106CFU/mL developed mild keratitis with a disease period of 6 to 8 days and did not develop a full corneal infection. Corneas infected with 107CFU/mL also had a disease course around 6 to 8 days and similar to the 106CFU/mL group, this group did not develop an active infection. Moderate keratomycosis was seen in the eyes infected with 108CFU/mL of F. solani. The disease process lasted around 14 to 16 days and developed similar to the clinical presentation, beginning with corneal infiltration, developing into corneal ulcers and followed by a period of corneal recovery resulting in corneal scar. Corneas infected with 109CFU/mL, had a disease course that lasted greater than 21 days, and had a significantly more severe disease compared to the 108CFU/mL group. In the 109CFU/mL group, 5 corneas were perforated and 3 demonstrated descemetocele.
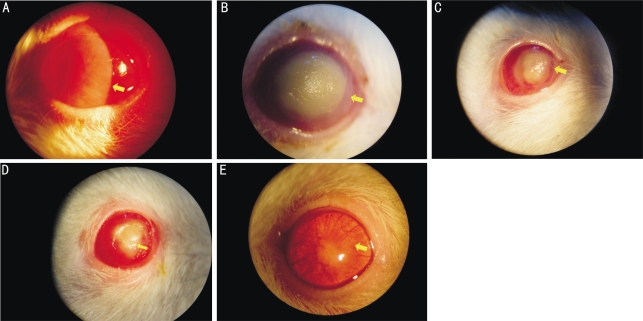
The disease course of F. solani-induced keratomycosis is depicted in Figures 1A to 1E. After 1 day of inoculation, a large amount of purulent secretion was seen at the palpebral margin and both conjunctival and ciliary hyperaemia was observed under a slitlamp biomicroscope. Corneal edema, corneal infiltration and grey nidus were also seen in the eyes (Figure 1A). After 3 days, the whole cornea developed a cloudy grey appearance and the nidus typically developed a yellow ulcer with compact texture and was swollen. The surface of the corneal ulcer was dry and rough with a moss-like appearance. The whole corneal margin was infiltrated with corneal neovascularization (CNV) (Figure 1B). After 6 days a dense corneal ulcer persisted, however, the area became smaller and the edges were covered by corneal epithelia. CNV developed near the edge of corneal ulcer (Figure 1C). After 10 days, the corneal epithelia were mostly restored, but the cornea itself remained opaque (Figure 1D). After 14 days, the corneal epithelium was reformed and although some slight swelling persisted. The outline of pupil and iris became visible within 14 days. CNV was visualized throughout the whole area of cornea (Figure 1E). No eye drops or drugs were used during healing process. The mock inoculated cornea was clear and in normal condition.
Figure 1. Clinical progression of F. solani-induced keratomycosis after inoculation with 108CFU/mL spore suspension of F. solani.
A: slitlamp photos showing corneal edema and infiltration after 1 day; B: yellow corneal ulcer with compact texture after 3 days; C: smaller corneal ulcer with epithelial recovery on the wound edge after 6 days; D: opaque cornea with more epithelial recovery after 10 days; E: complete corneal recovery after 14 days of the infection. Representative physical signs are shown as arrows pointed. The mock-inoculated corneas were normal after 3 days of infection
Pathological evaluation of keratomycosis
A, B, C and D groups were inoculated with F. solani at the concentration of 106, 107, 108 and 109CFU/mL individually. Visual scores (mean±SD) were evaluated based on the scoring system from Wu et al[4]. Statistical significance was tested by Student's t-test between different time points within each group (Tables 1 and 3). Depending on the visual scores, significant difference was tested between different time points (day 1 to day 14) within each group (Table 3), which indicate pathological progresses. In both group A and B, there was no significant score difference in the first six days of inoculation, indicating no significant pathological developments. In groups C and D, significant score difference was tested between day 1 and day 2 after inoculation, suggesting virulent pathological progresses. Continuously, visual score dropped dramatically from day 6 to day 14 after inoculation, showing that the recovery processes was undergoing in group C. In group D however, the recovery processes were delayed as compared to group C, which might be due to severe corneal perforations. The statistical data above further showed that the inoculation dose at the concentration of 108CFU/mL had apparent pathological progresses in this established rat model.
Table 3. Visual scores in different groups on different days after inoculation.
| Groups | Day 1 | Day 2 | Day 3 | Day 6 | Day 10 | Day 14 |
| Group A (106CFU/mL) | 5.88±0.83 | 6.50±0.44 | 6.00±0.93 | 5.00±1.071 | 3.38±0.52 | 2.75±0.46 |
| Group B (107CFU/mL) | 6.88±0.83 | 7.63±0.74 | 7.50±0.76 | 6.25±0.891 | 4.13±0.88 | 3.38±0.52 |
| Group C (108CFU/mL) | 7.75±0.711 | 9.12±0.60 | 8.50±0.53 | 8.00±0.531 | 7.00±0.371 | 5.50±1.31 |
| Group D (109CFU/mL) | 8.63±0.551 | 10.13±0.64 | 10.25±0.711 | 9.25±0.461 | 8.63±0.58 | 8.13±0.81 |
1indicates significant difference. CFU/mL=colony-forming unit per milliliter
Pathogenic examination
Pathogenic examinations were performed 1, 3 and 6 days after inoculation. No F. solani hyphae were found in mock-inoculated corneas at any of the time points. Positive F. solani hyphae in the necrotic corneal tissues were observed 1 and 3 days after inoculation, although no corneas were positive 6 days after inoculation. The isolated pathogens from corneal ulcer revealed that the corneas were infected with the original agent, F. solani, and the results of Gram staining were negative, which excluded the possibility of mixed infection or bacterial infection.
Histopathology
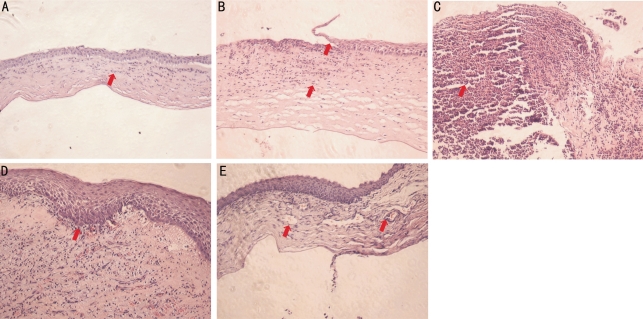
Mock-inoculated corneas did not show signs of infection, but did undergo some histological changes from the initial scratching procedure. There were several inflammatory cells infiltrated into the stroma 1 day after mock-inoculation (Figure 2A). HE stain of infected corneas in group C (108CFU/mL) presented an apparent inflammatory process, started with incomplete corneal epithelia coverage (Figure 2B) after 1 day of inoculation, and a large amount of inflammatory cells that infiltrated into the stroma after 3 days of inoculation. Additionally, the corneal stroma swelled and formed a prominent ulcer (Figure 2C). In the sections of 6 and 10 days after inoculation, the number of inflammatory cells decreased dramatically and the corneal epithelial started to recover to fill up the epithelial defect (Figures 2D and 2E).
Figure 2. The histopathology of experimental keratomycosis.
A: HE stainings showing corneal inflammatory infiltration after mock-inoculation; B: corneal inflammatory infiltration with incomplete epithelial coverage after 1 day; C: severe corneal inflammatory infiltration with corneal swelling after 3 days; D: decreased corneal inflammatory infiltration with epithelial recovery after 6 days; E: markedly reduced corneal inflammatory infiltration with neovascularization after 10 days of the infection. Representative pathological signs are shown as arrow(s) pointed. The mock-inoculated corneas were normal after 3 days of infection. All images were taken under 10 fold objective in bright field
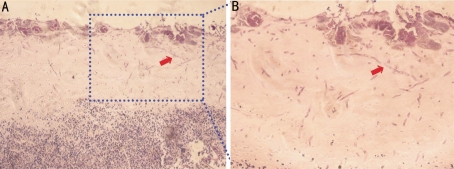
To visualize the infiltration pattern of F. solani in the experimental cornea, PAS staining was employed. The PAS staining were positive in the necrotic tissue in first three days after inoculation (Figure 3A). After this, the staining was negative, suggesting that the hyphae invaded the stroma in the early stage of inflammation but got cleared by inflammatory cells later on. The PAS staining clearly showed that the hyphae of F. solani infiltrated into the cornea in various levels of depth. In addition, the magnified images demonstrated the different shapes of the hyphae of F. solani presented in the corneal tissues (Figure 3B).
Figure 3. PAS staining shows the hyphae of F. solani, as arrow pointed after 3 days of infection.
A and B: taken under 10 and 20 fold objectives respectively in bright field
DISCUSSION
To establish an infectious animal model, several aspects needed to be thoroughly considered[5]: 1) the method of establishing the model should be similar to the infectious process in humans and should follow a similar clinical progression; 2) the method of creating the model should be standardized and repeatable; and 3) the extent of infectious disease should be controllable to allow for different disease analyses, including pathogenetic studies and drug treatment studies. Specifically, for the creation of a model for fungal keratitis, it should be emphasized that 1) the process of experimental corneal infection should mimic the clinical process of fungal keratitis; 2) the immune system of experimental animals should not be artificially affected; and 3) repeatable parameters to evaluate the severity of infection should be employed. Based on these criteria, we have evaluated the method described in this paper to four previously established models.
Direct method of intrastromal injection[6], is a simple way to establish interstitial keratitis. The disadvantage of this method is that the infection does not occur from the injection itself and the resultant infection may change or destroy the structure of stroma to some extent. Thus, this model showed dramatic changes in the corneal stroma in the early phase, which does not follow the slow initial development of the fungal infection as seen in the clinical scenario in the patients. Therefore, this method is not appropriate for basic science studies, including evaluation of the pathogenesis and immunologic mechanism of keratomycosis. However, this method may be beneficial for evaluating the effect of antifungal agents or to validating novel methods of clinical diagnosis.
The second method is similar to the before mentioned technique, although corneal buttons are used instead of contact lenses[7]. In this method, fungi are inoculated into the space created by the corneal button, which was solid and could generate a fixed space for pathogens adhesion. This button and the created space also provided a favorable environment for the growth of agents. Hence, this method is highly efficient, but the surgical technique is complicated therefore reducing the repeatability by other experimenters.
The final method, reported by Wu et al[4], is corneal scratching. This was a successful approach to establishing a mouse model of experimental fungal keratitis in which gentle scratching of the corneal surface generated primary injury, making it easier for fungi to attach. In the pre-experiment, we tried to incorporate this method into the rat model, but were unsuccessful because the corneal infection was mild and often was self-resolving.
Building on the results of the previous methods, we combined the contact lens and corneal scratching technique to establish a successful model of experimental keratomycosis. The clinical presentation of F. solani keratitis is always associated with corneal trauma. This trauma exposes the inner layer of corneal tissue to the pathogens, leading to the infection. This relationship suggests that adherence of pathogen to corneal tissues was the critical step of fungal infection. Accordingly, in this experiment, the corneal epithelia were firstly scraped away to generate a primary injury on the corneal surface. Secondly, slight nicks were created to increase the chance of fungal adherence to corneal tissues. The merits of this method were to increase the volume between contact lens and corneal tissues, while maintaining close contact between the pathogens and the host tissue. Additionally, contact lenses reducing tearing, which dilute and wash away the pathogens. The use of the contact lens also separated the pathogens from the physiological functions of immunoglobulin and different enzymes in tears, while ensuring a humid environment and warmer temperature, which are essential for growth of inoculated agents. Our data suggested that the combination of corneal scratching and contact lens use were efficient and successful. These methods are fairly simple to implement and are more repeatable than previous methods, we believe. In addition, the immune system of the animals is not augmented during the procedure. By using an appropriate inoculum does of F. solani (108CFU/mL), this rat model of experimental keratomycosis can be used as an appropriate platform for basic studies.
Previous histological examinations evaluated the infectious corneal tissues from human patients[8]. While human histological studies are optimal for clinical research, it is not possible to evaluate the histological changes that occur throughout the entire course of the infection in humans. The histological assessment included in the evaluation of this rat model revealed three less distinct stages of infection. The first stage occurs approximately 1 day after inoculation. It is characterized by corneal swelling and the irregularities on the corneal surface. The second stage occurs about 2 to 5 days after inoculation and is typified by corneal stromal necrosis and corneal ulcer formation. The final stage is usually from 6 to 14 days after inoculation and is characterized by gradual resolution of the corneal ulcer with concomitant CNV in the corneal tissues. Therefore, our histopathologic results supported the observations of clinical findings in the experimental model.
Results of pathogenic examining showed that PAS staining could detect the existence of F. solani hyphae from the treated corneal tissues 1 to 3 days after inoculation, but failed to detect any after 6 days of inoculation. This may explained by the fact that in the third stage (after 6 days of inoculation), the edge of corneal ulcer was healed and correspondingly it was difficult to sample the infectious tissue without corneal injury. Additionally, the immune response and inflammation reactions were activated when stimulated by pathogens, and therefore the pathogens were killed and partially removed.
In summary, the combination of corneal scratching and contact lens use, successfully established a rat model of F. solani keratitis. The experimental data supported the primary assumption that this combination method imitated human fungal keratitis, and is repeatable and easy to employ. Because this model follows several classical principles of human infection, this model will be useful for scientific studies in cases of keratomycosis.
Footnotes
Foundation item: Shanghai Science and Technology Commission (No. 08JC1419600)
REFERENCES
- 1.Xie L, Zhong W, Shi W, Sun S. Spectrum of fungal keratitis in north China. Ophthalmology. 2006;113(11):1943–1948. doi: 10.1016/j.ophtha.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 2.Chowdhary A, Singh K. Spectrum of fungal keratitis in North India. Cornea. 2005;24(1):8–15. doi: 10.1097/01.ico.0000126435.25751.20. [DOI] [PubMed] [Google Scholar]
- 3.Leck AK, Thomas PA, Hagan M, Kaliamurthy J, Ackuaku E, John M, Newman MJ, Codjoe FS, Opintan JA, Kalavathy CM, Essuman V, Jesudasan CA, Johnson GJ. Aetiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of fungal keratitis. Br J Ophthalmol. 2002;86(11):1211–1215. doi: 10.1136/bjo.86.11.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu TG, Keasler VV, Mitchell BM, Wilhelmus KR. Immunosuppression affects the severity of experimental Fusarium solani keratitis. J Infect Dis. 2004;190(1):192–198. doi: 10.1086/421300. [DOI] [PubMed] [Google Scholar]
- 5.O'Day DM, Head WS, Robinson RD, Yang R, Shetlar D, Wang MX. Contact lens-induced infection: a new model of Candida albicans keratitis. Invest Ophthalmol Vis Sci. 1999;40(7):1607–1611. [PubMed] [Google Scholar]
- 6.Avunduk AM, Beuerman RW, Varnell ED, Kaufman HE. Confocal microscopy of Aspergillus fumigatus keratitis. Br J Ophthalmol. 2003;87(4):409–410. doi: 10.1136/bjo.87.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong X, Shi W, Zeng Q, Xie L. Roles of adherence and matrix metalloproteinases in growth patterns of fungal pathogens in cornea. Curr Eye Res. 2005;30(8):613–620. doi: 10.1080/02713680590968196. [DOI] [PubMed] [Google Scholar]
- 8.Vemuganti GK, Garg P, Gopinathan U, Naduvilath TJ, John RK, Buddi R, Rao GN. Evaluation of agent and host factors in progression of mycotic keratitis: a histologic and microbiologic study of 167 corneal buttons. Ophthalmology. 2002;109(8):1538–1546. doi: 10.1016/s0161-6420(02)01088-6. [DOI] [PubMed] [Google Scholar]