Abstract
AIM
To investigate the effect of protein kinase C (PKC) on transforming growth factor-β2 (TGFβ2) and dopamine in retinal Müller cells of guinea pig myopic eye.
METHODS
Myopia was induced by translucent goggles in guinea pig, whose retinal Müller cells were cultured using the enzyme-digesting method. Retinal Müller cells were divided into 5 groups: normal control, myopia, myopia plus GF109203X, myopia plus PMA, myopia plus DMSO. PKC activities were detected by the non-radioactive methods. TGFβ2 and tyrosine hydroxylase (TH) proteins were analyzed by Western Blotting in retinal Müller cells. Dopamine was determined by the high-performance liquid chromatography-electrochemical detection in suspensions.
RESULTS
After 14 days deprived, the occluded eyes became myopic with ocular axle elongating. Müller cells of guinea pigs were obtained using enzyme digestion. Compared with normal control group, the increase in PKC activity and the up-regulation in TGFβ2 expression were found in retinal Müller cells of myopic eyes, with the decrease of TH and dopamine content (P<0.05). After PKC activated by PMA, TGFβ2 and TH content were up-regulated with the increase of dopamine content (P<0.05). While the PKC activities was inhibited by GF109203X, proteins of TGFβ2 and TH were down-regulated in the myopic eyes, with the decrease of dopamine content (P<0.05).
CONCLUSION
TGFβ2 and dopamine are modulated by PKC in Müller cells of the myopic eyes in guinea pig.
Keywords: protein kinase C, retinal Müller cell, transforming growth factor-β2, dopamine, myopia
INTRODUCTION
It has been demonstrated that the formation and development of myopia result from active remodeling of sclera under the regulation of retina[1]. Transforming growth factor-β2 (TGFβ2)[2] and dopamine[3] are retinal signal factors, which participate in regulating the development of myopic eyes. Müller cells are the primary glial cells in the retina, which constitute regulatory network “neuron-glia” together with retinal neurons, and participate in retinal physiological and pathological processes. It is reported that protein kinase C (PKC) plays an important role in Müller cells signaling in the retina[4]. In the present study, the expressions of TGFβ2, dopamine and tyrosine hydroxylase (TH) were studied in Müller cells isolated from myopic guinea pig eyes induced by form deprivation and primaryly cultured Müller cells of retina. In addition, these indexes were intervened by the activator (PMA) and inhibitor (GF109203X) of PKC, and then to analyze whether PKC has regulatory role on these myopic signaling factors.
MATERIALS AND METHODS
Materials
Healthy triad color guinea pigs (clean grade, n=35, aged 3 weeks, weighed 180-220g) are supplied by Experimental Animal Center of Xiangya Medical College of Central South University. Twenty-five guinea pigs are chosen to establish myopic eye model. The contralateral uncovered eye is considered as self control, and the remnant guinea pigs (n=10) are regarded as normal control. After the eye is covered for 14 days, 0.5g/L tropicamide is used to dilate the pupil so as to examine test eye refraction. Axial length is measured by A scan for 3 times, and the average value is obtained. After 14 days of deprivation, the axial length of deprived eye in the guinea pig prolongs, and myopia is formed. There was a significant difference in the refraction between the deprived eye (-3.21±0.42D) and its control eye (P<0.05). In addition, There was a significant difference in the axial length between deprived eye (8.37±0.14mm) and its control eye (P<0.05, Table 1).
Table 1. Change of refraction and axial length in guinea pigs.
| Groups | n | Refraction (D) | Axial length (mm) |
| Normal control | 10 | +0.45±0.20a | 8.13±0.10a |
| Self control | 25 | +0.62±0.18a | 8.12±0.08a |
| Deprivation | 25 | -3.21±0.42 | 8.37±0.14 |
aP<0.05 vs deprived eye
(mean±SD)
Primary culture of retinal Müller cells refer to reference[5]. The eyeballs of guinea pigs were enucleated, and the anterior segments removed. Then, the neural retinas were isolated and placed into an enzymatic solution containing 0.25% trypsin in Dulbecco's modified eagle medium (DMEM) for 10 minutes at 37°C under 5% CO2. Retinal samples were cut into pieces by microscopic ophthalmic scissors, beaten upon through pipettes, and then made into cell suspensions. The isolated cells were then maintained in DMEM containing 20% fetal bovine serum and 0.1% penicillin/streptomycin at 37°C under 5% CO2. After 24 hours, the cultural medium was changed for the first time, and the suspension cells were washed out. And then the medium was changed every 3 or 4 days. The cultured cells were passaged, when the density of adherent cell reached 3×105cm−2. After passage, the cell density was 5×104cm−2. Cells were identified by immunocytochemical analysis with antibodies against the Müller cell markers vimentin and glial fibrillary acidic protein (Santa Cruz, CA, USA).
Methods
PMA and GF109203X (Merck, Germany) are dissolved in dimethyl sulphoxide (DMSO), and added into the cultured second generation of retinal Müller cells for 3 hours. The concentration of PMA and GF109203X is 100nmol/L and 1μmol/L, separately. Müller cells are divided into 5 groups: normal control, myopia, myopia+GF109203X, myopia+PMA and myopia+DMSO.
Operating procedure is based on the instruction of non-radioactive PKC activity assay kit (Promega). Müller cells are scraped, and homogenated by 0.5mL pre-cooling PKC extract. Sample is centrifugated for 5 minutes with 14000r/min at 4°C, and the supernate fluid is preserved. And then, the fluid is added into 1mL DEAE cellulose column after pre-equilibration of PKC extract. After the column is washed, the composition containing PKC is eluted by 5mL PKC extract containing 200mmol/L NaCl. After the samples to be tested are mixed with PepTag® PKC reaction buffer, PepTag® C1 peptide (PKC specific phosphorylation substrate), combined PKC activation solution and peptide protection fluid, the reaction is terminated after incubation for 30 minutes, and then the sample is kept in dark place at 4°C. Deionized water replacing the sample is regarded as the negative control and the group without adding PepTag® C1 peptide is considered as the blank control. Preparation of 8g/L agarose gel, 10μL sample is applied each hole, 100V electrophoresis for 15 minutes (phosphorylated C1 peptide moves to positive pole, while non-phosphorylated C1 peptide moves to negative pole), and then the sample is observed under viltalight lamp. The phosphorylated strap is cut; collosol is heated at 95°C, and then transferred into 96-well microtiter plates. The final volume is 250μL. Shade selection at 570nm, and then optical absorption value is detected. PKC activity of every sample is caculated.
The specific methods of TGF Β2 and TH detection refer to reference[7]. The first antibody is rabbit anti-TGFβ2 polyclonal antibody and rabbit anti-TH polyclonal antibody (Santa Cruz, U.S.A), working concentration 1:500. Target strap is performed grey value analysis by Bandscan 5.0 image analysis software, GAPDH as the internal control, the relative expression of target protein is calculated. Dopamine content detected By HPLC[4]. Dopamine content in Müller cell culture fluid is expressed with µg/L.
Ststistical Analysis
All the data was expressed as mean±SD, and analyzed with SPSS11.0 software. One-way ANOVA was used to analyze eye refraction, axial length, proteins of TGFβ2 and TH, PKC activity, and dopamine content in the guinea pig.
RESULTS
PKC activity in Müller cells
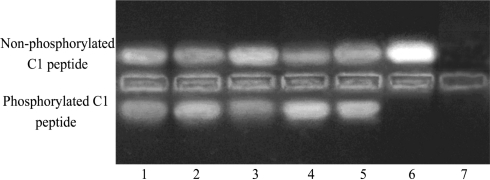
PKC activity of Müller cells was significantly increased in myopia group compared to that of normal control group (P<0.05). After PMA was added, PKC activity of Müller cells in myopic eye was further significantly elevated compared to that of myopia group (P<0.05). After GF109203X was added, PKC activity of Müller cells wa significantly decreased compared to that of myopia group (P<0.05). DMSO had no effects on PKC activity, and there was no difference compared to myopia group (P>0.05, Figure 1, Table 2).
Figure 1. PKC activity in Müller cells.
1: Normal control; 2: Myopia; 3: Myopia+GF109203X; 4: Myopia+PMA; 5: Myopia+DMSO; 6: Negative control; 7: Blank control
Table 2. PKC activity, TGFβ2, TH and dopamine in retinal Müller cells.
| Groups | PKC activity(units/mL) | TGFβ2 | TH | Dopamine (ng/mL) |
| Normal control | 0.14±0.05 | 0.15±0.03 | 0.60±0.12 | 26.6±5.8 |
| Myopia | 0.30±0.05a | 0.46±0.07a | 0.23±0.05a | 15.2±3.3a |
| Myopia + GF109203X | 0.08±0.03a,c | 0.11±0.02a,c | 0.11±0.04a,c | 9.7±3.1a,c |
| Myopia + PMA | 0.68±0.08a,c | 0.71±0.11a,c | 0.89±0.16a,c | 37.8±5.6a,c |
| Myopia + DMSO | 0.32±0.04a | 0.47±0.05a | 0.21±0.06a | 15.0±3.3a |
aP<0.05 vs normal control, cP<0.05 vs myopia, and vs myopia + DMSO
mean±SD
TGF β2, TH and dopamine in Müller cells
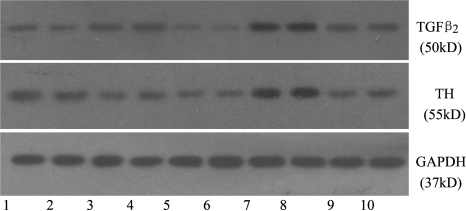
TGFβ2 and TH were expressed in Müller cells of normal control group as well as myopia group. TGFβ2 was up-regulated, while the expression of TH showed down-regulation in myopia group, which was significantly different from that of control group (P<0.05). Dopamine content secreting from Müller cells in the myopia group had significant different compared to that of normal control group (P<0.05, Figure 2, Table 2). After GF109203 was added, the expressions of TGFβ2 and TH showed down-regulation in Müller cells, and dopamine content was decreased, which were significantly different from that of myopia group (P<0.05). After PMA was added, the expressions of TGFβ2 and TH showed up-regulation in Müller cells, and dopamine content was increased (P<0.05). DMSO did not affect the expressions of TGFβ2 and TH and dopamine content (Table 2).
Figure 2. TGFβ2 and TH proteins in Müller cells (Westren blotting).
1,2: Normal control; 3,4: Myopia; 5,6: Myopia+GF109203X; 7,8: Myopia+PMA; 9,10: Myopia+DMSO
DISCUSSION
From the study on myopia using animal models, it is found that TGFβ2[2] and dopamine[3] participate in the development of form deprivation myopia. Seko et al[2] has reported that the content of TGFβ2 is increased in the posterior pole of retina-retinal pigment epithelium-choroid complex and sclera of the deprived eyes in chicken, suggesting that TGFβ2 participate in the development of myopia. However, TGFβ mRNA and protein are reduced in retina of the deprived eyes in chicken. The reduced expression of TGFβ protein mainly appears in the cellular layer of photoceptor. In addition, Jobling et al[6] consider tree shrew as the experimental animal and investigate the changes of three isomers of TGFβ in retina in the development of myopia. The results show that over 95% of the total TGFβ content in the guinea pig is TGFβ2[9]. Form deprivation in the early stage has no significant effect on TGFβ2 content in retina, and TGFβ2 content begins to decrease on day five[6].
Dopamine is one of the neurotransmitters in retina. Both form deprivation and lens-induced defocus induce dopamine content decreased in retina. Intravitreal injection of dopamine[7] and its agonist apomorphine has significant inhibitory action on the occurrence of myopia, suggesting that retinal dopamine participate in the formation of myopia. It needs to further study whether TGFβ2 and dopamine is involved in the occurrence of myopia and which retinal cells can be regulated by form deprivation to secret TGFβ2 and dopamine.
In vivo and in vitro researches demonstrated that TGFβ2[8] and TH[9],[10] protein are expressed in Müller cells of retina. In our study, it shows that cultured Müller cells of retina express TGFβ2, TH protein and secrete dopamine. The synthetic TGFβ2 protein is increased, while TH and dopamine content is decreased in myopic eye, suggesting that Müller cells of retina can synthesize and secrete TGFβ2 and dopamine in the guinea pig. In addition, the contents of TGFβ2 and dopamine are reduced in Müller cells during establishment of form deprivation myopia. The research by Jobling et al[6] shows that dynamic changes of TGFβ2 content exist in the retina of tree shrew, and the total content of TGFβ2 is reduced in the retina on day 5 after form deprivation. However, in our study we focus on the TGFβ2 content in retinal Müller cells. Our result indicates that the following possibilities exist: first of all, in addition to Müller cells, other retinal cells also can synthesize TGFβ2, furthermore, TGFβ2 content synthesized by these cells are reduced during myopia occurs, leading to the total content of TGFβ2 is reduced in the myopia retina; Second, during the formation of FDM, time dynamic changes of TGFβ2 exist in retina; Third, species differences may exist. In our study, variation tendencies of TH and dopamine in Müller cells of myopia is in accordance with that of retinal dopamine content in the guinea pig, suggesting that dopamine synthesized by Müller cells participate the occurrence of form deprivation myopia.
It has been demonstrated that PKC participates in regulation of TGFβ2[11] and TH[12] expression. Besides, PKC signal conduction pathway exists in Müller cells of retina[5],[6]. In our study, PKC activity in Müller cells of retina is detected by non-radioactivity method in the guinea pig. The results show that PKC activity in Müller cells of retina is increased in myopia. The expressions of TGFβ2 and TH up-regulate after PMA further activate PKC activity, and secretion of dopamine is increased. However, the expressions of TGFβ2 and TH down-regulate after GF109203X inhibits PKC activity, and secretion of dopamine is decreased. Therefore, synthesis and secretion of TGFβ2 and dopamine is regulated by PKC signal pathway in Müller cells of myopia in the guinea pig. PKC activity and TGFβ2 covary in Müller cells of myopia in the guinea pig, indicating that TGFβ2 in Müller cells may be regulated mainly by PKC signal pathway. However, variation tendency of PKC activity is opposite to that of TH and dopamine, suggesting that synthesis and secretion of dopamine in Müller cells may involve other signal pathways.
In brief, Müller cells may participate in the formation of myopia in the guinea pig, and it is a significant source for signaling factors of TGFβ2 and dopamine in the retina. And the synthesis of TGFβ2 and dopamine are regulated by PKC signal pathway.
Footnotes
Foundation item: National Natural Science Foundation of China (No. 30600694).
REFERENCES
- 1.Jostrup R, Shen W, Burrows JT. Identification of myopia-related marker proteins in tilapia retinal, RPE, and choroidal tissue following induced form deprivation. Curr Eye Res. 2009;34(11):966–975. doi: 10.3109/02713680903244138. [DOI] [PubMed] [Google Scholar]
- 2.Mathis U, Schaeffel F. Transforming growth factor-beta in the chicken fundal layers: an immunohistochemical study. Exp Eye Res. 2010;90(6):780–790. doi: 10.1016/j.exer.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Junfeng M, Shuangzhen L, Wenjuan Q, Fengyun L, Xiaoying W, Qian T. Levodopa inhibits the development of form-deprivation myopia in guinea pigs. Optom Vis Sci. 2010;87(1):53–60. doi: 10.1097/OPX.0b013e3181c12b3d. [DOI] [PubMed] [Google Scholar]
- 4.Swiatek-De Lange M, Stampfl A, Hauck SM, Zischka H, Gloeckner CJ, Deeg CA, Ueffing M. Membrane-initiated effects of progesterone on calcium dependent signaling and activation of VEGF gene expression in retinal glial cells. Glia. 2007;55(10):1061–1073. doi: 10.1002/glia.20523. [DOI] [PubMed] [Google Scholar]
- 5.Mao JF, Liu SZ, Qin WJ. Expression of TGFβ2, VIP and DA in retinal Müller cells of guinea pig myopic eye. Yanke Yanjiu. 2008;26(11):801–804. [Google Scholar]
- 6.Jobling AI, Wan R, Gentle A. Retinal and choroidal TGF-beta in the tree shrew model of myopia: isoform expression, activation and effects on function. Exp Eye Res. 2009;88(3):458–466. doi: 10.1016/j.exer.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Gao Q, Liu Q, Ma P. Effects of direct intravitreal dopamine injections on the development of lid-suture induced myopia in rabbits. Graefes Arch Clin Exp Opnthalmol. 2006;244(10):1329–1335. doi: 10.1007/s00417-006-0254-1. [DOI] [PubMed] [Google Scholar]
- 8.Gomes dos Santos AL, Bochot A, Tsapis N. Oligonucleotide-polyethylenimine complexs targeting retinal cells: structural analysis and application to anti-TGFbeta-2 therapy. Pharm Res. 2006;23(4):770–781. doi: 10.1007/s11095-006-9748-0. [DOI] [PubMed] [Google Scholar]
- 9.Kubrusly RC, da Cunha MC, Reis RA. Experssion of functional receptors and transmitter enzymes in cultured Müller cells. Brain Res. 2005;1038(2):141–149. doi: 10.1016/j.brainres.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 10.da Silva RT, Hokoç JN, de Mello FG. Differential immunodetection of L-DOPA decarboxylase and tyrosine hydroxylase in the vertebrate retina. Int J Dev Neurosci. 2009;27(5):469–476. doi: 10.1016/j.ijdevneu.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Moreno-Alvarez P, Sosa-Garrocho M, Briones-Orta MA. Angiotensin II increases mRNA levels of all TGF-beta isoforms in quiescent and activated rat hepatic stellate cells. Cell Biol Int. 2010;34(10):969–978. doi: 10.1042/CBI20090074. [DOI] [PubMed] [Google Scholar]
- 12.Zhang D, Kanthasamy A, Yang Y. Protein kinase C delta negatively regulates tyrosine hydroxylase activity and dopamine synthesis by enhancing protein phosphatase-2A activity in dopaminergic neurons. J Neurosci. 2007;27(20):5349–5362. doi: 10.1523/JNEUROSCI.4107-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]