Abstract
AIM
To detect and compare the levels of matrix metalloproteinases (MMPs) secreted by primary and recurrent human pterygium fibroblasts (HPFs).
METHODS
Primary and recurrent HPFs as well as human conjunctival fibroblasts (HCF) were cultured in RPMI 1640 medium at the same conditions. The protein levels of MMP-1, MMP-3 and MMP-9 were determined by enzyme-linked immune sorbent assay (ELISA), respectively.
RESULTS
1) The protein level of MMP-1 in serum-free supernatant from cultured primary and recurrent HPFs was higher than that in normal HCFs (P<0.05); similarly, the protein level of MMP-1 in serum-free supernatant from cultured primary HPFs was higher than that in recurrent HCFs (P<0.05). 2) The protein level of MMP-3 in serum-free supernatant from cultured primary HPFs was higher than that in normal HCFs (P<0.05); meanwhile, the protein level of MMP-3 in serum-free supernatant from cultured recurrent HPFs was lower when compared with that in primary HPFs and normal HCFs (P<0.05). 3) MMP-9 was not detected in primary and recurrent HPFs in the conditioned medium.
CONCLUSION
The protein levels of MMP-1 and MMP-3 in supernatant secreted by primary HPFs are different from recurrent HPFs. Different pathological mechanisms may exist between primary and recurrent pterygia.
Keywords: primary and recurrent pterygia, matrix metalloproteinase
INTRODUCTION
The zinc-dependent matrix metalloproteinases (MMPs) are a family of enzymes that can modify or degrade extracellular matrix (ECM)[1], mainly degrade collagen. MMPs play an important role in the process of physiological and pathological angiogenesis, invasion and metastasis of tumors as well as many eye diseases. In the process of cell abnormal proliferation and angiogenesis generation in primary and recurrent pterygia, modification or degradation of extracellular matrix may be related to MMPs. Previous reports have demonstrated that the overexpression of MMPs in fibroblasts from primary pterygium head[2],[3] may be the primary cause of pterygium progression and degradation of Bowman's layer.
Currently, fewer investigations on recurrent pterygium have been conducted. The pathological mechanisms of recurrent pterygium are still unclear. Especially, the studies on MMPs in recurrent pterygium are less reported at home and abroad, or just restricted to immunohistochemical studies. Clinical observation has also revealed that recurrent pterygium has more obvious proliferation of fibrous tissue and more serious tissue adhesion. Explanation for the recurrence of pterygium after the removal of primary pterygium now is accelerated proliferation of residual transformed fibroblasts and attack again to cornea. However, which situation is benefit for the secretion of MMPs in recurrent HPFs and whether MMP secretion level in recurrent HPFs is higher than that in primary HPFs are still unclear. Due to the abnormal fibroblast growth as one of the major components in pterygium, we have explored the protein level of MMPs secreted by human fibroblasts from primary and recurrent pterygia, which will provide a basis for further understanding the pathogenesis of primary and recurrent human pterygia.
MATERIALS AND METHODS
Pterygium Specimen
Primary and recurrent pterygium specimens were harvested from patients who were received ocular surgery. The mean age of these patients was 54.52 ± 11.30 years old (range from 40 to 68 years). The progress of primary pterygium was from 5 to 20 years and the recurrent pterygium was more than 1 year. No special preoperative drug history was offered to these patients. Normal conjunctival tissues without eye diseases were obtained from the donated eyes of corneal transplant in the eye bank of hospital. All of these donators have matched age with our enrolled patients.
Primary culture of fibroblasts in vitro
The eyeball should be sterilized when the tissue specimen of normal conjunctiva was obtained. The conjunctival epithelium was cleaned up and washed to remove the blood by using 0.01 M PBS. The tissue specimen was cut into small pieces. Approximately 5 times volume of 1:3 dilution of 0.25% trypsin (Shanghai Bioengineering Co., China) was added to the specimen species for the digestion for 8 minutes at room temperature. Then, RPMI 1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS) was added to terminate the digestion. The tissue pieces and solution were centrifuged at 1000r/min for 8 minutes and the supernatant was removed. The precipitate of tissue explants and cells were seeded into a culture flask with a well-distributed distance of 5mm, and then turn over the flask. Defined amount of 10% FBS medium was added the culture flask and the culture flask was placed in an incubator supplemented with 5% CO2 at 37°C for 24 hours until the tissue explants were attached. The culture flasks were turned over and continue to culture in RPMI 1640 medium composed of 10% FBS, 100U/mL penicillin and 100U/mL streptomycin. After the incubation of primary culture for five days, the medium was changed every 3 days. The innoculation of tissue explants and the medium condition was observed by an inverted phase contrast microscope (ZEIZSS Axiovert200).
Subculture of fibroblasts in vitro
When the primary cells were cultured up to 80% confluence, 0.25% trypsin was added to digest the cells. The retracted cytoplasm and increased intercellular gap of these cells were observed under the inverted phase contrast microscope. Then 10% FBS was added to terminate the digestion immediately, and then detached the adherent cells from the wall of the culture flasks. The cell suspension was centrifuged at 1000r/min for 10 minutes and the supernatant was removed. RPMI 1640 medium containing 10% FBS was added to suspend the cells with 1:3 dilution for the cell passage.
Purification of cultured fibroblasts
According to epithelial cells and fibroblasts with different tolerance to trypsin, fibroblasts were usually strip off the wall first, while epithelial cells need the longer time for detaching the wall of the culture flask. Under the microscope after adding 0.25% trypsin, when the retracted cytoplasm of fibroblasts and increased intercellular gap were observed, the cell digestion was terminated and the growth area of fibroblasts was pipetted slightly to separate the fibroblasts from epithelial cells. In addition, due to the fast proliferation of fibroblasts, the fibroblasts can be successfully purified during the second generation cell culture.
Identification of fibroblasts
Light microscope: The characteristics and morphology of these cells growing at different stages were observed, photographed and recorded by an inverted phase contrast microscope. The streptavidin-biotin peroxidose complex (SABC) (Wuhan Boster Biological Engineering Co.) was used for the detection of antigen vimentin and cell identification according to the kit instructions.
Collection and preservation of cell culture supernatant
The third passage of normal conjunctival, primary and recurrent pterygium fibroblasts were suspended in RPMI 1640 medium enriched with 10% FBS. Fibroblasts were seeded in 24-well plates at a density of 3×10[5]cells/mL. Each well was filled with 1mL of medium containing cells. After incubated for 24 hours, the original medium was discarded. Serum-free RPMI 1640 medium with reduced color was added into these fibroblasts and each well was 1mL. Each group (primary and recurrent HPFs as well as HCFs) was set up for 3 holes. The cells were cultured in an incubator supplemented with 5% CO2 at 37°C. After incubated for 48 hours, the supernatant was harvested through centrifugation at 3500r/min for 15 minutes. The supernatant was stored at -20°C.
MMP-1, MMP-3, MMP-9 in the supernatant from cultured primary and reccurrent pterygium and normal conjunctival fibroblasts were assayed by ELISA according to the kit instructions (Wuhan Boster Biological Engineering Co.). 1) According to the experimental data for the estimated content of the sample factor, the appropriate diluted times (1:2 or 1:10 diluted with provided sample diluent) were determined. Make concentration of factors to be detected in the sample after diluted in the best detection range ELISA kit required; 2) Totally 0.1mL of the sample and different concentration standard products for each were added to the holes in the enzyme panels. The reaction temperature and time were controlled according to the kit instructions; 3) 0.1mL of each antibody against human biotin was added to the hole; 4) 0.1mL of affinity meat-peroxidase compound was added to the hole; 5) 0.1mL of color development liquid was added to the hole at the light-free condition; 6) 0.1mL of color termination solution was added to the hole, and then the color turned into yellow from blue. The optical density (OD) at 450nm was determined by a photospectrometer.
Statistical Analysis
SAS 8.1 software was used for statistical analysis and the results are expressed as mean±SD. The mean among multiple groups was compared by One-Way ANOVA analysis, and then two groups were compared by LSD-t tests to calculate the P value. A significant difference was considered at P value less than 0.05.
RESULTS
Feature and Identification of Cultured Fibroblasts in Vitro by Microscope
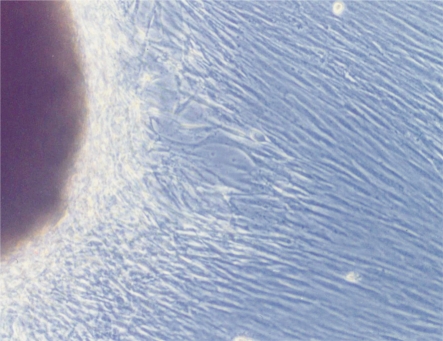
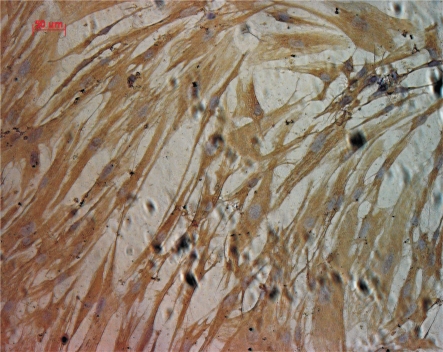
After tissue implantation for 5 days, a few cells grew out from tissue edge in a long spindle or triangle shape. As the cell culture time was gradually increased, the cells were predominant in long spindle shape. Meanwhile, the cells revealed a vortex form, radial arrangement pattern and unclear edge when the cells were 100% confluence, as shown in Figure 1. Immunohistochemistry staining analysis revealed brown cytoplasm and positive expression of vimentin in acetone-fixed cells, as shown in Figure 2. Based on these characteristics, these cells could be identified as fibroblasts.
Figure 1. Fibroblasts grew out from tissue edge (×200).
Figure 2. Immunohistochemistry examination of vimentin. The positive expression of vimention revealed by brown cytoplasm (×200).
Protein Levels of MMPs in Supernatant from Cultured Fibroblasts in Vitro
Protein level of MMP-1
The protein level of MMP-1 in serum-free supernatant from cultured primary and recurrent HPFs was higher than that in normal HCFs (P<0.05). Similarly, the protein level of MMP-1 in serum-free medium of cultured primary HPFs was higher than that in recurrent HCFs (P<0.05, Table 1).
Table 1. Protein level of MMP-1 in serum-free supernatant from cultured cells (pg/mL).
| Groups | Protein level of MMP-1 |
| Group A: normal HCFs | 3147.77 ± 74.00 |
| Group B: primary HPFs | 26927.77 ± 1134.82a |
| Group C: recurrent HPFs | 4945.70 ± 131.53a,c |
F=1203.9, P<0.001; aP< 0.05 vs group A; cP<0.05 vs group B
(n=3, mean±SD)
Protein level of MMP-3
The protein level of MMP-3 in serum-free supernatant from cultured primary HPFs was higher than that in normal HCFs (P<0.05). However, the protein level of MMP-3 did not exhibit an increase in serum-free supernatant from cultured recurrent HPFs when compared with primary HPFs and normal HCFs (P<0.05, Table 2).
Table 2. Protein level of MMP-3 in serum-free supernatant from cultured cells (pg/mL).
| Groups | Protein level of MMP-3 |
| Group A: normal HCFs | 12489.53 ± 3125.96 |
| Group B: primary HPFs | 52593.47 ± 1031.03a |
| Group C: recurrent HPFs | 4032.33 ± 1037.99a,c |
F=508.38, P<0.001; aP< 0.05 vs group A; cP<0.05 vs group B
(n=3, mean±SD)
Protein level of MMP-9
MMP-9 protein was not detected in primary and recurrent HPFs and HCFs in conditioned medium.
DISCUSSION
Previous studies have demonstrated a layer of fibroblasts in the head end of pterygium tissue can penetrate to the cornea between Bowman's layer and epithelial basement membrane, and then form a gray area, which will be helpful for further penetration of pterygium head to the cornea. Correspondingly, the elastic layer debris is pushed into the rear end, and the head of the pterygium will adhere with the matrix in shallow layer, which results in further development of pterygium due to the invasion and penetration of conjunctival fibroblasts to the cornea. Previous reports have demonstrated that primary pterygium has high correlation with the expression of MMPs. The overexpression of MMPs in fibroblasts from pterygium may be the primary cause of degradation of Bowman's layer and pterygium progression.
In the present study, the protein level of MMP-1 in serum-free supernatant from cultured primary and recurrent HPFs was higher than that in normal HCFs (P<0.05), which was consistent with the previous report that human pterygium tissues exhibit an enhanced MMP-1 expression detected by immunohistochemical analysis[4]. These results have attracted our extensive interests to explore the important role of MMP-1 in the pathogenesis of pterygium. Meanwhile, the protein level of MMP-1 in serum-free supernatant from cultured primary HPFs was also higher than that in recurrent HCFs (P<0.05). Thus, MMP-1 may be not mainly derived from the recurrent HPFs or does not play an important role in the recurrence of pterygium due to the removal of primary pterygium. Nowadays, the comparison of the expression level of MMP-1 in fibroblasts from primary and recurrent pterygia has not been reported.
Similarly, the protein level of MMP-3 in serum-free supernatant from cultured primary HPFs was higher than that in normal HCFs (P<0.05); but the protein level of MMP-3 did not exhibit a higher level in serum-free supernatant from cultured recurrent HPFs when compared with that in normal HCFs. Correspondingly, MMP-3 may be not mainly derived from recurrent HPFs either or is not the important factor for the recurrence of pterygium after the removal of primary pterygium.
Bowman's layer and stromal areas of cornea primarily consisting of collagen I are the targets of MMP-1. The pterygium cells invading over Bowman's layer produce elevated MMP-1 expression, which probably is the main MMP-1 responsible for the dissolution of Bowman's layer. Pterygium cells may also cause activation of fibroblasts at the head of the pterygium, leading to the initial cleavage of fibrillar collagen in Bowman's layer by the production of MMP-1 deriving from fibroblasts and further invasion of pterygium to cornea[4]. MMP-3 can degrade stroma. These results are well agreement with clinical pterygium-induced lesion in cornea. Enhanced protein levels of MMPs in pterygium may be related to transformed fibroblasts phenotype from pterygial tissues under the UV irradiation. In addition, it may be also associated with the secretion and activation of cytokines from pterygial tissues.
MMP-9 protein was not detected in primary and recurrent HPFs and HCFs in conditioned medium. Similar results are also reported in pterygium tissue by Schellini SA by immunohistochemistry[5]. Further studies are needed to explore mechanisms of its secretion and synthesis.
IL-1, IL-6 and IL-8 reveal an obvious increase when stromal cells in comea or whole cornea were exposed to UV. This phenomenon has also demonstrated in epithelial cells of pterygium[6]. In the presence of inflammatory cell factor such as IL-1β in cultured primary pterygium fibroblasts, the mRNA and protein expression levels of MMP-1 and MMP-3 in primary pterygium fibroblasts can significantly increase when compared with cultured human conjunctival fibroblasts[1]. Moreover, IL-6 and IL-8 can reveal a similar induction effect on the expression of MMPs. HPFs may secrete increased MMPs through above partial mechanisms.
Production of pterygium involves in factors such as transformed fibroblasts phenotype, the zinc-dependent matrix metalloproteinases (MMPs), allergic inflammation, imbalance in regulation in apoptosis and oxidative damage and so on. Many cytokines have important role in the process of formation of pterygium.
In conclusion, the levels of MMP-1 and MMP-3 in supernatant secreted by primary HPFs exhibit a different pattern from recurrent HPFs. Therefore, different pathogenesis may exist between primary and recurrent pterygia.
Footnotes
Foundation item: National Nature Science Foundation of China (No. 30371513); Science and Technology Project Foundation of Guangzhou City (No. 2007J1-C0101)
REFERENCES
- 1.Solomon A, Li DQ, Lee SB, Tseng SC. Regulation of collagenase, stromelysin, and urokinase-type plasminogen activator in primary pterygium body fibroblasts by inflammatory cytokines. Invest Ophthalmol Vis Sci. 2000;41(8):2154–2163. [PubMed] [Google Scholar]
- 2.Di Girolamo N, Wakefield D, Coroneo MT. Differential expression of matrix metalloproteinases and their tissue inhibitors at the advancing pterygium head. Invest Ophthalmol Vis Sci. 2000;41(13):4142–4149. [PubMed] [Google Scholar]
- 3.An MX, Wu KL, Lin SC, Hu SX. Inhibitory effect of tetrandrine on matrix metalloproteinases in pterygium fibroblasts in culture. Guoji Yanke Zazhi. 2007;7(4):938–940. [Google Scholar]
- 4.Dushku N, John MK, Schultz GS, Reid TW. Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol. 2001;119(5):695–706. doi: 10.1001/archopht.119.5.695. [DOI] [PubMed] [Google Scholar]
- 5.Schellini SA, Hoyama E, Oliveira DE, Bacchi CE, Padovani CR. Matrix metalloproteinase-9 expression in pterygium. Arq Bras Oftalmol. 2006;69(2):161–164. doi: 10.1590/s0004-27492006000200005. [DOI] [PubMed] [Google Scholar]
- 6.Di Girolamo N, Kumar RK, Coroneo MT, Wakefield D. UVB-mediated induction of interleukin-6 and -8 in pterygia and cultured human pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2002;43(11):3430–3437. [PubMed] [Google Scholar]