Abstract
Pancreatic cancer is the fourth leading cause of cancer deaths in developed countries. Smoking is an established risk factor for this malignancy but the underlying mechanisms are poorly understood. Previous reports have provided evidence that nicotinic acetylcholine receptors (nAChRs) and beta-adrenergic receptors (β-ARs) stimulate the growth and migration of pancreatic cancer cells. But a potential cooperation of these two receptor families in the regulation of pancreatic cancer has not been studied to date. Using two pancreatic cancer cell lines and immortalized pancreatic duct epithelia in vitro, our current data show, that all three cell lines synthesized and released the catecholamine neurotransmitters noradrenaline and adrenaline upon exposure to nicotine and that this activity was regulated by α3, α5, and α7-nAChRs. In accord with the established function of these catecholamines as β-AR agonists, nicotine-induced cell proliferation was blocked by the β-AR antagonist propranolol. Nicotine-induced proliferation was also abolished by the α7-nAChR antagonist α-bungarotoxin while catecholamine production in response to nicotine was blocked by gene knockdown of the α3, α5, and α7-nAChRs. The nicotinic agonists acetylcholine, nicotine, and its nitrosated carcinogenic derivative NNK induced the phosphorylation of CREB, ERK, Src and AKT and these responses were inhibited by propranolol. Our findings identify this hitherto unknown autocrine catecholamine loop as an important regulatory cascade in pancreatic cancer that may prove a promising new target for cancer intervention.
Keywords: Nicotinic receptors, beta-adrenergic receptor, autocrine growth factors, pancreatic cancer, neurotransmitters
Introduction
Pancreatic cancer is the fourth leading cause of cancer deaths with a morality rate near 100 % within two years of diagnosis (1). The major impediment to effective clinical outcomes for this malignancy is its delayed diagnosis and resistance to existing cancer therapeutics (2, 3). Novel strategies for more successful prevention and therapy of pancreatic cancer are therefore urgently needed.
Pancreatic ductal adenocarcinoma (PDAC) accounts for the majority of pancreatic cancer cases and is thought to arise from pancreatic duct epithelia (3). Smoking is a documented risk factor for pancreatic cancer (4), with smokers demonstrating a twofold increase in the risk of developing PDAC (5). Tobacco smoke is composed of over 4000 chemicals, including the nicotine-derived carcinogenic nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) (6). It has been shown that NNK causes PDAC in rats (7) and hamsters (8). While nicotine acts as the primary psychoactive component responsible for smoke addiction, it is thought to be non-carcinogenic. However, several laboratories have reported that nicotine activates numerous cellular signaling pathways downstream of nicotinic acetylcholine receptors (nAChRs), resulting in the stimulation of cell proliferation, angiogenesis, and metastasis of several cancers, including PDAC (9–12).
Nicotinic acetylcholine receptors constitute a heterogeneous family of ion channels that were initially thought to be restricted to the central and peripheral nervous system. However, recent studies have identified the expression of this receptor family in numerous non-neuronal cells, including the pancreas (13). In the nervous system and adrenal glands, nAChRs regulate the synthesis of the catecholamine neurotransmitters noradrenaline and adrenaline and their release into the extracellular environment and systemic circulation (14, 15). Both catecholamines are agonists for β-adrenergic receptors (β-ARs). Binding of adrenaline or noradrenaline to these receptors activates the stimulatory G protein (Gαs) which in turn activates adenylyl cyclase, the single rate limiting step for the formation of intracellular cAMP (16, 17). Moreover, studies by our laboratory have shown that PDAC cells express β-ARs with β2-ARs predominating, and that cAMP signaling downstream of these receptors stimulates the proliferation and migration of these cells (18–20).
In the current experiments, we have tested the hypothesis that analogous to their function in the CNS, nAChRs regulate the synthesis and release of noradrenaline and adrenaline in PDAC cells and pancreatic duct epithelial cells and that this autocrine catecholamine loop activates multiple cellular pathways that are overexpressed in pancreatic cancer.
Materials and Methods
Chemicals, primers and antibodies
Lipofectamine 2000 Reagent, stealth-183 for the CHRNA7 gene, stealth-1079 for the CHRNA3 gene, stealth-873 for the CHRNA5 gene, stealth-1973 for the CHRNA4 gene, stealth RNAi Negative Control Low GC Duplex, and Opti-MEM I reduced serum medium 1X were all purchased from Invitrogen Corporation (Carlsbad, CA, USA). The primer used to interfere with the α7 subunit mRNA was sense, GGA AGC UUU ACA AGG AGC UGG UCA A and antisense, UUG ACC AGC UCC UUG UAA AGC UUC C. The primer used to interfere with the α3 subunit mRNA was sense, GCU CUU CCA UGA ACC UCA AGG ACU A and antisense, UAG UCC UUG AGG UUC AUG GAA GAG C. The primer used to interfere with the α5 subunit mRNA was sense, GGG AGC AAA GGA AAC AGA ACC GAC A and antisense, UGU CGG UUC UGU UUC CUU UGC UCC C. The primer used to interfere with the α4 subunit mRNA was sense, GAC CGC AUC UUC CUC UGG AUG UUC A and antisense, UGA ACA UCC AGA GGA AGA UGC GGU C.
The TE Buffer 1X was purchased from Promega Corporation (Madison, WI, USA). The 2-Cat Elisa Kits were purchased from Rocky Mountain Diagnostics Incorporation (Colorado Springs, CO, USA). ELISA kits for Akt [pS473], ERK1/2 [pTpY185/187], and CREB [pS133] were purchased from Invitrogen Corporation (Carlsbad, CA, USA). The c-Src Kinase assay was purchased from MBL International (Woburn, MA, USA).
The antibodies Akt (60 kDa), p-Akt (60 kDa), Src (60 kDa), p-Src (60 kDa), ERK ½ (44/42 kDa), p-ERK ½ (44/42 kDa), p-CREB (43 kDa), anti-rabbit and anti-mouse were all purchased from Cell Signaling (Danvers, MA, USA). The primary antibodies anti-CREB (43 kDa) and anti-nicotinic acetylcholine receptor alpha4 (55 kDa) were purchased from Millipore (Billerica, MA, USA). The nicotinic acetylcholine receptor subunits α7 (56 kDa), α3 (57 kDa), α5 (53 kDa), and β-actin (42 kDa) antibodies were purchased from Abcam (Cambridge, MA, USA). Nicotine and propranolol were both purchased from Sigma-Aldrich (St. Louis, MO, USA). The site-selective α7-nAChR antagonist, α-bungarotoxin, was purchased from Calbiochem (Gibbstown, NJ, USA). The lysis buffer used to extract proteins along with Pierce ECL western blotting substrate were purchased from Thermo Scientific (Rockford, IL, USA).
Cell culture
The human pancreatic ductal adenocarcinoma cell lines Panc-1 and BxPC-3 were purchased from the American Type Culture Collection (Manassas, VA, USA). The immortalized human pancreatic duct epithelial cell line, HPDE6-C7, was clonally established after transduction of the HPV16-E6E7 genes into primary cultures of pancreatic duct epithelial cells and was a kind gift from Dr. Tsao (Division of Cellular and Molecular Biology, Department of Pathology, Ontario Cancer Institute/Princess Margaret Hospital, University of Toronto, Toronto, ON, Canada). All cell lines have been authenticated at the beginning of the current study by RADIL (Research Animal Diagnostic Laboratory, Columbia, MO, USA) by species-specific PCR evaluation. The Panc-1 cell line was maintained in DMEM medium supplemented with 10 % Fetal Bovine Serum (FBS). BxPC-3 cells were maintained in RPMI 1640 medium supplemented with 10 % FBS. HPDE6-C7 cells were maintained in Keratinocyte Serum Free Medium (KSFM) supplemented with 25 mg / 500 ml Bovine Pituitary Extract (BPE) and 2.5 µg / 500 ml Epidermal Growth Factor (EGF) (GIBCO Invitrogen Corporation, Grand Island, NY, USA). All cell lines were grown without antibiotics in an atmosphere of 5 % CO2, 99 % relative humidity, and 37 °C.
Analysis of intracellular and secreted adrenaline and noradrenaline
All three cell lines were maintained in their respective complete medium until reaching 65 % confluence, at which time they were switched to basal medium for 24 hours starvation. Cells were then switched into fresh basal media and were divided into two groups. The first groups of cells were either untreated or treated with 1 µM nicotine for 1, 5, 15, or 30 minutes. The second groups of cells were either untreated or treated with 10 pM, 500 pM, 1 nM, 500 nM, 1 µM, or 10 µM nicotine for 30 minutes. The culture media, containing secreted catecholamines were then collected in 15 ml test tubes. The cells which contained synthesized intracellular catecholamines were lysed and harvested into 1.5 ml eppendorf tubes after a one time wash with warm 1X PBS. Quantitative analyses of intracellular and secreted adrenaline and noradrenaline of five samples per treatment group were conducted using 2-Cat ELISA kits following the vendor’s recommendations. Absorbance of samples was read using an uQuant Bio-Tek Instrument ELISA reader at 450 nm primary wavelength with a 630 nm reference wavelength.
Gene knockdown of the α3, 4, 5, and 7-nAChRs
Cells from all three cell lines were grown for 24 hours in their respective complete media. At that time, cells were switched to Opti-MEM I media and were divided into several groups. Groups 1 and 2 from each cell line were left untreated in Opti-MEM I media for 24 hours. Group 3 was transfected for 24 hours with stealth RNAi Negative Control Low GC Duplex. Groups 4 and 5 were transfected with either stealth-183, 1079, 873, or 1973 for the CHRNA7, 3, 5, and 4 genes respectively for 24 hours in Opti-MEM I media. Once the 24 hours transfection was complete, all cells were switched into their respective basal media. Groups 1, 3, and 4 were left untreated for 30 minutes in basal media whereas groups 2 and 5 were treated with 1 µM nicotine for 30 minutes in basal media. All transfections were done using Lipofectamine 2000 reagent following the instructions of the manufacturer. Cell lysates were then harvested and collected in 1.5 ml eppendorf tubes after one time wash with warm 1X PBS for adrenaline and noradrenaline analyses by immunoassays as described above. The transfection efficiency was monitored by western blots using α3, 4, 5 and 7-nAChRs as primary antibodies and actin as a loading control following the procedure outlined below. Following background subtraction, mean densities of 2 rectangular areas of standard size per band from three independent westerns were determined and mean values and standard deviations (n = 6) of protein expression were calculated.
Assessment of cell proliferation by MTT assay
Cells from the three cell lines were seeded in 6-well plates at a density of 50,000 cells per well in their respective phenol red free complete media. Cells were then left untreated, or treated with 1 µM nicotine for 72 hours, 200 nM α-bungarotoxin for 72 hours, 1 µM propranolol for 72 hours, 200 nM α-bungarotoxin for 10 minutes followed by 1 µM nicotine for 72 hours, or 1 µM propranolol for 10 minutes followed by 1 µM nicotine for 72 hours. The MTT (3-[4,5- dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide) colorimetric assay (Sigma-Aldrich St Louis, MO, USA) was used to assess cell proliferation following instructions by the vendor. The MTT assay is based on the nicotinamide adenine dinucleotide-dependent enzymatic reduction of the tetrazolium salt MTT to form formazan in metabolically active, viable cells. Absorbance of samples was read using an uQuant Bio-Tek Instrument ELISA reader at 570 nm primary and 650 nm reference wavelengths.
Quantitative assessment of phosphorylation of signaling proteins by ELISA assays
Cells from the three cell lines were left to grow in their respective complete media until reaching 65 % confluence. Cells were then left untreated, or treated with 1 µM nicotine for 72 hours, 1 µM propranolol for 72 hours, or 1 µM propranolol for 10 minutes followed by 1 µM nicotine for 72 hours in complete media. The cells were then lysed and harvested into 1.5 ml eppendorf tubes after a one time wash with warm 1X PBS. Quantitative analyses of Akt, CREB, Src, and ERK1/2 phosphorylation of five samples per treatment group were conducted using Akt, CREB, c-Src, and ERK1/2 ELISA kits respectively following the vendors’ recommendations. Absorbance of samples was read using an uQuant Bio-Tek Instrument ELISA reader at 450 nm primary wavelength with a 630 nm reference wavelength.
Western blot analysis
Cells from HPDE6-C7, BxPC-3, and Panc-1 were grown in their respective complete medium until reaching 65 % confluence. Cells were then switched to their respective basal media without any supplements or antibiotics for 24 hours starvation. The cells were then switched to fresh basal media and were divided into three groups. Group 1 was either untreated, or treated with 1 µM nicotine for 10, 15, 30 or 60 minutes. Group 2 was either untreated, or treated with 1 nM NNK for 10, 15, 30 or 60 minutes. Group 3 was either untreated, or treated with 10 µM acetylcholine for 10, 15, 30 or 60 minutes. Protein samples were prepared using lysis buffer (50-mmol / L Tris-HCl, 1 % NP-40, 150-mmol / L NaCl, 1-mmol / L phenylmethysulfonylfluoride, 1-mmol / L Na3VO4, 1-mmol / L NaF, and 1 µg / mL of aprotinin, leupeptin, and pepstatin). After heat denaturation, protein samples were electrophoresed using 12 % SDS gels (Invitrogen) and blotted onto membranes. The membranes were blocked (5 % nonfat dry milk solution) for one hour at room temperature. Membranes were then incubated overnight at 4 °C with the following primary antibodies: Akt, p-Akt, Src, p-Src, CREB, p-CREB, ERK ½, and p-ERK 1/2. The membranes were then washed (0.5 % Tween 20 / TBS) and incubated with their respective fluorescent secondary antibodies for two hours. Protein bands were then visualized with enhanced chemiluminescence reagent (Pierce ECL Western Blotting Detection Substrate).
Statistical analysis of data
GraphPad Instat 3 software (GraphPad Instant biostatistics, San Diego, CA, USA) was used to test significant differences between different treatment groups. Statistical tests used included non-parametric One Way ANOVA and Tukey-Kramer multiple comparison tests. In addition, ImageJ from NIH was used for mean density determination of bands. Data of the immunoassays and MTT assays are expressed as mean and +/− standard deviation of five samples per treatment group. The EC50 values of the concentration-response curves were determined by nonlinear regression analysis. Densitometry data of western blots are expressed as mean values and standard deviations of 2 density determinations per band from three independent westerns per antibody (n = 6).
Results
Effects of nicotine on catecholamine neurotransmitter levels
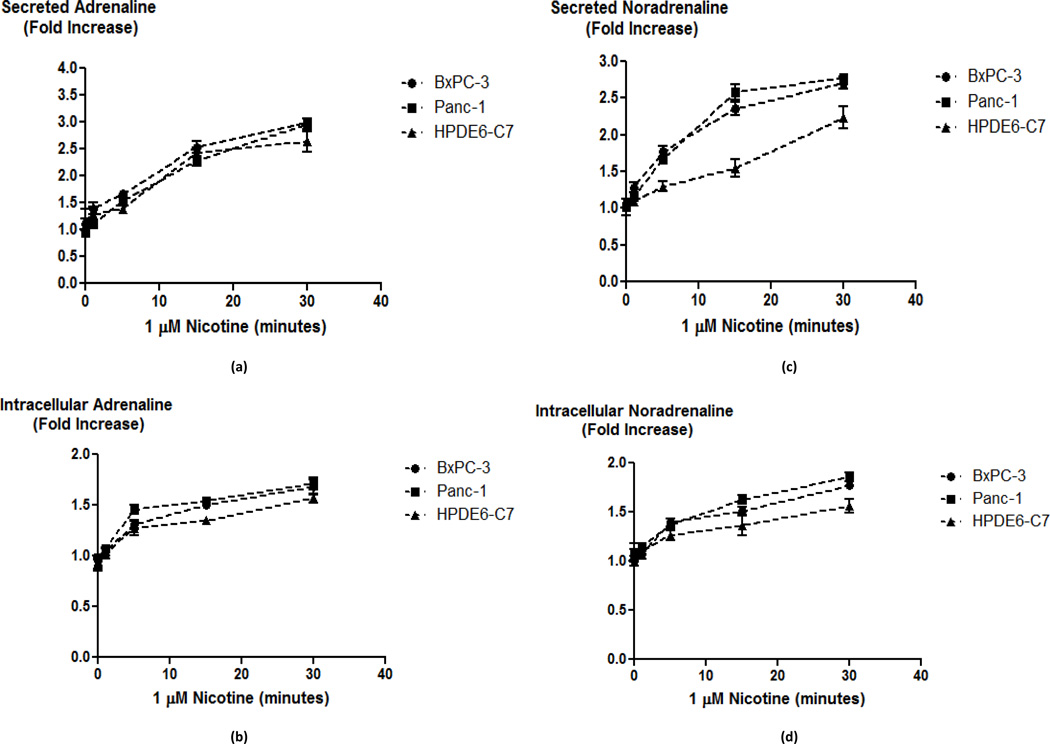
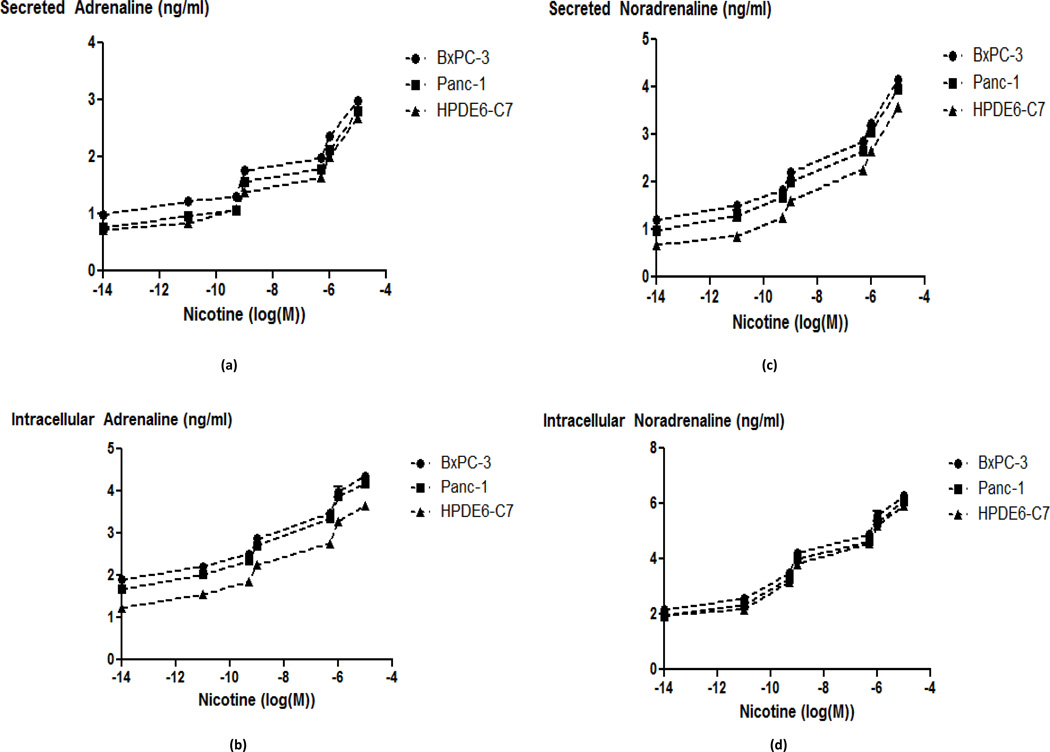
One important role of the α7-nAChR in the nervous system is the regulation of the synthesis and release of neurotransmitters, including adrenaline and noradrenaline (14, 21). While our laboratory has previously shown that β-ARs regulate the proliferation and migration of pancreatic cancer cells in vitro (18–20), the function of nAChRs expressed in these cells is poorly understood. We therefore tested the hypothesis that pancreatic ductal adenocarcinoma cells and normal pancreatic duct epithelial cells, synthesize and release their own catecholamine neurotransmitters and that this activity is regulated by nAChRs. In support of this hypothesis, our immunoassays detected noradrenaline and adrenaline in cell lysates as well as culture media of both pancreatic cancer cell lines and the immortalized pancreatic duct epithelial cells. The intracellular as well as secreted levels of noradrenaline and adrenaline increased significantly (p < 0.0001) in a time-dependent manner when the cells were exposed to nicotine (1 µM) for 1 to 30 minutes (Fig. 1). The immortalized duct epithelial cell line HPDE6-C7 was less responsive to nicotine than the cancer cells (p < 0.0001 at the 30 minute time point) as indicated by lower levels of intracellular and secreted catecholamines at all-time points investigated (Fig. 1). Exposure of the cells for 30 minutes to nicotine at concentrations from 10 pM through 10 µM additionally revealed concentration-dependent increases in intracellular and secreted catecholamines in all three cell lines (Fig. 2). In accord with the differences in responsiveness observed in the time courses (Fig. 1), the EC50 values of nicotine for intracellular and secreted noradrenaline and adrenaline were in the nanomolar range for the two cancer cell lines while they were in the 10–100 nanomolar range for HPDE6-C7 cells (Fig. 2).
Fig. 1.
Secreted (a) and intracellular (b) adrenaline levels; and secreted (c) and intracellular (d) noradrenaline levels in HPDE6-C7, BxPC-3 and Panc-1 cells treated with 1 µM nicotine from 5 to 30 minutes. Representative data points are mean and +/− SD from five samples per treatment group.
Fig. 2.
Secreted (a) and intracellular (b) adrenaline levels; and secreted (c) and intracellular (d) noradrenaline levels in HPDE6-C7, BxPC-3 and Panc-1 cells treated with nicotine concentrations from 10 pM through 10 µM for 30 minutes. The EC50 values were as follows: HPDE6-C7 intracellular adrenaline: 100 nM, secreted adrenaline: 98 nM, intracellular noradrenaline: 14 nM, secreted noradrenaline: 250 nM; Panc-1 intracellular adrenaline: 460 pM, secreted adrenaline: 350 pM, intracellular noradrenaline: 7.3 nM, secreted noradrenaline: 214 pM; BXPC-3 intracellular adrenaline: 469 pM, secreted adrenaline: 3.24 nM, intracellular noradrenaline: 2.6 nM, secreted noradrenaline: 6.7 nM. Representative data points are mean and +/− SD from five samples per treatment group.
Effects of nAChR knockdown on neurotransmitter levels and nAChR protein
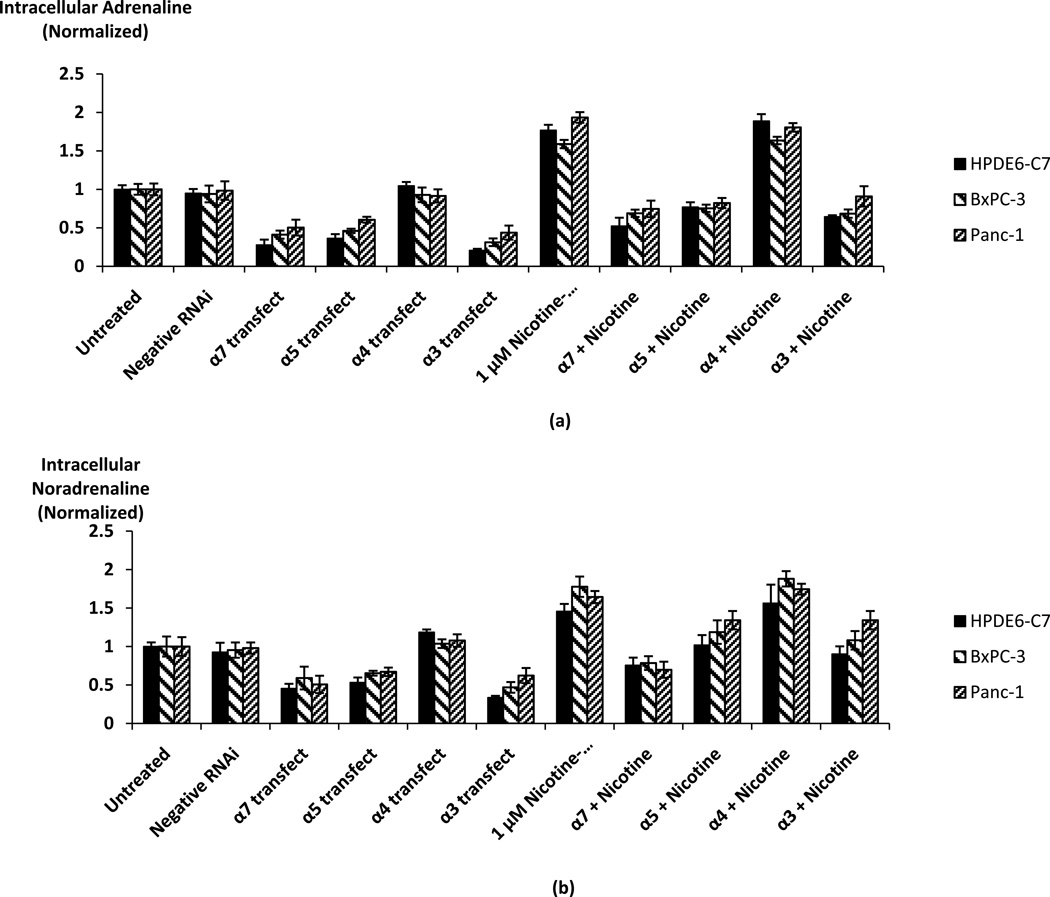
To assess the potential regulatory role of the α3, α4, α5, and α7-nAChRs for catecholamine synthesis in pancreatic cancer and pancreatic duct epithelial cells, the three cell lines were transfected with stealth α3, α4, α5, or α7 RNAi constructs. Immunoassays showed that transfections with the α3, α5, and α7 RNAi constructs significantly inhibited (p < 0.0001) the stimulatory effect of nicotine on adrenaline and noradrenaline synthesis in each of the three cell lines and reduced catecholamine synthesis below base levels in control cells (Fig. 3). These findings confirm regulatory roles of the α3, α5, and α7-nAChRs for catecholamine production by these cells. By contrast, gene knockdown of the α4-nAChR did not significantly reduce catecholamine synthesis in either cell line (Fig. 3). As no reduction in catecholamine synthesis was observed in the cells transfected with the negative control RNAi, these findings indicate that the cells also produce one or both of the physiological agonists for these nAChRs (acetylcholine, choline) that continuously stimulated base level catecholamine synthesis.
Fig. 3.
Assessment of the regulatory roles of alpha3, 4, 5, and 7-nicotinic acetylcholine receptors on the nicotine-induced stimulation of catecholamine production in HPDE6-C7, BxPC-3, and Panc-1 cells. Receptor knockdown of alpha3, 5, and 7 significantly (p < 0.001) reduced nicotine induced stimulation of intracellular (a) adrenaline and (b) noradrenaline. By contrast, alpha4 receptor knockdown showed no significant effect on catecholamine production. The columns in the graphs are mean and +/− SD from five samples per treatment group.
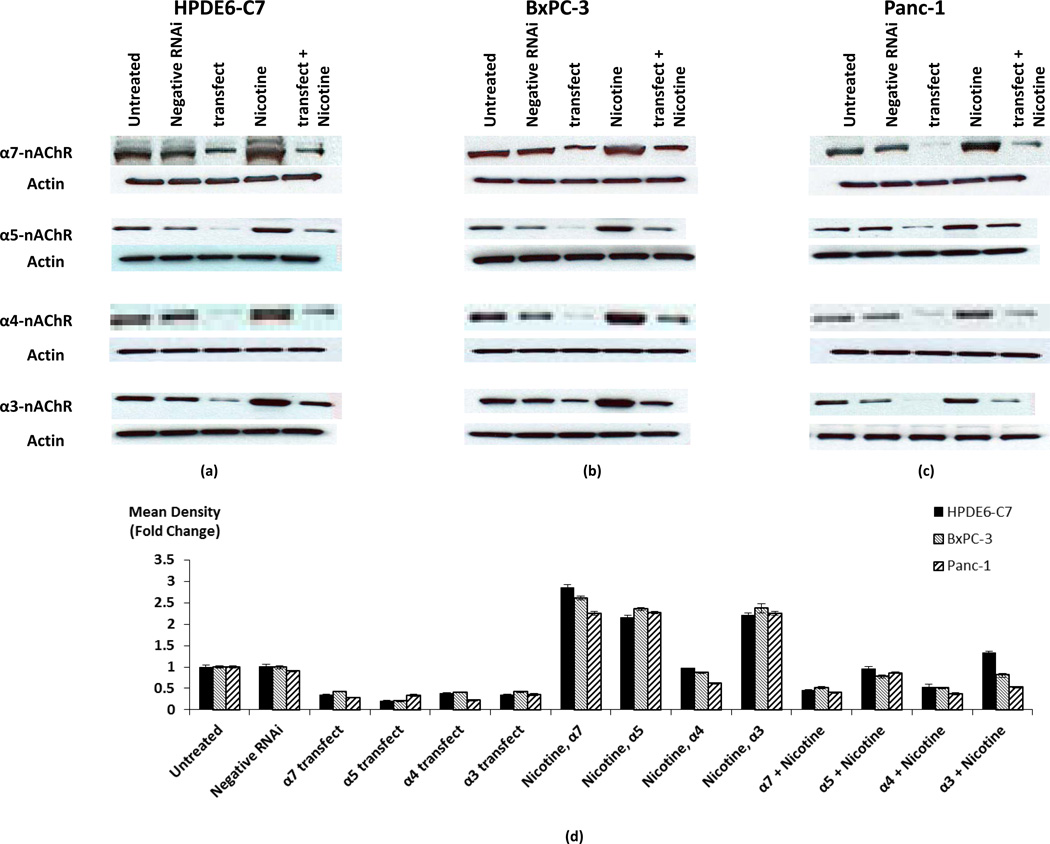
Results of western blots showed significant increases (p < 0.0001) in the expression of all investigated nAChR proteins in all three cell lines exposed for 30 minutes to 1 µM nicotine (Fig. 4). Furthermore, transfection of these cells with stealth RNAi for each of the investigated nAChR constructs significantly decreased (p < 0.0001) their protein expression in cells with and without nicotine exposure (Fig. 4). By contrast, transfection of cells with stealth RNAi negative control low GC showed no significant change in the protein expression of this receptor (Fig. 4), confirming the specificity of the observed gene knockdowns.
Fig. 4.
Western blots showing the effects of gene knockdown on protein expression of alpha3, 4, 5, and 7-nicotinic acetylcholine receptors in HPDE6-C7 (a), BxPC-3 (b) and Panc-1 (c) cells in the presence and absence of nicotine (1 µM for 30 minutes). The house keeping protein β-actin was used as a control to ensure equal loading of proteins. The columns in the graph (d) represent means and +/− SD of two mean density readings per band from three independent western blots (n = 6) expressed as fold changes in expression of α3, 4, 5 and 7-nAChRs.
Activation of multiple signaling proteins by nAChR agonists and inhibition by the beta-blocker propranolol
Binding of an agonist to β-ARs activates adenylyl cyclase, leading to the formation of cAMP and phosphorylation of the transcription factor CREB by activated protein kinase A (17). In addition, activated protein kinase A transactivates the EGFR pathway in pancreatic cancer cells and pancreatic duct epithelia, leading to the phosphorylation of the extracellular signal regulated kinases ERK1/2 (19). We therefore assessed the phosphorylation of CREB and ERK by western blots in our three cell lines after exposures from 10 to 60 minutes to nicotine (1 µM), NNK (1 nM) or acetylcholine (10 µM). We also monitored the phosphorylation of Src family tyrosine kinases and of the serine / threonine protein kinase B, AKT, because both are frequently overexpressed in pancreatic cancer (22, 23). As Fig. 5 shows, 1 µM nicotine induced the phosphorylation of all four signaling proteins in both pancreatic cancer cell lines and immortalized pancreatic duct epithelial cells. In accord with findings that the affinity of NNK to the α7-nAChR is about 1000 times greater than that of nicotine (24), similar responses of the four investigated signaling proteins were observed when the cells where exposed to 1 nM NNK (Fig. 5). The physiological nAChR agonist, acetylcholine, binds to these receptors at a significantly lower affinity than nicotine (14). We therefore used acetylcholine at a 10 µM concentration when assessing its effects on the phosphorylation status of the signaling proteins under investigation. As shown in Fig. 5 at this concentration, acetylcholine had similar inducing effects on all four signaling proteins as nicotine and NNK. The quantitative assessment of nicotine-induced activation of these signaling proteins and the inhibitory effects of propranolol were achieved by immunoassays. As Fig. 6 shows, the phosphorylation of ERK, CREB, Src and AKT were each significantly (p < 0.001) induced by nicotine and these responses were completely blocked by propranolol (p < 0.0001). Propranolol also significantly (p < 0.01) reduced base level phosphorylation of these signaling proteins in cells not exposed to nicotine (Fig. 6).
Fig. 5.
Western blots assessing phosphorylation of Akt, Src, CREB, and ERK1/2 in HPDE6-C7 (a), BxPC-3 (b) and Panc-1 (c) cells treated with 10 µM acetylcholine, 1 µM nicotine and 1 nM NNK at different time exposures. All agents increased phosphorylation of all four proteins as compared with the untreated groups. The un-phosphorylated proteins Akt, Src, CREB, and ERK1/2 were used as controls to ensure equal loading of proteins.
Fig. 6.
ELISA assays showing phosphorylation levels of Src (a), ERK1/2 (b), Akt (c), and CREB (d) for HPDE6-C7, BxPC-3 and Panc-1. Nicotine significantly (p < 0.001) induced the phosphorylation of all signaling proteins investigated. Propranolol, a beta-blocker, significantly (p < 0.001) reduced base level and nicotine induced phosphorylation of all four proteins. Representative data points are mean and +/− SD from five samples per treatment group.
Regulation of cell proliferation by neurotransmitter receptors
MTT assays were conducted to evaluate cell proliferation of HPDE6-C7, BxPC-3, and Panc-1 cells induced by nicotine (1 µM) in the presence and absence of the general β-AR antagonist, propranolol (1 µM) or the site-selective α7-nAChR antagonist, α-bungarotoxin (α-BTX, 200 nM). Cells from each of the three investigated cell lines exposed to nicotine for 72 hours showed significant increases (p < 0.0001) in cell proliferation. This response to nicotine was reduced below base levels (p < 0.0001) by pre-exposure of cells to either α-BTX or propranolol (Fig. 7a), indicating that the α7-nAChR as well as β-ARs were involved in the observed nicotine-induced cell proliferation. In addition, base level cell proliferation in the absence of nicotine was significantly (p < 0.0001) reduced by α-BTX or propranolol (Fig. 7a), suggesting regulatory functions of both receptor types in non-exogenously stimulated cells.
Fig. 7.
Cell proliferation of HPDE6-C7, BxPC-3 and Panc-1 cells assessed by MTT assays, showing inhibition of nicotine-induced cell proliferation by the site-selective α7-nAChR antagonist, α-bungarotoxin (α-BTX), and by the beta-blocker propranolol (a). Columns in the graph represents mean +/− SD of fiver samples per treatment group.
Schematic diagram of the proposed stimulation of pancreatic cancer cells by the catecholamine loop that is jointly regulated by the α3, 5, and 7-nAChRs and β-ARs (b).
Discussion
Our data show, for the first time, that pancreatic cancer cells and normal, pancreatic duct epithelia, express an autocrine catecholamine loop that stimulates their proliferation and is jointly regulated by the nAChRs α3, α5, α7 and by β-ARs. The catecholamines noradrenaline and adrenaline, that were synthesized and released by two investigated pancreatic cancer cell lines and a cell line of immortalized pancreatic duct epithelia, are commonly known as stress neurotransmitters because they are synthesized in the adrenal medulla and released into the systemic blood circulation in response to psychological stress (15). In addition, both of these neurotransmitters are synthesized and released from nerves of the sympathicus (25), thus regulating vital functions in multiple organs. Furthermore, noradrenaline and adrenaline have excitatory and anti-inflammatory functions in the brain where they are synthesized and released by neurons (21). It is well documented that nicotine increases catecholamine production at these neuroendocrine sites, thus increasing the systemic levels of noradrenaline and adrenaline (26, 27). Accordingly, a previous study by our laboratory showed a significant nicotine-induced growth promotion of pancreatic cancer xenografts associated with increased levels of noradrenaline, adrenaline and cAMP in blood and xenograft tissues as well as an induction of p-CREB and p-ERK in xenograft tissues. These changes were interpreted as indirect effects of such systemic neuroendocrine responses (28). Our current in vitro experiments are not influenced by this systemic neuroendocrine effect of nicotine and unequivocally show that two pancreatic cancer cell lines as well as immortalized pancreatic duct epithelia synthesized and released their own noradrenaline and adrenaline in response to nicotine. The important role of the catecholamine neurotransmitters in the observed nicotine-induced stimulation of cell proliferation was confirmed by abolishment of this response by propranolol, an antagonist for β1- as well as β2-ARs. As assessed by both, gene knockdown and pharmacological blockage with α-BTX, the α7-nAChR regulated catecholamine production as well as cell proliferation in response to nicotine. Interestingly, gene knockdown of the α3 and α5-nAChRs also significantly reduced catecholamine production in all three cell lines, indicating cooperative function of these two nAChRs with the α7-nAChR. By contrast, the α4-nAChR did not appear to participate in the regulation of catecholamine production. These findings are in accord with reports that the α3, α5, and α7-nAChRs cooperate in regulating the proliferation of oral keratinocytes (29) whereas the α4-nAChR regulates the production of γ-aminobutyric acid (GABA) in human small airway epithelial cells (30). The α3, α5 and α7-nAChRs thus function as the upstream regulator of this novel autocrine regulatory loop with β-ARs as the effectors of released noradrenaline and adrenaline (Fig. 7b). Our findings are in accord with recent observations that the α7-nAChR regulates the synthesis and release of noradrenaline and adrenaline in small airway epithelial cells (30) and colon cancer cells (31) and suggest this autocrine catecholamine loop as a novel target for pancreatic cancer intervention. This interpretation is supported by our findings that multiple phosphorylated signaling proteins that are frequently overexpressed in pancreatic cancer were simultaneously induced in each of the investigated cell lines by exposure to nicotine while the beta-blocker propranolol reversed these responses and additionally reduced the phosphorylation of all investigated signaling protein levels below base levels. These findings indicate that the observed phosphorylation of signaling proteins were events downstream of beta-adrenergic receptors. Inhibitors of ERK, Src, AKT and EGFR tyrosine kinases alone or in combination are currently being explored as "targeted therapeutics' for pancreatic cancer, an approach that necessitates treatment of the patient with multiple inhibitors (2, 3). Our current data suggest single agent therapy with the beta-blocker propranolol as a novel alternative to this strategy.
While CREB, Src and AKT are traditionally considered downstream effectors of the EGFR pathway in pancreatic cancer (3), in vitro studies with pancreatic cancer cell lines and immortalized pancreatic duct epithelia have shown that ERK is also phosphorylated simultaneously with CREB in response to beta-adrenergic agonists following the PKA-dependent transactivation of the EGFR (19). In addition, studies in ovarian cancer cells have identified the phosphorylation of Src in these cells as a cAMP-dependent event in response to stress neurotransmitters (32).
The nitrosated carcinogenic nitrosamine NNK is a nAChR agonist with a thousand-fold higher affinity to the α7-nAChR than nicotine (24, 29). In the current experiments, exposures of the cells to NNK were therefore conducted at a 1 nM concentration as opposed to 1 µM nicotine used in the accompanying experiments. In turn, the documented lower affinity of acetylcholine to nAChRs than nicotine (14) was the reason why we exposed our cell lines to 10 µM acetylcholine. As our western blots show, the induction of the investigated signaling proteins was similar with all three agents at the concentrations used. Exposure of the cells to 1 µM nicotine for 30 minutes additionally upregulated the protein expression of nAChRs in the investigated three cell lines. This response is in accord with the reported rapid increase of nAChR numbers in response to nicotine or other agonists (33). Similar effects of nicotine on the α7-nAChR have been described in the brain and are thought to be caused by post-translational and post-transcriptional mechanisms (34).
Smoking is a documented risk factor for the development of pancreatic cancer (4). However, this malignancy also develops in a significant number of nonsmokers. While some of these cases are preceded by diabetes or pancreatitis, two additional known risk factors for pancreatic cancer (4, 35), our current findings suggest that psychological stress may also contribute to the development and progression of this disease. While in our experiments the production of catecholamines and resulting induction of multiple signaling proteins that regulate cell proliferation, migration and apoptosis was induced by the exogenous addition of nAChR agonists to the cells, psychological stress triggers a systemic increase in noradrenaline and adrenaline via activation of the pituitary/adrenal system (15). In analogy to reports that experimentally induced psychological stress promotes the growth and metastasis of ovarian cancer via beta-adrenergic, cAMP-dependent signaling (36), psychological stress may therefore also facilitate the development of pancreatic cancer and promote the progression of this malignancy, thus impairing therapeutic outcomes (37, 38). However, further studies are needed to address this potential aspect of pancreatic cancer regulation.
In summary, our data suggest that the autocrine catecholamine loop expressed in pancreatic cancer cells and in normal pancreatic duct epithelial cells that is jointly governed by the α3, α5, and α7-nAChRs and β-ARs as an important regulatory network that controls multiple signal transduction pathways known to be hyperactive in pancreatic cancer. As shown in Fig. 7, this entire cascade could theoretically be inhibited by beta-blockers, or by agents that inhibit the activation of adenylyl cyclase and associated formation of cAMP. While the α7-nAChR has been suggested as a drug target for the therapy of non-small cell lung cancer (39), the vital functions of this receptor in the nervous system renders the use of α7-nAChR antagonists for cancer therapy problematic. On the other hand, beta-blockers have been safely used for decades as cardiovascular therapeutics and the beta-blocker propranolol prevented the development of NNK-induced pancreatic cancer in hamsters (38). A recent report has also identified significantly better clinical outcomes in breast cancer patients treated with beta-blockers (40). These findings are in accord with data that have shown stimulation of breast cancer cell proliferation by beta-adrenergic agonists in vitro (41, 42). In addition, it has been shown that γ-aminobutyric acid (GABA) inhibited the proliferation and migration of pancreatic cancer cells in vitro via GABA-B receptor mediated inhibition of adenylyl cyclase (20). GABA also reversed the growth promoting effects of nicotine on pancreatic cancer xenografts by reducing tumor cAMP levels (28). GABA has been safely used as a nutritional supplement for many years and selective GABA-B-receptor agonists are widely used for the pharmacological management of spastic pain after spinal injuries and spinal surgery. Further studies are now warranted to explore the potential usefulness of these agents for the improvement of clinical outcomes in pancreatic cancer therapy.
Acknowledgments
Financial Support: National Cancer Institute (R01CA042829, RO1CA130888)
Footnotes
Conflict of Interest: None to declare
References
- 1.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 2.Almhanna K, Philip PA. Defining New Paradigms for the Treatment of Pancreatic Cancer. Curr Treat Options Oncol. 2011 doi: 10.1007/s11864-011-0150-8. (ahead of print) [DOI] [PubMed] [Google Scholar]
- 3.Strimpakos A, Saif MW, Syrigos KN. Pancreatic cancer: from molecular pathogenesis to targeted therapy. Cancer Metastasis Rev. 2008;27:495–522. doi: 10.1007/s10555-008-9134-y. [DOI] [PubMed] [Google Scholar]
- 4.Silverman DT. Risk factors for pancreatic cancer: a case-control study based on direct interviews. Teratog Carcinog Mutagen. 2001;21:7–25. doi: 10.1002/1520-6866(2001)21:1<7::aid-tcm3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Wittel UA, Hopt UT, Batra SK. Cigarette smoke-induced pancreatic damage: experimental data. Langenbecks Arch Surg. 2008;393:581–588. doi: 10.1007/s00423-007-0273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hecht SS. Cigarette smoking: cancer risks, carcinogens, and mechanisms. Langenbecks Arch Surg. 2006;391:603–613. doi: 10.1007/s00423-006-0111-z. [DOI] [PubMed] [Google Scholar]
- 7.Rivenson A, Hoffmann D, Prokopczyk B, Amin S, Hecht SS. Induction of lung and exocrine pancreas tumors in F344 rats by tobacco-specific and Areca-derived N-nitrosamines. Cancer Res. 1988;48:6912–6917. [PubMed] [Google Scholar]
- 8.Schuller HM, Jorquera R, Reichert A, Castonguay A. Transplacental induction of pancreas tumors in hamsters by ethanol and the tobacco-specific nitrosamine 4- (methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 1993;53:2498–2501. [PubMed] [Google Scholar]
- 9.Jull BA, Plummer HK, 3rd, Schuller HM. Nicotinic receptor-mediated activation by the tobacco-specific nitrosamine NNK of a Raf-1/MAP kinase pathway, resulting in phosphorylation of c-myc in human small cell lung carcinoma cells and pulmonary neuroendocrine cells. J Cancer Res Clin Oncol. 2001;127:707–717. doi: 10.1007/s004320100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dasgupta P, Chellappan SP. Nicotine-mediated cell proliferation and angiogenesis: new twists to an old story. Cell Cycle. 2006;5:2324–2328. doi: 10.4161/cc.5.20.3366. [DOI] [PubMed] [Google Scholar]
- 11.Davis R, Rizwani W, Banerjee S, et al. Nicotine promotes tumor growth and metastasis in mouse models of lung cancer. PLoS One. 2009;4:e7524. doi: 10.1371/journal.pone.0007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chipitsyna G, Gong Q, Anandanadesan R, et al. Induction of osteopontin expression by nicotine and cigarette smoke in the pancreas and pancreatic ductal adenocarcinoma cells. Int J Cancer. 2009;125:276–285. doi: 10.1002/ijc.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154:1558–1571. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindstrom J, Anand R, Gerzanich V, Peng X, Wang F, Wells G. Structure and function of neuronal nicotinic acetylcholine receptors. Prog Brain Res. 1996;109:125–137. doi: 10.1016/s0079-6123(08)62094-4. [DOI] [PubMed] [Google Scholar]
- 15.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 16.Wallukat G. The beta-adrenergic receptors. Herz. 2002;27:683–690. doi: 10.1007/s00059-002-2434-z. [DOI] [PubMed] [Google Scholar]
- 17.Lefkowitz RJ. The superfamily of heptahelical receptors. Nat Cell Biol. 2000;2:E133–E136. doi: 10.1038/35017152. [DOI] [PubMed] [Google Scholar]
- 18.Weddle DL, Tithoff P, Williams M, Schuller HM. Beta-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis. 2001;22:473–479. doi: 10.1093/carcin/22.3.473. [DOI] [PubMed] [Google Scholar]
- 19.Askari MD, Tsao MS, Schuller HM. The tobacco-specific carcinogen, 4- (methylnitrosamino)-1-(3-pyridyl)-1-butanone stimulates proliferation of immortalized human pancreatic duct epithelia through beta-adrenergic transactivation of EGF receptors. J Cancer Res Clin Oncol. 2005;131:639–648. doi: 10.1007/s00432-005-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuller HM, Al-Wadei HA, Majidi M. GABA(B) receptor is a novel drug target for pancreatic cancer. Cancer. 2008;112:767–778. doi: 10.1002/cncr.23231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNamee EN, Ryan KM, Griffin EW, et al. Noradrenaline acting at central beta-adrenoceptors induces interleukin-10 and suppressor of cytokine signaling-3 expression in rat brain: implications for neurodegeneration. Brain Behav Immun. 24:660–671. doi: 10.1016/j.bbi.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Yokoi K, Hawke D, Oborn CJ, et al. Identification and validation of SRC and phospho- SRC family proteins in circulating mononuclear cells as novel biomarkers for pancreatic cancer. Transl Oncol. 2011;4:83–91. doi: 10.1593/tlo.10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsons CM, Muilenburg D, Bowles TL, Virudachalam S, Bold RJ. The role of Akt activation in the response to chemotherapy in pancreatic cancer. Anticancer Res. 2010;30:3279–3289. [PMC free article] [PubMed] [Google Scholar]
- 24.Schuller HM, Orloff M. Tobacco-specific carcinogenic nitrosamines. Ligands for nicotinic acetylcholine receptors in human lung cancer cells. Biochem Pharmacol. 1998;55:1377–1384. doi: 10.1016/s0006-2952(97)00651-5. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann BB. Catecholamines and symathomimetic drugs. In: Goodman-Gilman A, Rall TW, Nies AS, Taylor P, editors. The Pharmacological Basis of Therapeutics. New York: Pergamon Press; 1990. pp. 187–220. [Google Scholar]
- 26.Benowitz NL. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Progr Cardiovasc Dis. 2003;46:91–111. doi: 10.1016/s0033-0620(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 27.Benowitz NL. Neurobiology of nicotine addiction: implications for smoking cessation treatment. Am J Med. 2008;121:S3–S10. doi: 10.1016/j.amjmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Al-Wadei HA, Plummer HK, 3rd, Schuller HM. Nicotine stimulates pancreatic cancer xenografts by systemic increase in stress neurotransmitters and suppression of the inhibitory neurotransmitter gamma-aminobutyric acid. Carcinogenesis. 2009;30:506–511. doi: 10.1093/carcin/bgp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arredondo J, Chernyavsky AI, Grando SA. Nicotinic receptors mediate tumorigenic action of tobacco-derived nitrosamines on immortalized oral epithelial cells. Cancer Biol Ther. 2006;5:511–517. doi: 10.4161/cbt.5.5.2601. [DOI] [PubMed] [Google Scholar]
- 30.Al-Wadei HA, Al-Wadei MH, Masi T, Schuller HM. Chronic exposure to estrogen and the tobacco carcinogen NNK cooperatively modulates nicotinic receptors in small airway epithelial cells. Lung Cancer. 2009;69:33–39. doi: 10.1016/j.lungcan.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Wong HP, Yu L, Lam EK, Tai EK, Wu WK, Cho CH. Nicotine promotes cell proliferation via alpha7-nicotinic acetylcholine receptor and catecholamine-synthesizing enzymes-mediated pathway in human colon adenocarcinoma HT-29 cells. Toxicol Appl Pharmacol. 2007;221:261–267. doi: 10.1016/j.taap.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson MB, Armaiz-Pena G, Takahashi R, et al. Stress hormones regulate interleukin-6 expression by ovarian carcinoma cells through a SRC-dependent mechanism. J Biol Chem. 2007;282:29919. doi: 10.1074/jbc.M611539200. [DOI] [PubMed] [Google Scholar]
- 33.Gopalakrishnan M, Molinari EJ, P SJ. Regulation of human alpha4beta2 neuronal nicotinic acetylcholine receptors by cholinergic channel ligands and second messenger pathways. Molec Pharmacol. 1997;52:524–534. [PubMed] [Google Scholar]
- 34.Govind AP, Vezina P, Green WN. Nicotine-induced upregulation of nicotinic receptors: Underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol. 2009;78:756–765. doi: 10.1016/j.bcp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowenfels AB, Maisonneuve P. Risk factors for pancreatic cancer. J Cell Biochem. 2005;95:649–656. doi: 10.1002/jcb.20461. [DOI] [PubMed] [Google Scholar]
- 36.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 37.Schuller HM. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat Rev Cancer. 2009;9:195–205. doi: 10.1038/nrc2590. [DOI] [PubMed] [Google Scholar]
- 38.Schuller HM, Al-Wadei HA. Neurotransmitter receptors as central regulators of pancreatic cancer. Future Oncol. 2010;6:221–228. doi: 10.2217/fon.09.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paleari L, Cesario A, Fini M, Russo P. alpha7-Nicotinic receptor antagonists at the beginning of a clinical era for NSCLC and mesothelioma? Drug Discov Today. 2009;14:822–836. doi: 10.1016/j.drudis.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Powe DG, Voss MJ, Zanker KS, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628–638. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cakir Y, Plummer HK, 3rd, Tithof PK, Schuller HM. Beta-adrenergic and arachidonic acid-mediated growth regulation of human breast cancer cell lines. Int J Oncol. 2002;21:153–157. [PubMed] [Google Scholar]
- 42.Plummer HK, 3rd, Yu Q, Cakir Y, Schuller HM. Expression of inwardly rectifying potassium channels (GIRKs) and beta-adrenergic regulation of breast cancer cell lines. BMC Cancer. 2004;4:93. doi: 10.1186/1471-2407-4-93. [DOI] [PMC free article] [PubMed] [Google Scholar]