Abstract
Retinal diseases are featured with the common result of retinal cell apoptosis that will cause irreversible vision loss. Various attempts have been made for the solution against cell death. However, few approaches turn out to be effective. With the progress in mesenchymal stem cells (MSCs) research, MSCs were considered as a promising source for cell replacement or neuroprotection in retinal disorders. MSCs have the property of self-renewal and are multipotent cells derived from various mesenchymal tissues, which were demonstrated being capable of differentiating into multilineage tissue cells. Some works were also done to differentiate MSCs into retinal cells. MSCs could be induced to express retinal cell markers under certain stimuli. Recent studies also suggest that MSCs should be an ideal source for neuroprotection via the secretion of a variety of neurotrophins. Engineered MSCs were also used as vehicles for continuous delivery of neurotrophins against retinal degeneration with encouraging results. Since there are still barriers on the differentiation of MSCs into functional retinal cells, the use of MSCs for neuroprotection in retinal diseases seems to be a more practicable approach and worthy of further investigations.
Keywords: mesenchymal stem cells, differentiation, neurotrophin, retina
INTRODUCTION
Age-related macular degeneration, retinitis pigmentosa, Stargardt's disease and glaucoma which lead to the apoptosis of photoreceptors or retinal ganglion cells are common retinal diseases resulting in irreversible vision loss. Current therapies for these diseases mainly focus on the etiology or palliative ways to slow down the process of retinal cell death. However, except for glaucoma at the early stage, low efficacy was found in these therapies. Although the breakthroughs in gene therapy brought new hope for approaches against these diseases, there is still a long way to go before clinical application. With the progress in mesenchymal stem cells (MSCs) research, MSCs become a promising source for a variety of diseases[1]-[5] due to the properties such as plasticity, neuroprotection and immunomodulation etc. Furthermore, in contrast to embryonic stem cells MSCs possess many advantages such as self-renewal, easiness of isolation and expansion in vitro, free from ethical problems, etc. In particular, autologous derivation gives MSCs great potential for retinal diseases and has caught attentions from worldwide researchers. Therefore, the feasibility of using MSCs for the diverse retinal disorders was investigated. So far encouraging outcomes have been achieved, although there are still problems remained to be solved. The current concerns on MSCs for retinal degeneration lie in two main aspects, differentiation and neuroprotection.
Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are multipotent and self-renewing stem cells derived from bone marrow, adipose tissue, umbilical cord, placenta and other mesenchymal tissues, which could be induced to differentiate into bone marrow, cartilage, muscle, lipid, myocardial cells, glial cells and neurons[6]-[9], and play an important role in tissue repair or regeneration[10]. MSCs are easy to isolate and expand rapidly after a short period of dormancy. An in vitro culture of MSCs takes less than one week to pass the next generation. Though the isolated MSCs could maintain a normal karyotype and telomerase activity even at passage 12[11], the aged MSCs underwent deterioration in biological functions including the plasticity and the ability in tissue repair[12],[13]. Therefore, most studies on the therapeutic potential of MSCs were conducted at early passages as long as the cells were purified.
For the identification of MSCs, there are no standard methods because MSCs are heterogenous populations consisting of a variety of subsets. There are even no universally-accepted criteria for the definition of MSCs among the investigators. According to the recommendation by the International Society for Cellular Therapy, three principles are included in the definition of MSCs, which are adherence to plasticity, specific surface antigen expression and multipotent differentiation potential[14]. Therefore, the identification of MSCs is based on these principles. The classical method for the identification includes the detection of cells positive for mesenchymal markers CD90, CD105, CD73, CD66, CD29 and CD44, and negative for hematopoietic markers CD34, CD45, together with an induction to differentiate into mesodermal lineages[15]. This classical method is prevalent in researches, but it is a time-consuming process in contrast to a PCR based detection[16].
The plasticity of MSCs has been verified in various researches, and it is even considered as a necessary test for the identification of MSCs. The differentiation of MSCs into adipocytes could be induced by 1-methy-3-isobutylxanthine, dexamethasone, insulin and indometacin with a result that lipid vacuoles continued to develop over time inside the induced cells and adipocyte specific products such as peroxisome proliferation-activated receptor γ2, lipoprotein lipase, and the fatty acid binding protein aP2 were expressed[11]. While the osteogenic differentiation of MSCs could be induced by dexamethasone, β-phosphoglycerol, ascorbic acid phosphate (vitamin C) and 10% v/v fetal bovine serum[17]. When MSCs were seeded in a 3D constructs with the presence of TGF-β superfamily members, the cells expressed cartilage specific markers and turned to be extracellular matrix rich in proteoglycans and collagen type II, suggesting a chondrogenic differentiation of the MSCs[18]. Furthermore, MSCs could be induced to express tyrosine hydroxylase, γ-amino butyric acid, β-microtubulin, nestin and glial fibrillary acid protein[19]-[23], which suggests a neuronal differentiation of MSCs.
Molecules responsible for the differentiation of MSCs into particular tissue cells varied depending on the cell lineage that MSCs tend to differentiate. Investigations into the mechanism for the differentiation of MSCs are necessary for the aim of directional differentiation. For these reasons, an overall study of the diversity of proteome between MSCs and induced MSCs might provide a way for a profound insight into the mechanism in regard to the differentiation of MSCs. Receptor tyrosine kinase is a critical protein which regulates the proliferation, growth and differentiation of MSCs. It works in a ligand-receptor model and activates the down stream pathway resulting in a series of biological effects such as phosphorylation of key proteins and differentiation of MSCs. The differentiation of MSCs into bone forming cells is stimulated by epidermal growth factor (EGF) rather than platelet-derived growth factor (PDGF). Though over 90% of the down stream proteins activated by both cytokines are the same, the two cell factors induce quite different biological effects. PI3K pathway is uniquely activated by PDGF. PI3K inhibitor could retreat the differentiated effect of PDGF, suggesting that PI3K might be a control point for the differentiation[24]. Thus, it underlined the significance of an overall investigation into the network related to the differentiation of MSCs.
MSCs possess the common features of stem cells such as self-renewal and plasticity, but there are debates that transdifferentiation could be a more appropriate description for the multilineage differentiation[25]. In addition, there are also controversies that the differentiation of MSCs is probably a result of cell fusion[26]. The expression of a variety of cell-specific markers and even the gain of cell function are results from cell fusion that leads to the multiplication of chromosome and the expression of particular proteins. Actually, such phenomenon is more often seen in hematopoietic stem cells[27],[28]. When MSCs were co-cultured with bronchial epithelium, some of the co-cultured MSCs rapidly integrated into the monolayer of bronchial epithelium, changed the morphology into bronchial epithelium-like shape, and expressed bronchial epithelium-specific markers. Moreover, some of the integrated cells fused with bronchial epithelium. Nuclear fusions were also detected among these fused cells[29]. It is necessary to mention that cell fusion is not a rare incident. Therefore, cell fusion presents the question to some extent that whether the expression of particular markers in MSCs is really differentiation.
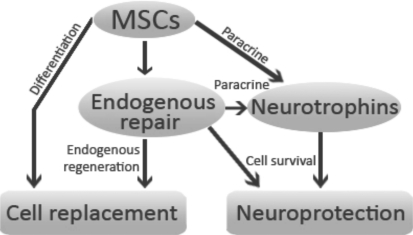
Although there are questions on the plasticity of MSCs, it is true that MSCs play an important role in tissue repair (Figure 1). MSCs could migrate and integrate into the wound tissue and promote the process of wound repair. It is probable that the wound area benefits from the cytokines secreted from MSCs or the differentiation of MSCs into the tissue-specific cells and replaces the wound tissue. MSCs could even transfer across the blood-brain barrier and promote the repair in the central nervous system. Transplantation of MSCs in stroke patients significantly helped functional recovery without side effects during the observations[30],[31]. Although the exact mechanism that MSCs affect the recovery process is still obscure, it might be related to the secretion of neurotrophic factors hence the survival of neurons, the expression of stromal-derived factor (SDF-1) located in the wound tissue together with the migration and integration of MSCs into these areas. What's more, the MSCs induced endogenous cell proliferation and axon reconstruction[32]. Although only a small amount of MSCs were detected migration, integration and differentiation, the improvement of the morphology and function in the injured tissue was usually significant. One explanation is probable that MSCs promote the endogenous cell repair. MSCs could promote endogenous neuron stem cells proliferation, migration, integration and differentiation, after being grafted into the dentate gyrus of hippocampus in immunodeficiency mice[33].
Figure 1. Cell replacement and neuroprotection are the potential applications of MSCs for neurodegenerative diseases.
Cell replacement could be achieved by the differentiation of MSCs or MSCs-associated endogenous regeneration. Neuroprotection results from the secretion of neurotrophins by both MSCs and host cells or other mechanisms during the process of endogenous repair
Another characteristic for MSCs is the immunomodulation, which also catches the attention for the potential use in immunosuppression. MSCs could inhibit the proliferation of T cells and the expression of cytokines from T cells[34]. Meanwhile, MSCs inhibit T cell proliferation in a dose-dependent manner[35]. Strong inhibition of cyclin D2 and up-regulation of cyclin-dependent kinase inhibitor p27kip1 in T cells could be induced by MSCs in an in vitro model, thus leading to the suppression of T cells from activation[36]. MSCs could ameliorate the pathological changes in asthma in an animal model by the expression of TGF-β which blocked pro-inflammatory Th2 cells response and modulated Th1/Th2 to a proper ratio[37]. The inhibition of T cell response plays an important role in the process that MSCs take part in immunomodulation. Nevertheless, this does not necessarily mean that the transplantation of allogenous MSCs would not result in rejection. An injection of MSCs into rat hippocampus and striatum induced severe immune response in the host, leading to the failure of survival of the engrafted cells[38]. Although allogenous MSCs could raise the T cell response in the host, the host's MSCs were capable of inducing immune tolerance[39]. Therefore, immunomodulation could be a better description for MSCs in terms of their immune properties than the general notion that MSCs are immunoprivileged.
Mesenchymal Stem Cells for Retinal Diseases
Both in vitro and in vivo studies were conducted in the investigation of MSCs for retinal diseases. Non-contact co-culture of MSCs with retinal cells or organotypic explants is a usual approach for an in vitro study of the differentiation of MSCs into retinal cells, due to the advantage that it is free from the possibility of cell fusion and that it has a relatively simple microenvironment in contrast to an in vivo study. Though there is a progressive necrosis of the retinal cells and exacerbation of the morphology in retinal explants cultured in vitro, a relative long-term survival of retinal explants could be achieved[40]. So it is possible for an investigation of intraocular transplantation of MSCs in an in vitro model, making the investigation more facilitated in contrast to an in vivo transplantation.
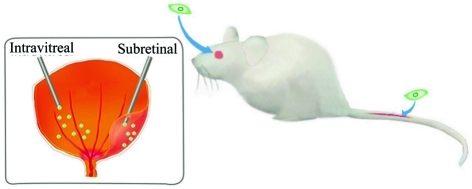
However, an in vivo transplantation of MSCs is also necessary for validation of the potential use or therapeutic effect of MSCs. The transplantation of MSCs in vivo for retinal diseases mainly refers to three approaches (Figure 2): systemic administration, subretinal injection and intravitreal injection. Subretinal injection of MSCs is preferred for the advantage of the immunoprivilege and also a direct contact with the outer layer of the retina in the subretinal space where it is easy for the migration, integration and differentiation of the transplanted cells. Given the restricted volume capable of transplantation and the destruction of tissue integrity, intravitreal transplantation is also considered as an alternative for a local administration of MSCs. In contrast with subretinal transplantation, an intravitreal injection of MSCs allows a larger volume hence a larger amount of cells could be transplanted. Besides, an intravitreal transplantation of MSCs could reduce the damage to retinas. The inner limiting membrane (ILM) is a natural barrier that blocks the MSCs from migration and incorporation into neural retina. An in vitro model of intravitreal injection of MSCs demonstrated that MSCs could hardly penetrate the ILM[41]. Likewise, the intravitreally injected MSCs could attach to the surface of the retina, but few cells passed across the barrier[42]. A few injected cells integrated into neural retina even after the basement membrane of the ILM were digested by collagenase, demonstrating that the integrity of the inner basal lamina was not sufficient to block the migration. However, when the Müller cells were suppressed by glial-specific toxins, it dramatically enhanced the integration of the implanted cells into retina[43], showing that glial endfeet were the major contribution to the prevention of grafted cells penetrating into the retina. So an intravitreal injection of MSCs seems more suitable for the investigation of neurotrophic properties of MSCs, by which neurotrophins secreted from MSCs could penetrate into the retina, while MSCs could not. Thus, the migration, integration and differentiation of MSCs into retinal cells could be ignored, as long as there is a normal Müller cell reactivity. MSCs could migrate into multiple organs after a systemic administration[44]. However, the migration of MSCs into the host retina after systemic administration might require some stimuli, e.g. the damage in RPE or the retinal vascular leakage. Because blood-retinal barrier (BRB) at a normal integrity could be a possible factor that prevents MSCs from migration into the host retina.
Figure 2. Major approaches for administration of MSCs (green) in retinal disorders.
Both systemic and local administrations of MSCs were used in animal models (right). For local administration, intravitreal and subretinal injections were conducted according to different purposes (left)
Mesenchymal Stem Cells and Retinal Repair
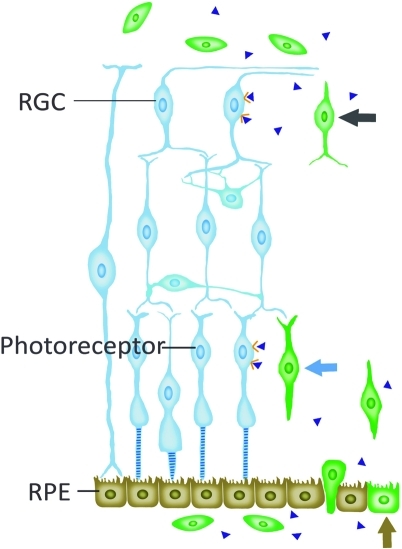
Retinal damage plays an important role in the homing and integration of grafted cells due to the high expression of chemoattractants by injured retinal cells[45]. Among these chemoattractants, SDF-1 was considered as a major contribution to the migration of MSCs. The interaction between SDF-1 and its receptor CXCR4 made MSCs respond to tissue damage[46]. Therefore, during the retinal damage, MSCs also migrate to the injured retina and are involved in the repair via the secretion of SDF-1 from retinal cells. A systemic administration of MSCs in a laser-induced choroidal neovascularization animal model resulted in recruitment and differentiation of MSCs into multiple cell types participating in the process of neovascularization in the damaged spot[47]. MSCs could migrate to retina, differentiate into photoreceptor-like cells (Figure 3) as well as glial-like cells and ameliorate the BRB breakdown in diabetic rats after tail vein injections[48]. Nevertheless, a systemic administration of MSCs did not result in the migration of grafted cells into host retina in an intraocular hypertension animal model induced by photocoagulation of trabecular meshwork. While a quite different result that a small amount of MSCs migrated to retina after intravitreal injection was observed in the same animal model[49]. Intraocular hypertension leads to retinal damage, but it is not sufficient for the migration of MSCs after systemic administration due to the integrity
Figure 3. Migration and integration of MSCs (green) after administration.
The grafted cells differentiated into RPE-like cells (brown arrow), photoreceptor-like cells (blue arrow) and RGC-like cells (green arrow). The host retina also benefits from MSCs-induced neurotrophins (blue triangles) up-regulation. RPE=retinal pigment epithelium; RGC=retinal ganglion cells
of BRB. Sodium iodate-induced RPE damage up-regulated the expression of SDF-1 by RPE and promoted the migration of MSCs. Moreover, the chemotaxis effect of MSCs was retrieved after incubation with SDF-1 antagonist[50]. Chemoattractants expressed by retinal cells and BRB breakdown might be two prerequisites for the migration and integration of MSCs into host retina after systemic administration. However, BRB breakdown did not seem to be a necessary factor for the migration and integration of MSCs after intravitreal injection provided the existence of retinal damage.
Differentiation of Mesenchymal Stem Cells into Retinal Cells
MSCs-based retinal regeneration is one of the most promising therapies for retinal diseases, which needs successful generation of retinal cells from MSCs. Some growth factors involved in the development of retinal cells might also be essential to the differentiation of MSCs into retinal cells. These molecules include basic fibroblast growth factor (bFGF), brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), epidermal growth factor (EGF), etc. Basic fibroblast growth factor is critical for the expression of opsin in neonatal retinal cells by enhancing the proliferation of precursor cells and differentiation of immature photoreceptors[51]. EGF played a role in neurites formation[52]. NGF promoted the survival of retinal cells[53],[54]. BDNF was involved in the differentiation of ganglion cells[55]. Therefore, these growth factors were used in the attempts for the differentiation of MSCs into retinal cells in vitro. MSCs could be induced to express microtubule-associated protein 2, β-tubulin III, glial fibrillary acidic protein (GFAP), protein kinase C alpha (PKC-α) and recoverin in the medium supplemented with BDNF, NGF and bFGF. Besides, neurofilament 200 could also be detected in the growth factors pretreated MSCs after co-culture with retinal explants[56]. Growth factors involved in the early development of retinal cells provide a microenvironment for the differentiation of MSCs into retinal cells. What's more, the growth factors present in the culture medium regulate the differentiation of MSCs. It was reported that approximately 30% MSCs could be induced to express rhodopsin and cellular retinoic acid-binding protein 1, and another 6%∼10% MSCs could be induced to express nestin, suggesting a retinal or neuronal differentiation of MSCs after an in vitro induction by activin A, taurine and EGF[57]. However, further investigations are necessary for a higher production of these differentiated cells to reach the final goal of directional differentiation of MSCs.
Although it would be relatively easy to determine key factors responsible for the differentiation of MSCs into retinal cells in an in vitro model in contrast to an in vivo study, only an in vitro induction is not sufficient to make MSCs differentiate into functional retinal cells due to the simple microenvironment. Therefore, in vivo studies were also conducted for assessing the differentiation potential of MSCs during retinal damage. Subretinal injection of MSCs into sodium iodate-induced retinal degeneration animal model resulted in a small amount of cells migrating and incorporating into neuronal retina and expressed rhodopsin, GFAP and cytokeratin, though the majority of injected cells remained in the subretinal space[58]. Morphological changes and differentiation also occurred in mechanically injured retina after intravitreal injection[59]. Furthermore, functional recovery was observed in RCS rats that received subretinal injections of MSCs[60].
Plenty of studies have demonstrated that MSCs could be induced to differentiate into retinal cells in vitro and in vivo, some of which even showed the enhancement of function in these differentiated cells. However, there are still questions faced with the differentiation of MSCs into retinal cells, e.g. selection of an effective induction approach and time point control for a directional differentiation. Though MSCs could be induced to differentiate into retinal cells or retinal progenitors under certain stimuli, MSCs are of homeostasis and tend to maintain themselves in an undifferentiated state. MSCs could differentiate into retinal ganglion precursor-like cells when co-cultured with E13.5 retinal progenitor cells (RPCs) for 3 days, but the effect did not persist after a longer time of co-culture, suggesting the self-feedback regulation of MSCs to an undifferentiated condition[61]. The retrieval of MSCs from differentiation presents a problem in respect of a stable differentiation status after induction and makes it difficult to select a time point for detection of a particular marker. These may be part of the reasons for different outcomes during the induction of MSCs to differentiate into retinal cells[62]-[64]. Alhough there are questions on the differentiation of MSCs into retinal cells, hosts could benefit from the transplantation of MSCs. Myelin formation by xenogenic oligodendrocyte precursors after the retinal transplantation could be enhanced by adjunctive MSCs[64]. Umbilical cord-derived MSCs were neurotrophic and could promote the survival of axotomised RGC and regeneration of axon[63], which indicated other potential applications of MSCs besides the plasticity.
Neurotrophic Effect of Mesenchymal Stem Cells in Retinal Disorders
Neurotrophins which are beneficial to central nervous system (CNS) disorders probably play similar roles in retinal degeneration due to the same derivation of CNS and retina during the development, especially in RGCs degeneration. GDNF-loaded biodegradable microspheres are neurotrophic for RGCs degeneration by enhancing the survival of RGCs, decreasing the activation of glial cells in retina and optic nerve and reducing the loss of inner plexiform layer thickness[65],[66] . Ciliary neurotrophic factor (CNTF) increased the survival of RGCs by signal transducer and activator of transcription 3 (STAT3) pathway and induced regeneration of cone outer segment[67],[68]. BDNF is another important neurotrophin for the survival of RGCs, which binds to the receptor TrkB, resulting in the activation of c-jun and suppression of caspase-2[69],[70]. The neurotrophins provide alternative ways for retinal disorders. However, the rescue effects on retinal cells depended on continuous release of neurotrophins[71]. A merely intravitreal injection of BDNF did not produce neurotrophic effect in an animal model[72],[73]. Thus, an appropriate way of neurotrophin delivery is necessary for achieving the therapeutic effect. Viral vector seems to be an ideal tool for the delivery of neurotrophic factors against retinal disorders, but the possibility of gene mutation and the lack of acceptance from patients make it still a long way for clinical application. MSCs have been reported to express a variety of neurotrophins which might be beneficial for retinal diseases after transplantation in vivo. Furthermore, the use of MSCs for the delivery of neurotrophins is free of the problems encountered with viral vector. In addition, neurotrophins secreted from MSCs are more varied than viral vector transfection and released in an injury-dependent manner rather than a continuous manner. So MSCs could be a promising source for retinal neuroprotection.
It has been frequently reported that MSCs are neurotrophic in CNS disorders. Although MSCs showed no indication of neuronal differentiation in co-culture with slice of neonatal brain cortex, neuroprotection could be achieved via the interactions between NGF and neurotrophin-3 (NT-3) secreted from MSCs and the receptors NGFR and TrkC[74]. For retinal diseases, only a limited amount of MSCs are capable of differentiation or even no differentiation occurs after transplantation, but histological improvement or functional recovery of the degenerative retina is usually detected after the administration of MSCs. It is a suggestion that MSCs are neurotrophic in retinal diseases. Thus the use of MSCs for retinal degeneration would be a more practical approach since there are still technical problems faced with the differentiation of MSCs into retinal cells.
Subretinal injection of MSCs in RCS rats induced hexagonal morphology of the grafted cells, the expression of pigment epithelium-derived factor (PEDF) as well as epithelial markers and more important a rescue effect for the degenerating photoreceptors[75]. Though the rescue of photoreceptors could be a result from the differentiation of MSCs into RPE hence the improvement of biological function in the RPE layer, the differentiation of MSCs could only be a partial explanation for the result. A similar experiment was conducted in rhodopsin knockout mice through subretinal transplantation of MSCs in which the photoreceptors were undergoing progressive apoptosis while the RPE kept intact. The engraftment demonstrated a significant rescue effect for the photoreceptors with only sparse distribution of MSCs in the retina[76]. Therefore, neurotrophic properties of MSCs play a significant role in protecting photoreceptors from apoptosis after subretinal injection.
Actually, there might be another possibility that the differentiation of MSCs into retinal cells leads to a rescue effect after transplantation of MSCs. But the point is that only a very small amount of grafted cells could incorporate into retina and differentiate. Furthernore, there was hardly any evidence demonstrating that the differentiated cells are functional as normal ones. Inoue et al[77] reported no differentiation of MSCs into photoreceptors via subretinal injection in RCS rats during the observation, but a significant reduction of the loss of outer nuclear layer (ONL) thickness and the deterioration of electroretinogram were detected after the injection. Cultured medium from MSCs could also enhance the survival of photoreceptors in vitro. So it is possible that neurotrophins secreted from MSCs are responsible for the rescue effect. Various neurotrophins may be involved in the repair of retinal degeneration, e.g. CNTF, bFGF, BDNF, GDNF, PEDF.
Subretinal transplantation of MSCs in light-damaged retina induced the inhibition of photoreceptor apoptosis and increased the amount of cells in ONL while expressing BDNF and bFGF[78]. Intravitreal injection of MSCs could slow down the rate of RGCs death with the expression of CNTF, GDNF, bFGF, HGFα, and BDNF in grafted MSCs for a long period in an experimental glaucoma model[42]. The expression of CNTF and bFGF in the retina of an ischemia/reperfusion model decreased in contrast to the normal retina, while the increased expression of both neurotrophic factors was detected after an intravitreal injection of MSCs. Moreover, the damaged retinal tissue was observed being rescued after the engraftment[79].
Another frequently used approach in the attempt for neuroprotection from MSCs is transfection of MSCs with neurotrophins, MSCs being made as vehicles for steady release of neurotrophins. Various neurotrophins were transfected into MSCs for the treatment of neurodegenerative diseases with encouraging outcomes. BDNF delivered from engineered MSCs was able to attenuate the loss of the RGC-5s following glutamate or H2O2 insults[80]. PEDF-transduced MSCs demonstrated the rescue effect for dystrophic retinas after engraftment[74]. However, most of the current engineered MSCs are viral transfected, leading to the consideration that whether the transfected MSCs would encounter the problems that viral vectors did. Levkovitch-Verbin et al[81] developed a non-transfected approach for the induction of MSCs into neurotrophic factors secreting MSCs (NTF-SCs) and found that both NTF-SCs and MSCs were beneficial to the survival of RGCs in rat eyes after optic nerve transection. The neurotrophic effect of MSCs targets not only on RGCs but also on other retinal cells, though RGCs could be the most promising target for neurotrophic therapy by MSCs. Systemic administration of MSCs in RCS rats resulted in a rescue effect in photoreceptors and a reduction of pathological retinal vascular generation. Moreover, the preservation of visual function and up-regulation of neurotrophic factors in the host retina were detected after the introduction of MSCs[82].
Neurotrophins are beneficial to retinal degeneration, so the up-regulation of neurotrophins in both grafted cells and host retinas are responsible for the neurotrophic effect. However, it is still not clear that how the engrafted cells and the host interact with each other, which neurotrophin plays the major role in the rescue effect and whether there is a synergistic effect with a combination of these neurotrophins. Therefore, these remain for further investigations.
Conclusion
MSCs are a promising source for either cell replacement or neuroprotection of retinal degeneration. Great success has been made in the attempt for clinical use of MSCs. MSCs have been demonstrated to be able to differentiate into photoreceptor-like cells and RGC-like cells and express a variety of neurotrophins beneficial to the survival of retinal cells under a pathologic condition. Therefore, two major routes for clinical application of MSCs are cell replacement and neuroprotection. For the cell replacement, technical problem of massive differentiation of MSCs into functional cells makes the attempt still a long way to explore. While the neuroprotection is quite different, an administration of MSCs to rescue the degenerating retinal cells is currently available by autologous transplantation without rejection. However, further studies for the mechanism that MSCs exert neurotrophic effects on retinal cells should be carried out. Other issues from the administration of MSCs such as the teratoma or proliferative vitreoretinopathy should also be paid particular attention to, though these were seldom reported during the observations.
Footnotes
Foundation item: National Natural Science Foundation of China (No.81070715); Innovative Platform Foundation of Fujian Province,China (No.2010Y2003)
REFERENCES
- 1.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci USA. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, van de Water Jeroen AJM, Mohapatra G, Figueiredo JL, Martuza RL, Weissleder R, Shah K. Assessment of therapeutic efficacy and fate of engineered humanmesenchymal stem cells for cancer therapy. Proc Natl Acad Sci USA. 2009;106:4822–4827. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, Prockop DJ. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci USA. 2008;105:4638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho SR, Kim YR, Kang HS, Yim SH, Park CI, Min YH, Lee BH, Shin JC, Lim JB. Functional recovery after the transplantation of neurally differentiated mesenchymal stem cells derived from bone barrow in a rat model of spinal cord injury. Cell Transplant. 2009;18:1359–1368. doi: 10.3727/096368909X475329. [DOI] [PubMed] [Google Scholar]
- 7.Ladak A, Olson J, Tredget EE, Gordon T. Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Exp Neurol. 2011;228:242–252. doi: 10.1016/j.expneurol.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Abdallah BM, Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther. 2008;15:109–116. doi: 10.1038/sj.gt.3303067. [DOI] [PubMed] [Google Scholar]
- 9.Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 10.Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666–1669. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- 11.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 12.Crisostomo PR, Wang M, Wairiuko GM, Morrell ED, Terrell AM, Seshadri P, Nam UH, Meldrum DR. High passage number of stem cells adversely affects stem cell activation and myocardial protection. Shock. 2006;26:575–580. doi: 10.1097/01.shk.0000235087.45798.93. [DOI] [PubMed] [Google Scholar]
- 13.Wilson A, Shehadeh LA, Yu H, Webster KA. Age-related molecular genetic changes of murine bone marrow mesenchymal stem cells. BMC Genomics. 2010;11:229. doi: 10.1186/1471-2164-11-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 15.Halfon S, Abramov N, Grinblat B, Ginis I. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev. 2011;20:53–66. doi: 10.1089/scd.2010.0040. [DOI] [PubMed] [Google Scholar]
- 16.Boucher SE. Simplified PCR assay for detecting early stages of multipotent mesenchymal stromal cell differentiation. Methods Mol Biol. 2011;698:387–403. doi: 10.1007/978-1-60761-999-4_28. [DOI] [PubMed] [Google Scholar]
- 17.Naito H, Tojo T, Kimura M, Dohi Y, Zimmermann WH, Eschenhagen T, Taniguchi S. Engineering bioartificial tracheal tissue using hybrid fibroblast-mesenchymal stem cell cultures in collagen hydrogels. Interact Cardiovasc Thorac Surg. 2011;12:156–161. doi: 10.1510/icvts.2010.253559. [DOI] [PubMed] [Google Scholar]
- 18.Erickson IE, Huang AH, Chung C, Li RT, Burdick JA, Mauck RL. Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue Eng Part A. 2009;15:1041–1052. doi: 10.1089/ten.tea.2008.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barzilay R, Kan I, Ben-Zur T, Bulvik S, Melamed E, Offen D. Induction of human mesenchymal stem cells into dopamine-producing cells with different differentiation protocols. Stem Cells Dev. 2008;17:547–554. doi: 10.1089/scd.2007.0172. [DOI] [PubMed] [Google Scholar]
- 20.Barzilay R, Ben-Zur T, Bulvik S, Melamed E, Offen D. Lentiviral delivery of LMX1a enhances dopaminergic phenotype in differentiated human bone marrow mesenchymal stem cells. Stem Cells Dev. 2009;18:591–601. doi: 10.1089/scd.2008.0138. [DOI] [PubMed] [Google Scholar]
- 21.Wislet-Gendebien S, Leprince P, Moonen G, Rogister B. Regulation of neural markers nestin and GFAP expression by cultivated bone marrow stromal cells. J Cell Sci. 2003;116:3295–3302. doi: 10.1242/jcs.00639. [DOI] [PubMed] [Google Scholar]
- 22.Suon S, Yang M, Iacovitti L. Adult human bone marrow stromal spheres express neuronal traits in vitro and in a rat model of Parkinson's disease. Brain Res. 2006;1106:46–51. doi: 10.1016/j.brainres.2006.05.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tropel P, Platet N, Platel JC, Noël D, Albrieux M, Benabid AL, Berger F. Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:2868–2876. doi: 10.1634/stemcells.2005-0636. [DOI] [PubMed] [Google Scholar]
- 24.Kratchmarova I, Blagoev B, Haack-Sorensen M, Kassem M, Mann M. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science. 2005;308:1472–1477. doi: 10.1126/science.1107627. [DOI] [PubMed] [Google Scholar]
- 25.Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004;18:980–982. doi: 10.1096/fj.03-1100fje. [DOI] [PubMed] [Google Scholar]
- 26.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 28.Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- 29.Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, Peister A, Wang MY, Prockop DJ. Differetiation, cell fusion and nuclear fusion during ex vivio repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci USA. 2003;100:2397–2402. doi: 10.1073/pnas.0437997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 31.Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY, STARTING collaborators A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- 32.Tang Y, Yasuhara T, Hara K, Matsukawa N, Maki M, Yu G, Xu L, Hess DC, Borlongan CV. Transplantation of bone marrow-derived stem cells: a promising therapy for stroke. Cell Transplant. 2007;16:159–169. [PubMed] [Google Scholar]
- 33.Munoz JR, Stoutenger BR, Robinson AP, Spees JL, Prockop DJ. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci USA. 2005;102:18171–18176. doi: 10.1073/pnas.0508945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE, Koc ON. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 36.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 37.Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, Hodges MG, Jelinek I, Madala S, Karpati S, Mezey E. Bone marrow stromal cells use TGF-β to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci USA. 2010;107:5652–5657. doi: 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coyne TM, Marcus AJ, Woodbury D, Black IB. Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia. Stem Cells. 2006;24:2483–2492. doi: 10.1634/stemcells.2006-0174. [DOI] [PubMed] [Google Scholar]
- 39.Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson TV, Martin KR. Development and characterization of an adult retinal explant organotypic tissue culture system as an in vitro intraocular stem cell transplantation model. Invest Ophthalmol Vis Sci. 2008;49:3503–3512. doi: 10.1167/iovs.07-1601. [DOI] [PubMed] [Google Scholar]
- 41.Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, Hodges MG, Jelinek I, Madala S, Karpati S, Mezey E. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci USA. 2010;107:5652–5657. doi: 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu S, Tanabe T, Dezawa M, Ishikawa H, Yoshimura N. Effects of bone marrow stromal cell injection in an experimental glaucoma model. Biochem Biophys Res Commun. 2006;344:1071–1079. doi: 10.1016/j.bbrc.2006.03.231. [DOI] [PubMed] [Google Scholar]
- 43.Johnson TV, Bull ND, Martin KR. Transplantation prospects for the inner retina. Eye. 2009;23:1980–1984. doi: 10.1038/eye.2008.376. [DOI] [PubMed] [Google Scholar]
- 44.Assis AC, Carvalho JL, Jacoby BA, Ferreira RL, Castanheira P, Diniz SO, Cardoso VN, Goes AM, Ferreira AJ. Time-dependent migration of systemically delivered bone marrow mesenchymal stem cells to the infarcted heart. Cell Transplant. 2010;19:219–230. doi: 10.3727/096368909X479677. [DOI] [PubMed] [Google Scholar]
- 45.Castanheira P, Torquetti LT, Magalhãs DR, Nehemy MB, Goes AM. DAPI diffusion after intravitreal injection of mesenchymal stem cells in the injured retina of rats. Cell Transplant. 2009;18:423–431. doi: 10.3727/096368909788809811. [DOI] [PubMed] [Google Scholar]
- 46.Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A, Lu M, Raginski K, Vanguri P, Smith A, Chopp M. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27:6–13. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- 47.Hou HY, Liang HL, Wang YS, Zhang ZX, Wang BR, Shi YY, Dong X, Cai Y. A therapeutic strategy for choroidal neovascularization based on recruitment of mesenchymal stem cells to the sites of lesions. Mol Ther. 2010;18:1837–1845. doi: 10.1038/mt.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Z, Li K, Yan X, Dong F, Zhao C. Amelioration of diabetic retinopathy by engrafted human adipose-derived mesenchymal stem cells in streptozotocin diabetic rats. Graefes Arch Clin Exp Ophthalmol. 2010;248:1415–1422. doi: 10.1007/s00417-010-1384-z. [DOI] [PubMed] [Google Scholar]
- 49.Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51:2051–2059. doi: 10.1167/iovs.09-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Reca RG, Atmaca-Sonmez P, Ratajczak MZ, Ildstad ST, Kaplan HJ, Enzmann V. Retinal pigment epithelium damage enhances expression of chemoattractants and migration of bone marrow–derived stem cells. Invest Ophthalmol Vis Sci. 2006;47:1646–1652. doi: 10.1167/iovs.05-1092. [DOI] [PubMed] [Google Scholar]
- 51.Hicks D, Courtois Y. Fibroblast growth factor stimulates photoreceptor differentiation in vitro. J Neurosci. 1992;12:2022–2033. doi: 10.1523/JNEUROSCI.12-06-02022.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morrison RS, Kornblum HI, Leslie FM, Bradshaw RA. Trophic stimulation of cultured neuron from neonatal rat brain by epidermal growth factor. Science. 1987;238:72–75. doi: 10.1126/science.3498986. [DOI] [PubMed] [Google Scholar]
- 53.Karlsson M, Mayordomo R, Reichardt LF, Catsicas S, Karten H, Hallböök F. Nerve growth factor is expressed by postmitotic avian retinal horizontal cells and supports their survival during development in an autocrine mode of action. Development. 2001;128:471–479. doi: 10.1242/dev.128.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheedlo HJ, Bartosh TJ, Wang Z, Srinivasan B, Brun-Zinkernagel AM, Roque RS. RPE-derived factors modulate photoreceptor differentiation: a possible role in the retinal stem cell niche. In Vitro Cell Dev Biol Anim. 2007;43:361–370. doi: 10.1007/s11626-007-9051-3. [DOI] [PubMed] [Google Scholar]
- 55.Barde YA, Davies AM, Johnson JE, Lindsay RM, Thoenen H. Brain derived neurotrophic factor. Prog Brain Res. 1987;71:185–189. doi: 10.1016/s0079-6123(08)61823-3. [DOI] [PubMed] [Google Scholar]
- 56.Tomita M, Mori T, Maruyama K, Zahir T, Ward M, Umezawa A, Young MJ. A comparison of neural differentiation and retinal transplantation with bone marrow-derived cells and retinal progenitor cells. Stem Cells. 2006;24:2270–2278. doi: 10.1634/stemcells.2005-0507. [DOI] [PubMed] [Google Scholar]
- 57.Kicic A, Shen WY, Wilson AS, Constable IJ, Robertson T, Rakoczy PE. Differentiation of marrow stromal cells into photoreceptors in the rat eye. J Neurosci. 2003;23:7742–7749. doi: 10.1523/JNEUROSCI.23-21-07742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gong L, Wu Q, Song B, Lu B, Zhang Y. Differentiation of rat mesenchymal stem cells transplanted into the subretinal space of sodium iodate-injected rats. Clin Experiment Ophthalmol. 2008;36:666–671. doi: 10.1111/j.1442-9071.2008.01857.x. [DOI] [PubMed] [Google Scholar]
- 59.Tomita M, Adachi Y, Yamada H, Takahashi K, Kiuchi K, Oyaizu H, Ikebukuro K, Kaneda H, Matsumura M, Ikehara S. Bone marrow-derived stem cells can differentiate into retinal cells in injured rat retina. Stem Cells. 2002;20:279–283. doi: 10.1634/stemcells.20-4-279. [DOI] [PubMed] [Google Scholar]
- 60.Lund RD, Wang S, Lu B, Girman S, Holmes T, Sauvé Y, Messina DJ, Harris IR, Kihm AJ, Harmon AM, Chin FY, Gosiewska A, Mistry SK. Cells Isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells. 2007;25:602–611. doi: 10.1634/stemcells.2006-0308. [DOI] [PubMed] [Google Scholar]
- 61.Sun XR, Ge J, Jiang RZ, Duan S, Song G, Duan YH, Liu BQ, Zhang YH. Induced differentiation of bone marrow mesenchymal stem cells towards retinal ganglion precursor cells. Cell Research DOI. 10(1038) [Google Scholar]
- 62.Hill AJ, Zwart I, Tam HH, Chan J, Navarrete C, Jen LS, Navarrete R. Human umbilical cord blood-derived mesenchymal stem cells do not differentiate into neural cell types or integrate into the retina after intravitreal grafting in neonatal rats. Stem Cells Dev. 2009;18:399–409. doi: 10.1089/scd.2008.0084. [DOI] [PubMed] [Google Scholar]
- 63.Zwart I, Hill AJ, Al-Allaf F, Shah M, Girdlestone J, Sanusi AB, Mehmet H, Navarrete R, Navarrete C, Jen LS. Umbilical cord blood mesenchymal stromal cells are neuroprotective and promote regeneration in a rat optic tract model. Exp Neurol. 2009;216:439–448. doi: 10.1016/j.expneurol.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 64.Arriola A, Kiel ME, Shi Y, McKinnon RD. Adjunctive MSCs enhance myelin formation by xenogenic oligodendrocyte precursors transplanted in the retina. Cell Res. 2010;20:728–731. doi: 10.1038/cr.2010.63. [DOI] [PubMed] [Google Scholar]
- 65.Jiang C, Moore MJ, Zhang X, Klassen H, Langer R, Young M. Intravitreal injections of GDNF-loaded biodegradable microspheres are neuroprotective in a rat model of glaucoma. Mol Vis. 2007;13:1783–1792. [PubMed] [Google Scholar]
- 66.Ward MS, Khoobehi A, Lavik EB, Langer R, Young MJ. Neuroprotection of retinal ganglion cells in DBA/2J mice with GDNF-loaded biodegradable microspheres. J Pharm Sci. 2007;96:558–568. doi: 10.1002/jps.20629. [DOI] [PubMed] [Google Scholar]
- 67.Li Y, Tao W, Luo L, Huang D, Kauper K, Stabila P, Lavail MM, Laties AM, Wen R. CNTF induces regeneration of cone outer segments in a rat model of retinal degeneration. PLoS One. 2010;5:e9495. doi: 10.1371/journal.pone.0009495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ji JZ, Elyaman W, Yip HK, Lee VW, Yick LW, Hugon J, So KF. CNTF promotes survival of retinal ganglion cells after induction of occular hypertension in rats: the possible involvement of STAT3 pathway. Eur J Neurosci. 2004;19:265–272. doi: 10.1111/j.0953-816x.2003.03107.x. [DOI] [PubMed] [Google Scholar]
- 69.Kurokawa T, Katai N, Shibuki H, Kuroiwa S, Kurimoto Y, Nakayama C, Yoshimura N. BDNF diminishes caspase-2 but not c-jun immunoreactivity of neurons in retinal ganglion cell layer after transient ischemia. Invest Ophthalmol Vis Sci. 1999;40:3006–3011. [PubMed] [Google Scholar]
- 70.Bähr M. Live or let die: retinal ganglion cell death and survival during development and in the lesioned adult CNS. Trends Neurosci. 2000;23:483–490. doi: 10.1016/s0166-2236(00)01637-4. [DOI] [PubMed] [Google Scholar]
- 71.Pease ME, Zack DJ, Berlinicke C, Bloom K, Cone F, Wang Y, Klein RL, Hauswirth WW, Quigley HA. Effect of CNTF on retinal ganglion cell survival in experimental glaucoma. Invest Ophthalmol Vis Sci. 2009;50:2194–2200. doi: 10.1167/iovs.08-3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ko ML, Hu DN, Ritch R, Sharma SC, Chen CF. Patterns of retinal ganglion cell survival after brain-derived neurotrophic factor administration in hypertensive eyes of rats. Neurosci Lett. 2001;305:139–142. doi: 10.1016/s0304-3940(01)01830-4. [DOI] [PubMed] [Google Scholar]
- 73.Lewis GP, Linberg KA, Geller SF, Guérin CJ, Fisher SK. Effects of the neurotrophins brain-derived neurotrophic factor in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 1999;40:1530–1544. [PubMed] [Google Scholar]
- 74.Pisati F, Bossolasco P, Meregalli M, Cova L, Belicchi M, Gavina M, Marchesi C, Calzarossa C, Soligo D, Lambertenghi-Deliliers G, Bresolin N, Silani V, Torrente Y, Polli E. Induction of neurotrophin expression via human adult mesenchymal stem cells: implication for cell therapy in neurodegenerative diseases. Cell Transplant. 2007;16:41–55. doi: 10.3727/000000007783464443. [DOI] [PubMed] [Google Scholar]
- 75.Arnhold S, Heiduschka P, Klein H, Absenger Y, Basnaoglu S, Kreppel F, Henke-Fahle S, Kochanek S, Bartz-Schmidt KU, Addicks K, Schraermeyer U. Adenovirally transduced bone marrow stromal cells differentiate into pigment epithelial cells and induce rescue effects in RCS rats. Invest Ophthalmol Vis Sci. 2006;47:4121–4129. doi: 10.1167/iovs.04-1501. [DOI] [PubMed] [Google Scholar]
- 76.Arnhold S, Absenger Y, Klein H, Addicks K, Schraermeyer U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefes Arch Clin Exp Ophthalmol. 2007;245:414–422. doi: 10.1007/s00417-006-0382-7. [DOI] [PubMed] [Google Scholar]
- 77.Inoue Y, Iriyama A, Ueno S, Takahashi H, Kondo M, Tamaki Y, Araie M, Yanagi Y. Subretinal transplantation of bone marrow mesenchymal stem cells delays retinal degeneration in the RCS rat model of retinal degeneration. Exp Eye Res. 2007;85:234–241. doi: 10.1016/j.exer.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y, Wang W. Effects of bone marrow mesenchymal stem cell transplantation on light-damaged retina. Invest Ophthalmol Vis Sci. 2010;51:3742–3748. doi: 10.1167/iovs.08-3314. [DOI] [PubMed] [Google Scholar]
- 79.Li N, Li XR, Yuan JQ. Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefes Arch Clin Exp Ophthalmol. 2009;247:503–514. doi: 10.1007/s00417-008-1009-y. [DOI] [PubMed] [Google Scholar]
- 80.Harper MM, Adamson L, Blits B, Bunge MB, Grozdanic SD, Sakaguchi DS. Brain-derived neurotrophic factor released from engineered mesenchymal stem cells attenuates glutamate- and hydrogen peroxide-mediated death of staurosporine-differentiated RGC-5 cells. Exp Eye Res. 2009;89:538–548. doi: 10.1016/j.exer.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levkovitch-Verbin H, Sadan O, Vander S, Rosner M, Barhum Y, Melamed E, Offen D, Melamed S. Intravitreal injections of neurotrophic factors secreting mesenchymal stem cells are neuroprotective in rat eyes following optic nerve transection. Invest Ophthalmol Vis Sci. 2010;51:6394–6400. doi: 10.1167/iovs.09-4310. [DOI] [PubMed] [Google Scholar]
- 82.Wang S, Lu B, Girman S, Duan J, McFarland T, Zhang QS, Grompe M, Adamus G, Appukuttan B, Lund R. Non-invasive stem cell therapy in a rat model for retinal degeneration and vascular pathology. PLoS One. 2010;5:e9200. doi: 10.1371/journal.pone.0009200. [DOI] [PMC free article] [PubMed] [Google Scholar]