Abstract
Attentional biases have been proposed to contribute to symptom maintenance in Posttraumatic Stress Disorder (PTSD), although the neural correlates of these processes have not been well defined; this was the goal of the present study. We administered an attention bias task, the dot probe, to a sample of 37 (19 control, 18 PTSD+) traumatized African-American adults during fMRI. Compared to controls, PTSD+ participants demonstrated increased activation in the dorsolateral prefrontal cortex (dlPFC) in response to threat cue trials. In addition, attentional avoidance of threat corresponded with increased ventrolateral prefrontal cortex (vlPFC) and dorsal anterior cingulate cortex (dACC) activation in the PTSD group, a pattern that was not observed in controls. These data provide evidence to suggest that relative increases in dlPFC, dACC and vlPFC activation represent neural markers of attentional bias for threat in individuals with PTSD, reflecting selective disruptions in attentional control and emotion processing networks in this disorder.
INDEX WORDS: Attention bias, PTSD, Threat, fMRI, Prefrontal cortex, Neuroimaging, Posttraumatic Stress Disorder, Anterior cingulate cortex, Dorsolateral prefrontal cortex, Cognition
1. Introduction
Emotion processing theories (Foa & Kozak, 1986) suggest that Posttraumatic Stress Disorder (PTSD), a condition that develops in a minority of psychologically traumatized individuals, is characterized by biases in information processing, including attention (Weber, 2008). Attention biases to emotional or trauma-related information may serve to maintain PTSD symptoms, leading to neglect of important environmental information and disrupting downstream cognitive processes.
The dot probe task (Mogg & Bradley, 1999) is an attention bias paradigm that offers advantages over frequently-used bias tasks such as the Stroop, allowing examination of direction of bias (toward or away from the cue). The few existing dot probe studies have yielded mixed findings (Bryant & Harvey, 1997; Dalgleish et al., 2003; Elsesser, Sartory, & Tackenberg, 2004; Pine et al., 2005; Fani et al., 2010), indicating biases toward threat (Bryant & Harvey, 1997; Fani, Tone, Phifer et al., 2011), away from threat (Pine et al., 2005), toward happy facial expressions (Fani, et al., 2010), and inconsistent or non-significant patterns of bias (Dalgleish, et al., 2003; Elsesser, et al., 2004, 2005) in different populations with PTSD. One possible explanation for the discrepancies among these findings is variability in the emotional salience of stimuli. Stimuli are likely to differ in the responses they elicit from viewers; for traumatized individuals, stimuli that are too general or too loosely related to their own traumas may be less effective than more trauma-relevant (and presumably, more arousing) stimuli in evoking attentional biases. These findings indicate that precise, adaptable attention bias measures carefully tailored to the population under study are needed to properly detect any existing biases.
Behavioral methods, however, represent only one way to measure attention bias. More objective methods, including recordings of neural responses during functional neuroimaging, provide an additional way to characterize attention bias in individuals with PTSD, and can be used to detect abnormalities in attention to emotional cues that may not be detected behaviorally. Functional neuroimaging studies employing selective attention paradigms have shed light on specific neural networks that are likely to be engaged during attention bias task performance; these studies have underscored the roles of limbic systems, and dorsal and ventral components of frontal systems, as individuals focus attention to targets and attempt to ignore distracting information. Among the regions highlighted most frequently are the anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (dlPFC), inferior frontal gyrus, and amygdala (for reviews, see Banich et al., 2009 and Bishop, 2008). Attentional tasks that require conflict monitoring and implementation of cognitive control tend to engage dlPFC and ACC regions, and activation in these regions appears to correspond with increases in task demands (Mitchell, 2010). Specifically, increased activation has been observed in dorsal aspects of the ACC (dACC) during attention to neutral task targets (Bush et al., 1998; Whalen et al., 1998), whereas emotionally-valenced distractors tend to engage ventral brain systems, including the ventral ACC (vACC; Mohanty et al., 2007), inferior frontal gyrus, including ventrolateral aspects of the PFC (vlPFC; Monk et al., 2006; Yamasaki, LaBar, & McCarthy, 2002), and amygdala (Yamasaki, et al., 2002). The amygdala is critically involved with rapid detection of emotionally-salient material, particularly, cues that signal threat (Ledoux & Muller, 1997); this region has connections to both dorsal and ventral prefrontal regions, which serve to modulate its response (for a review, see Ochsner & Gross, 2005). There is evidence to suggest that the amygdala demonstrates a heightened response to threat-relevant cues, even when these cues are not the focus of attention (Dolan & Vuilleumier, 2003). To summarize, the ACC, amygdala, dlPFC and vlPFC are key constituents within dorsal and ventral attention networks; these regions are differentially engaged during performance on tasks that require cognitive control in the face of distracting information.
Anxious psychopathology has been associated with disrupted function in these attentional systems. Dot probe studies of anxious individuals have indicated that threat biases correspond with activation in these dorsal and ventral networks, although findings have been somewhat inconsistent. Some authors have found anxiety to correspond with increased activation in the dlPFC to contrast conditions representing threat bias (Telzer et al., 2008), whereas others have found increased activation in the vlPFC (Britton et al., 2011; Monk, et al., 2006) and amygdala (Monk et al., 2008); in two of these studies, anxiety corresponded with an attention bias away from threat (Britton, et al., 2011; Monk, et al., 2006).
The few selective attention studies of PTSD populations have indicated the involvement of the ACC, dlPFC, and amygdala during task engagement, with considerable variability in magnitude and direction of findings, which could reflect the different types of distractor stimuli (emotional vs. neutral) included in these tasks (Bremner et al., 2004; Bryant et al., 2005; Felmingham et al., 2009; Pannu Hayes et al., 2009; Shin et al., 2001). Taken together, these studies confirm the involvement of ACC, dlPFC, vlPFC and amygdala during selective attention processes in PTSD populations; however, none of these studies were equipped to examine the attentional strategies that were deployed during task performance.
Therefore, this study was designed to examine attention biases in PTSD, manifest through behavioral response and neural response; we employed a dot probe task (Mogg & Bradley, 1999) that has been adapted for use with our highly-traumatized African-American population while examining concurrent neural responses using fMRI. We used photographs of angry, neutral, and happy emotional facial expressions as dot probe stimuli, given that: facial expressions are biologically salient signals in human communication (Ekman & Oster, 1979); angry facial expressions are relevant threat signals for this group of traumatized individuals, considering the high rates of interpersonal trauma experienced by participants in this population (Gillespie et al., 2009; Schwartz et al., 2005). We included photographs of African-American, as well as Caucasian, models to increase stimulus relevance for our study population.
Associations among patterns of attention bias, neural response, and PTSD were examined in this sample of trauma-exposed adults. Given the current data on neural correlates of selective attention processes in both healthy and anxious populations, we chose the ACC, dlPFC, vlPFC and amygdala as regions of interest (ROIs). We hypothesized that: 1) current PTSD would be associated with a significant attentional bias toward threat, measured through behavioral response; 2) in response to contrast conditions corresponding with attention bias to threat, individuals with PTSD would exhibit increased activation in the vlPFC and amygdala, and decreased activation in the dlPFC and dACC, compared to traumatized controls 3) an attention bias for threat (either toward or away from the cue) would correspond with greater activation in the vlPFC and amygdala in individuals with PTSD, versus controls
2. Method
2.1 Participants
Study procedures were approved by the institutional review boards of Emory and Georgia State Universities. A total of 48 adult females aged 20–62 years were recruited through an ongoing study of risk factors for PTSD; they were approached in general medical clinics of a publicly funded hospital that serves economically-disadvantaged individuals in inner-city Atlanta. Patients attending these clinics have been found to exhibit high rates of interpersonal trauma and post-traumatic symptoms that vary considerably in severity, as evidenced by previous studies sampling this population (Bradley et al., 2008; Schwartz, et al., 2005). Given that all face pairs in the attention bias task were of female faces, only female participants were recruited to provide an implicit control for potential gender effects on attentional biases.
Patients were deemed eligible for participation if they were able and willing to give informed consent and understand English, as determined by a study researcher. Participants were initially screened to assess for the presence of these exclusion criteria: current psychotropic medication use, current alcohol or substance abuse or dependence, medical or physical conditions that preclude MRI scanning (e.g., metal implants), a history of schizophrenia or other psychotic disorder, medical conditions that contribute significantly to psychiatric symptoms (such as dementia), history of head injury or loss of consciousness for longer than 5 minutes, or a history of neurological illness. They were given clinical assessments during a separate appointment. Table 1 details sample demographics and clinical characteristics.
Table 1.
Demographic and Clinical Characteristics
| Trauma Control (n=19) | PTSD (n=18) | ||
|---|---|---|---|
|
| |||
| Mean (SD) | Mean (SD) | t | |
| Age | 38 (13.1) | 34.7 (13.7) | .73 |
| PSS re-experiencing | 1.4 (1.8) | 5.3 (2.1) | −5.99** |
| PSS avoidance and numbing | 2.4 (3.3) | 10.1 (5.2) | −5.34** |
| PSS hyperarousal | 2.6 (3.5) | 8.1 (3.1) | −4.92** |
| PSS total | 6.4 (7.7) | 23.6 (8.3) | −6.34** |
| BDI total | 7.6 (6.8) | 16.8 (8.9) | −3.49* |
| TEI total | 4.4 (3.2) | 4 (1.7) | .44 |
| % | % | χ2/Cramer’s V | |
| Education | 4.3 | ||
| < 12th grade | 12.1 | 9.1 | |
| 12th grade/high school graduate | 21.2 | 12.1 | |
| GED | 3 | 0 | |
| Some college/technical school | 12.1 | 12.1 | |
| College/tech school graduate | 9.1 | 9.1 | |
| Monthly Income | 2.46 | ||
| $0 – 249 | 6.3 | 6.3 | |
| $250 – 499 | 6.3 | 9.4 | |
| $500 – 999 | 25 | 9.4 | |
| $1000–1999 | 12.5 | 15.6 | |
| $2000+ | 6.3 | 3.1 | |
p < .05
p < .01
2.2 Trauma and Symptom Assessment
At initial assessment, participants were administered the Traumatic Events Inventory (TEI) to detail frequency and type of trauma(s) experienced; consistent with prior research (Binder et al., 2008; Gillespie, et al., 2009), total level of trauma exposure was measured by a sum score reflecting the total number of different types of trauma (e.g., car accident, sexual assault, natural disaster) to which a participant had been exposed over the course of their life (TEI total score). The Beck Depression Inventory (BDI-II; Beck, Steer, & Brown, 1996) was administered to measure current depressive symptoms. The PTSD Symptom Scale (PSS; Falsetti et al., 1993) was administered to assess for the presence of PTSD based on DSM-IV criteria, similar to earlier studies (Fani et al., 2011; Jovanovic et al., 2010); based on these criteria, participants were classified as either trauma controls (TC) or PTSD+. Table 1 details the clinical attributes of this sample.
2.3 Task Description and Behavioral Data Analyses
A dot probe task (Mogg & Bradley, 1999) was presented during neuroimaging using E-prime software, version 1.1. Each trial began with the presentation of a central fixation cross for 500 ms, immediately followed by a pair of face photographs (both of the same model) that were presented simultaneously for 500 ms (see Figure 1). In each face pair, one face displays an emotional expression (either threatening or happy) and the other a neutral expression. After the offset of the face pair, an asterisk is presented in place of one of the faces for 1100ms. Participants indicate as quickly as possible with a forced-choice button press response whether the asterisk appeared on the left- or right-hand side of the screen. The probe appears on left or right side of the screen an equal number of times. To facilitate investigation of between-group differences in neural response to threatening, happy, and neutral faces (posed by either an African-American or Caucasian model, all female), forty blank trials were also presented as implicit baseline trials. All face pairs represented the same model. This task consisted of 200 randomly-ordered trials (64 positive-neutral face pairs, 64 threat-neutral face pairs, 32 neutral-neutral face pairs, and 40 blank trials). The faces used in this task were selected from three separate sets of stimuli; African-American faces were selected from the Center for Productive Aging (Minear & Park, 2004) and NimStim (Tottenham et al., 2009) databases and White faces were selected from a commonly-used version of the dot probe (Bradley, Mogg, & Lee, 1997). A total of 50% African-American and 50% Caucasian face pairs were used in this version of the dot probe.
Figure 1.
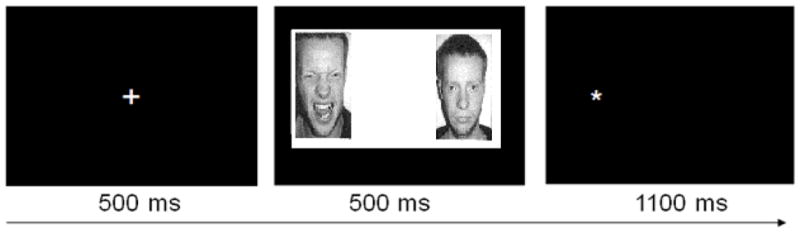
Schematic representation of dot probe trial structure
Emotion bias scores were calculated by subtracting response time to emotion-congruent stimuli (probes that replace neutral pictures) from response time to emotion-incongruent stimuli (probes that replace happy or angry/threatening pictures); these scores were further decomposed into threat and happy bias scores, both for all stimuli of each emotion type combined and separately for African-American (AA) and Caucasian (C) face pairs. Two multivariate analyses of variance (MANOVA) were conducted to examine potential differences in response to threatening, happy, and neutral cues between PTSD and TC groups. The first model examined between-group differences in mean response time to threat, happy, and neutral probes, and the second model examined between-group differences in threat bias score (overall, and separated by face race). Multivariate correlations were computed to examine correlations between attention bias scores (threat or happy bias) and BDI, TEI total and PSS total and subscale scores (re-experiencing, avoidance/numbing, and hyperarousal). A threshold of p <.05 was used to determine statistical significance for all behavioral data analyses.
2.4 MRI procedures
Scanning took place in a Siemens 3-Tesla scanner at Emory University Hospital. Participants viewed task stimuli via an adjustable mirror affixed to the 12 channel radiofrequency coil, which reflected a computer screen located at the end of the MRI aperture.
Following a shimming procedure and short calibration scan, a high-resolution T1-weighted structural scan was acquired using a MPRAGE sequence (176 slices, field of view=256 mm cubic voxels; 1×1×1 mm slice; TR= 2600ms; TE= 3.02 ms; TI= 900ms; flip angle= 8 degrees). During task administration, a total of 26 contiguous echo-planar, T2-weighted images parallel to the anterior-posterior commissure line were acquired (TR=2530 msec; TE=30 msec; field of view=240 mm; 64×64 matrix; 3.75×3.75×4.0 mm voxel). Statistical Parametric Mapping, version 5 (SPM5, Wellcome Department of Neurology, London, UK: http://www.fil.ion.ucl.ac.uk/spm/) was used for file conversion, image pre-processing and statistical analyses. Functional images were slice-time corrected with a high-pass filter applied, realigned to the first image in the session to correct for motion. The mean of the realigned undistorted images was then co-registered with the structural T1 volume, spatially normalized to standardized Montreal Neurological Institute (MNI) space based on the position of the anterior and posterior commissure and, finally, smoothed with an 8mm FWHM Gaussian kernel.
Due to excessive motion (7), and/or brain parenchyma abnormalities (4), 11 participants were excluded, leaving a total of 37 participants for fMRI analyses (19 TCs, 18 PTSD+) to be included in analyses. One other participant was excluded from behavioral analyses due to a high number of missed trials on the dot probe (over 20%), leaving a total of 36 participants (19 TCs, 17 PTSD+) to be included in behavioral data analyses. To examine blood-oxygen-level dependent (BOLD) signal change to task stimuli, a first-level, fixed-effects analysis was conducted by creating vectors for onset time of each condition, including threat/neutral, happy/neutral, and neutral/neutral trials. The primary t-contrast for examining BOLD signal change corresponding to threat bias was threat/neutral versus happy/neutral and neutral/neutral face pair trials (combined); each event within this contrast included face pair presentation and probe. In order to create models for these comparisons, box-car functions using 1, −1 contrast conventions were used to indicate voxels that had a higher activation level for the contrast condition. Random-effects, between-groups analyses were conducted to compare brain-wide responses of PTSD+ and TC groups to threat/neutral versus happy/neutral and neutral/neutral face pair conditions (combined) using t-tests. Random-effects, voxel-wide regression analyses for each diagnostic group were also conducted, in which threat bias score served as a predictor of hemodynamic response for threat/neutral versus happy/neutral and neutral/neutral face pair conditions (combined). An additional regression analysis was conducted to examine the relationship between trauma exposure (TEI total score) and hemodynamic response to threat. A non-linear transformation (http://www.bioimagesuite.org/Mni2Tal/index.html) was used to convert coordinates from MNI to Talairach (Rajeevan & Papademetris), and a Talairach daemon (Lancaster et al., 2000) was used to localize anatomical coordinates of voxels associated with statistically significant patterns of BOLD activation. Two different statistical thresholds were used to evaluate fMRI findings. First, a statistical threshold of p< .005 (uncorrected) and an extent threshold of ≥ 5 voxels per cluster were used to determine significant activations in whole-brain t-tests. Next, small volume correction was applied to significant clusters of activation within a priori specified regions of interest (defined using standard anatomical criteria from the Talairach and Tournoux stereotaxic atlas; Talairach & Tournoux, 1988), and Family-wise Error (FWE) correction was applied in order to control for multiple comparisons within those regions; a p<.05SVC_FWE threshold, extent threshold of ≥ 5 voxels per cluster was used to determine statistical significance.
3. Results
3.1 Demographic Data
No significant differences in frequency of trauma exposure were found between PTSD and TC groups, as measured by TEI total score (p>.05). As expected, PTSD and TC groups demonstrated statistically significant differences in PSS total and subscale scores, as well as depressive symptoms, measured by BDI total score (p<.05; see Table 1). Multivariate correlations revealed that PTSD symptoms, as measured by PSS total and subscale scores, were not significantly correlated with age, depressive symptoms, or trauma incidence (p>.05).
3.2 Behavioral Results
There were no significant differences between PTSD and TC groups for mean response time or variable response time to probes. Distribution of threat bias scores met assumptions of normality, according to the Shapiro-Wilk statistic (p>.05). MANOVA results indicated no significant main effects of diagnosis on mean response time for threatening, happy, or neutral faces or mean threat bias score (p>.05). Compared to TCs, participants with PTSD were slightly faster when responding to probes preceded by threat cues (in either position on the screen), but this difference was not statistically significant (p>.05). Participants with PTSD demonstrated a non-significant bias away from threat represented in Caucasian faces (Mean bias score= −19.5, SD=52.5), compared to TCs (Mean bias score= −8.9, SD=54.9). Similarly, no significant correlations were observed between threat bias (overall, or for AA or C faces) and PSS, BDI, and TEI total score (p>.05). Table 2 details mean response times and threat bias scores for each diagnostic group.
Table 2.
Mean Response Times and Attention Bias Scores for PTSD and Trauma Control Groups (N=36)
| Group | N | MRT to Threat | Threat bias | AA face Threat Bias | C face Threat Bias |
|---|---|---|---|---|---|
| Control | 19 | 522.68 (79.84) | −8.67 (43.44) | −8.46 (49.14) | −8.88 (54.89) |
| PTSD | 17 | 515.85 (65.79) | −6.49 (41.26) | 6.5 (52.44) | −19.49 (52.5) |
AA = African American
C = Caucasian
3.3 fMRI Results
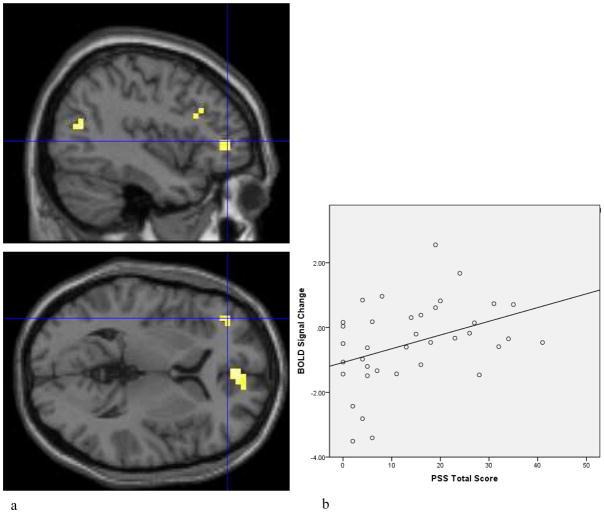
In a between-group comparison, participants with PTSD demonstrated increased neural activation to threat versus happy and neutral face pair trials in an a priori specified ROI, the dorsolateral prefrontal cortex (BA 46; p<.05SVC_FWE; see Table 3, Figure 2a); significant positive correlations were observed among dlPFC activation and PTSD symptoms, including PSS total (r=.39, p<.05; see Figure 2b), avoidance (r=.4, p<.05) and re-experiencing (r=.41, p<.05) symptoms. Compared to controls, PTSD+ individuals also demonstrated increased activation in the medial frontal gyrus, middle temporal gyrus and thalamus. Compared to PTSD+ individuals, trauma controls demonstrated no significant differences in activation within any ROIs, but demonstrated increased activation in the middle occipital, lingual and posterior cingulate gyri, as indicated in Table 3.
Table 3.
Anatomical Locations of Increased Activation in Response to Threatening versus Happy and Neutral Face Pair Trials (p<.005)
| PTSD versus TC group | ||||||
|---|---|---|---|---|---|---|
| x | y | z | k | t | Brodmann Area | Anatomical location |
| −44 | −65 | 18 | 12 | 3.71 | 39 | Middle temporal gyrus |
| 16 | −26 | 16 | 6 | 3.61 | Thalamus | |
| 4 | 51 | 1 | 10 | 3.37 | 10 | Medial frontal gyrus |
| −51 | −31 | 2 | 9 | 3.21 | 21 | Middle temporal gyrus |
| −40 | 43 | 2 | 5 | 3.03 | 46 | dlPFC |
| TC versus PTSD group | ||||||
| x | y | z | k | t | Brodmann Area | Anatomical location |
| −28 | −78 | −6 | 5 | 3.48 | 18 | Middle occipital gyrus |
| −16 | −54 | −1 | 12 | 3.44 | 19 | Lingual gyrus |
| 12 | −62 | 10 | 7 | 2.97 | 30 | Posterior cingulate |
| Correlation of threat bias scores with activation to threatening versus neutral and happy face pair trials | ||||||
| PTSD | ||||||
| Positive correlation | ||||||
| x | y | z | k | t | Brodmann Area | Anatomical location |
| −8 | −89 | 4 | 5 | 4 | 17 | Lingual gyrus |
| Negative correlation | ||||||
| x | y | z | k | t | Brodmann Area | Anatomical location |
| 20 | −56 | 47 | 10 | 3.67 | 7 | Precuneus |
| 20 | 6 | 48 | 10 | 3.28 | 6 | Medial frontal gyrus |
| −16 | 19 | −4 | 15 | 3.27 | Caudate | |
| −24 | 24 | 6 | 2.75 | Claustrum | ||
| 32 | −17 | 41 | 47 | 3.22 | 4 | Precentral gyrus |
| 36 | −10 | 37 | 3.09 | 6 | Precentral gyrus | |
| 32 | −45 | 32 | 2.90 | 40 | Parietal lobe | |
| −20 | −38 | 17 | 8 | 3.21 | Caudate | |
| −28 | 30 | −12 | 10 | 3.20 | 47 | vlPFC |
| −36 | −10 | 34 | 6 | 3.06 | 6 | Precentral gyrus |
| −44 | −27 | −2 | 5 | 3.06 | 22 | Insula |
| 51 | −10 | 37 | 11 | 3.05 | 4 | Precentral gyrus |
| −28 | −54 | 10 | 6 | 3.05 | 30 | Parahippocampal gyrus |
| −20 | −46 | 10 | 3.04 | 30 | Precentral gyrus | |
| 24 | −36 | 57 | 5 | 2.99 | 40 | Parietal lobe |
| 12 | −14 | 27 | 7 | 2.91 | Caudate | |
| −8 | 43 | 2 | 7 | 2.87 | 32 | Anterior cingulate cortex |
| TC | ||||||
| Positive Correlation | ||||||
| x | y | z | k | t | Brodmann Area | Anatomic Location |
| −20 | −8 | −13 | 7 | 3.76 | Parahippocampal gyrus | |
| −51 | −24 | −6 | 14 | 3.54 | 21 | Middle temporal gyrus |
| −51 | −12 | −9 | 3.50 | 22 | Superior temporal gyrus | |
| −59 | −43 | 2 | 6 | 3.41 | 21 | Middle temporal gyrus |
| −59 | −50 | 10 | 3.21 | 22 | Superior temporal gyrus | |
| Correlation of TEI score with activation to threatening versus neutral and happy face pair trials | ||||||
| Positive Correlation | ||||||
| x | y | z | k | t | Brodmann Area | Anatomic Location |
| 20 | −66 | −7 | 91 | 6.44 | 19 | Lingual gyrus |
| 32 | −70 | −10 | 6.20 | 18 | Lingual gyrus | |
| 12 | −74 | 0 | 4.18 | 18 | Lingual gyrus | |
| −28 | −67 | −10 | 150 | 5.54 | 19 | Fusiform gyrus |
| 4 | −65 | 14 | 5.33 | 31 | Posterior cingulate | |
| −12 | −69 | 18 | 4.56 | 18 | Cuneus | |
| −16 | −47 | −4 | 26 | 5.40 | Cerebellum | |
| −24 | −77 | 19 | 47 | 5.17 | 18 | Cuneus |
| −28 | −89 | 12 | 3.63 | 18 | Middle occipital gyrus | |
| 20 | −88 | 19 | 58 | 4.51 | 18 | Cuneus |
| 28 | −65 | 25 | 3.53 | 31 | Precuneus | |
| 12 | −84 | 23 | 3.19 | 18 | Cuneus | |
| 20 | −47 | −1 | 18 | 3.79 | 19 | Parahippocampal gyrus |
| 20 | −58 | 7 | 3.35 | 30 | Posterior cingulate | |
| Negative Correlation | ||||||
| x | y | z | k | t | Brodmann Area | Anatomic Location |
| −48 | −20 | −16 | 5 | 3.04 | 20 | Inferior temporal gyrus |
dlPFC = dorsolateral prefrontal cortex
vlPFC = ventrolateral prefrontal cortex
Figure 2.
a) Statistical parametric map of increased neural activation in the dorsolateral prefrontal cortex (dlPFC; Talairach x = −40, y = 43, z = 2, t= 3.03) to threatening versus happy and neutral face pair trials in PTSD versus trauma control participants. Activation is shown overlaid onto an averaged structural MRI. Figure presented at p < 0.005 (uncorrected) threshold. b) Contrast values indicating increased dlPFC activation to threatening versus neutral and happy face pairs in association with PTSD symptoms (PSS total score; r =.39, p<.05).
Within-group analyses indicated that, in the PTSD group, no positive correlations were observed between threat bias score and BOLD response to threat in any ROIs, although a small cluster of activation was observed in the lingual gyrus. However, threat bias score negatively correlated with activation in the vlPFC (BA 47; p<.05SVC_FWE; see Figure 3a); threat bias score also negatively correlated with activation in a dorsal region of the ACC (BA 32; (p<.05SVC_FWE; see Figure 3b), in addition to other non-hypothesized regions, such as the medial frontal gyrus, insula, precuneus, caudate, precentral gyrus, parahippocampal gyrus, and parietal lobe. Within the vlPFC cluster, overall threat bias score (r= −.38, p=.13) and threat bias score for African-American faces (r= −.45, p=.07) negatively corresponded with BOLD signal change; however, these correlations did not reach statistical significance. In the TC group, no positive correlations were observed between threat bias score and BOLD response to threat in any ROIs, although significant clusters of activation were observed in the parahippocampal gyrus, and middle and superior temporal gyri. There were no statistically significant negative correlations between threat bias score and BOLD response to threat within the TC group.
Figure 3.
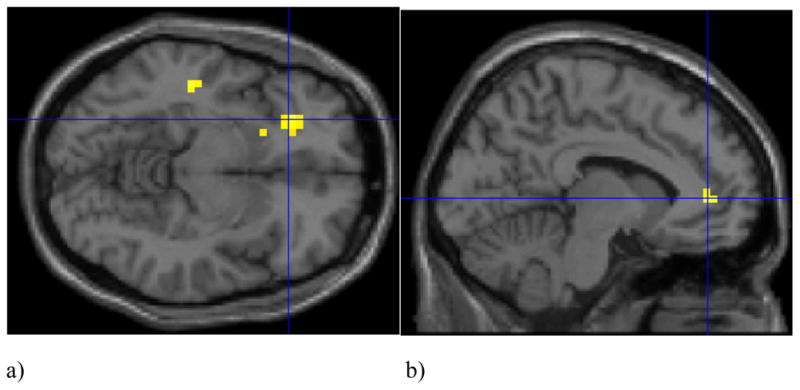
Statistical parametric maps indicating significant negative correlations between threat bias score and activation in the a) ventrolateral prefrontal cortex (vlPFC; Talairach x = −28, y = 30, z = −12, t= 3.2) and b) anterior cingulate cortex (ACC; Talairach x = −8, y = 43, z = 2, t=2.9) to threatening versus happy and neutral face pair trials in PTSD+ participants. Activations are shown overlaid onto an averaged structural MRI. Figure presented at p < 0.005 (uncorrected) threshold.
Trauma exposure (TEI total score) was not significantly correlated with activation to threat cues in any ROI. Trauma exposure was primarily positively correlated with BOLD signal in the visual cortex, and negatively correlated with activation in the inferior temporal gyrus (see Table 3).
4. Discussion
The present study examined associations between behavioral and neural correlates of attention bias for threat in a sample of traumatized individuals with and without PTSD. We observed that TC and PTSD groups demonstrated differential responses to threatening facial expressions in the context of this selective attention task. Relative to traumatized controls, individuals with PTSD showed increased activation in the dlPFC to threatening versus happy and neutral face pair trials. Among individuals with PTSD, threat bias score was negatively correlated with activation in the vlPFC and ACC to this contrast condition, whereas no significant correlations were observed between bias score and activation in any ROIs within the TC group.
No statistically significant between-group differences emerged in behavioral measures of attention bias; however, individuals with PTSD demonstrated a tendency to direct attention away from threatening Caucasian faces, relative to TCs.
Our hypothesis that PTSD and TC groups would demonstrate differential response in the dlPFC to threat cues in the context of this task was confirmed; however, the direction of dlPFC response contrasted with our predictions. The finding of increased dlPFC activation to threat cue trials in individuals with PTSD, relative to traumatized controls, is consistent with an earlier dot probe study of anxious individuals (Telzer, et al., 2008), and two studies of PTSD populations that used oddball paradigms (Bryant et al., 2005; Felmingham et al., 2009); in the latter two studies, the authors found that, during attention to target tones, PTSD groups similarly demonstrated increased activity in dorsal frontal regions, including the dACC and dlPFC, compared to controls.
One potential explanation for our finding of increased dlPFC response in PTSD+ participants relates to task demands. During this task, participants are instructed to attend to the location of neutral probes; as in the oddball task, participants are confronted with distractor images that have the potential to interfere with their attention to probes. It is plausible that angry emotional expressions presented in the context of this cognitive paradigm were distressing to this group of highly traumatized participants with PTSD. The act of responding quickly to neutral target images while being confronted with briefly-presented distracting images (particularly, images with emotional value or trauma-related salience) is likely to engage attentional control networks. Thus, the increased dlPFC activation we observed could reflect a higher expenditure of cognitive control resources to emotionally-evocative cues in individuals with clinically-significant PTSD, relative to traumatized individuals with little to no PTSD symptomatology.
Another explanation for these findings may be derived from recent conceptualizations of PTSD pathophysiology, which have highlighted the relevance of dorsal frontal networks in threat cue appraisal (reviewed in Etkin, Egner, & Kalisch, 2011). This framework indicates that specific components of the dorsal frontal network participate in emotional, as well as cognitive processing, and that these regions are directly related to the appraisal of fear-related cues (Etkin, et al., 2011). Further, some recent studies suggest that dorsal prefrontal regions may be positively coupled with limbic circuitry, and that these regions show similar enhancements in response during attention to threat-related cues; this was evident in one such study, which indicated that anxiety was associated with greater coupling of the dorsomedial prefrontal cortex and amygdala during biased attention for fearful facial expressions (Robinson et al., 2011). In the context of these findings, our observation of increased response to threat in the dlPFC, as well as a dorsomedial prefrontal region (BA 10), may reflect amplified threat evaluation circuits in this group of traumatized participants with post-traumatic psychopathology.
These conceptualizations have also outlined the salience of ventral prefrontal regions in the management of conflicting, or distracting, emotional information; recent reviews highlight the increasing number of studies that revealed altered function within these networks in PTSD populations (Etkin & Wager, 2007). We observed that, among individuals with PTSD, threat bias score was significantly, and negatively, correlated with BOLD signal in the vlPFC, indicating that activation in this region was associated with a bias away from threat. In comparison, there were no statistically significant associations between threat bias score and vlPFC activation in controls. Other lines of evidence have similarly observed anxiety-specific alterations in vlPFC activation during the processing of emotional distractors in selective attention tasks. Three other dot probe studies observed an increased vlPFC response to threatening facial expressions: two revealed corresponding associations between anxiety and threat bias (Britton, et al., 2011; Monk, et al., 2006), and the third found no significant anxiety-related differences in threat bias, measured behaviorally (Monk, et al., 2008). Increased vlPFC activation to distracting emotional information has been observed in selective attention (Yamasaki, et al., 2002) and response inhibition (Chiu, Holmes, & Pizzagalli, 2008) studies of healthy individuals, as well as working memory (Morey et al., 2008; Thomaes et al., 2011) studies of PTSD populations. Ventrolateral prefrontal regions have extensive connections with limbic areas, including the amygdala (Petrides & Pandya, 2002), and clearly participate in the processing of threatening or aversive cues; however, the functional role of the vlPFC in this network requires further clarification. Some lines of attention research suggest that the vlPFC participates in reflexive shifts of attention to biologically salient stimuli that are not the intended objects of attention (Corbetta & Shulman, 2002). Notably, some recent studies of attention and executive functioning have observed increased vlPFC activation in response to aversive or threatening emotional information in the context of cognitive interference tasks (e.g., Stroop tasks: Hart et al., 2010).
We also observed that, in individuals with PTSD, avoidant threat biases corresponded with increased activation in the dACC, which was an unexpected finding. Other studies have observed an increase in dorsal ACC response to distracting information in selective attention (Weissman et al., 2003) and interference paradigms (Egner et al., 2008). There appears to be increasing evidence to suggest that, during selective attention processes, this region is responsible for management of task-irrelevant material, irrespective of emotional valence. Given that increases in vlPFC and dACC activation corresponded with attentional avoidance in PTSD+, but not TC, individuals, these data reflect selective disruptions within networks that are responsible for managing task-irrelevant, distracting emotional information.
Behaviorally, no statistically significant differences in response time to threat cues were observed between diagnostic groups; however, individuals with PTSD demonstrated a tendency to avoid threat, represented in Caucasian faces. It is likely that power limitations precluded our ability to detect statistically significant associations among bias scores and PTSD symptoms; this relationship was evident in our earlier study, which included a considerably larger sample (Fani et al., 2011). Given that a non-significant association was observed between threat biases and trauma exposure, the inclusion of a non-traumatized control group would be useful toward disentangling the effects of trauma and PTSD on attention biases in this population. However, the presence of atypical neural response patterns to threatening faces in the absence of positive behavioral findings has been documented previously in some studies of anxious individuals (McClure et al., 2007; Monk, et al., 2008); thus, it is possible that the atypical responses observed in the dlPFC, dACC and vlPFC regions to threat represent biological markers of imbalanced attentional networks in PTSD. These data may suggest that more objective measures, such as fMRI, may be more sensitive than behavioral measures in detecting responses to threat cues presented in the context of attention bias tasks, particularly in the present population. Further, these neural correlates could potentially represent an intermediate neurocognitive phenotype, one that may be associated with allelic variations in genes that have been linked to risk for affective disorders (Domschke & Dannlowski, 2010); future investigations of associations between candidate genes for PTSD risk and neural response patterns during attention bias task performance are warranted to confirm this possibility.
Although associations between threat bias and activation in the parahippocampal region were common to both groups, our findings also indicated increased activation to threat in other brain regions, including the thalamus and middle temporal gyrus, in association with a PTSD diagnosis. Increased activation in temporal regions has been previously observed in PTSD participants during presentation of trauma-related reminders (Hopper et al., 2007; Lanius et al., 2002; Osuch et al., 2001), and the presence of these findings serves as a reminder that brain structures and regions frequently implicated in the disorder do not operate as isolated units, but in the context of functional systems. Middle temporal regions (particularly the hippocampus and parahippocampal gyrus) have been frequently implicated in dysfunctional encoding and memory retrieval in PTSD (Bremner, 2007), and some studies of attention and response inhibition in healthy individuals have similarly indicated that medial temporal regions are involved in these processes (Egner & Hirsch, 2005). These brain regions are worthwhile targets for investigation in future studies of attentional processes in PTSD.
There was no evidence for PTSD-related increases in amygdala function to threat cue trials in this study. A number of other studies have also failed to find any PTSD-specific alterations in amygdala activity to trauma-related cues (Bremner, Narayan, et al., 1999; Bremner, Staib, et al., 1999; Lanius, et al., 2002; Lanius et al., 2001; Sakamoto et al., 2005; Shin, et al., 2001). One possibility relates to the contextual demands of this attention bias task; other studies have similarly observed attenuated amygdala activation corresponding with increases in dlPFC activity in the presence of increasing cognitive processing load (Mitchell et al., 2007). In support of this notion, a recent meta-analysis of emotion processing neuroimaging studies revealed that amygdala response was attenuated in the context of increased attentional demands (Costafreda et al., 2008). Thus, in the present study, the increased dACC and dlPFC activation observed in concert with a lack of amygdala response may indicate efforts to overcompensate for emotional disruption caused by threatening facial expressions and disturbing trauma memories that these images might evoke. Further, the patterns of activation revealed in this study likely reflect processes engaged in response to the active attentional component of this task, unlike studies that simply required passive viewing of expressions of facial emotion. (Foa, Huppert, & Cahill, 2006).
Several study limitations are worth noting. First, the design of this study prohibited separate investigations of response to task targets and distractors; these two types of stimuli are likely to engage different components of attentional circuits. Although participants in this study represent an understudied population in the PTSD literature, the circumscribed demographic profile of this population may limit generalizability of these findings to other traumatized populations. In particular, this study included only female participants; given that only female face stimuli were used in this version of the dot probe, it was impossible to investigate potential interactive effects of gender and attentional biases. Similarly, a lack of White participants in this study precluded examination of stimulus- by participant-race interactions and their effects on attentional biases. Also, we did not investigate how trauma onset may interact with patterns of behavioral and neural response; given what is known about the deleterious cognitive and biological effects of early trauma exposure (Vermetten & Bremner, 2002), it is possible that individuals who first experienced trauma in childhood versus adulthood would exhibit different patterns of behavioral and neural response. Notably, lifetime trauma exposure did not relate to differential neural response to threat in any ROI. Thus, it does not appear that our findings are more relevant to cumulative trauma exposure than post-traumatic psychopathology; however, the addition of a non-traumatized control group would best permit differentiation of trauma- versus PTSD-specific effects on attentional biases. Finally, although we corrected for multiple comparisons within a priori specified ROIs, our (uncorrected) statistical threshold for whole-brain analyses (p<.005) may have increased risk for Type I error; thus, these findings warrant replication in future studies of attention bias using more conservative statistical thresholds.
The rich findings that emerged from this study have important implications for current information processing models of post-traumatic psychopathology. The alterations in dlPFC, ACC and vlPFC function observed in the present study complement findings from earlier studies of generally anxious individuals and illustrate their relevance to PTSD. The present data indicate enhanced activation in regions responsible for threat appraisal, control of attentional resources, and management of distracting emotional information during selective attention processing in PTSD. Disproportional allocation of cognitive control resources to emotional or trauma-relevant information perpetuates PTSD symptomatology by preventing adequate processing of other relevant environmental information and contemplative appraisal of the various thoughts and feelings associated with the trauma(s). This rigid attentional style can, in turn, lead to poor mental efficiency and impairment in cognitive processes such as working memory, since fewer cognitive resources will be available at any given time.
The investigation of attentional biases and associated dysregulation in neural systems in PTSD is a worthwhile endeavor, given the surprising lack of research in this area. The data presented here provide some insights into these processes that may guide or inform further research aimed at characterizing attentional biases in PTSD. Particularly, the present findings underscore the need for research utilizing a combination of techniques to detect attentional biases in individuals with this disorder. Finally, there is an unfortunate lack of research on economically underprivileged individuals, who experience a disproportionately high amount of trauma throughout their lives (Gillespie, et al., 2009; Schwartz, et al., 2005) but are typically not the focus of PTSD neuroimaging research. The inclusion of these groups in studies of information processing biases in PTSD is invaluable for informing appropriate treatments for this often neglected population.
Highlights.
Behavioral and neuroimaging methods were combined to examine attention bias in a traumatized sample
PTSD+ individuals demonstrated increased activation to threat in a cognitive control brain region
Threat cue avoidance corresponded with increased activation in an emotion processing region in PTSD
Disproportional allocation of cognitive control resources to trauma cues may perpetuate PTSD
Acknowledgments
This work was primarily supported by National Institutes of Mental Health (MH071537 to KJR) and (F32MH095456 to NF). Support was also received from Emory and Grady Memorial Hospital General Clinical Research Center, NIH National Centers for Research Resources (M01RR00039), the Burroughs Wellcome Fund (KJR), and the Georgia State University Brains and Behavior Fellowship (NF). We would like to thank Justine Phifer, Asante Kamkwalala, and Lauren Sands for their technical support, and Karin Mogg and Brendan Bradley for their assistance in developing the dot probe measure.
Footnotes
Financial Disclosures: All authors have no conflicts of interest, financial or otherwise, to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banich MT, Mackiewicz KL, Depue BE, Whitmer AJ, Miller GA, Heller W. Cognitive control mechanisms, emotion and memory: a neural perspective with implications for psychopathology. Neurosci Biobehav Rev. 2009;33(5):613–630. doi: 10.1016/j.neubiorev.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown G. Manual for Beck Depression Inventory II (BDI-II) San Antonio, Texas: Psychology Corporation; 1996. [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ. Neural mechanisms underlying selective attention to threat. Ann N Y Acad Sci. 2008;1129:141–152. doi: 10.1196/annals.1417.016. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Lee SC. Attentional biases for negative information in induced and naturally occurring dysphoria. Behav Res Ther. 1997;35(10):911–927. doi: 10.1016/s0005-7967(97)00053-3. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65(2):190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD. Functional neuroimaging in posttraumatic stress disorder. Exp Rev Neurotherapeutics. 2007;7:393–405. doi: 10.1586/14737175.7.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156(11):1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Vythilingam M, Afzal N, Schmahl C, Elzinga B, et al. Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biol Psychiatry. 2004;55(6):612–620. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Britton JC, Bar-Haim Y, Carver FW, Holroyd T, Norcross MA, Detloff A, et al. Isolating neural components of threat bias in pediatric anxiety. J Child Psychol Psychiatry. 2011 doi: 10.1111/j.1469-7610.2011.02503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA, Felmingham KL, Kemp AH, Barton M, Peduto AS, Rennie C, et al. Neural networks of information processing in posttraumatic stress disorder: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:111–118. doi: 10.1016/j.biopsych.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Harvey AG. Attentional bias in posttraumatic stress disorder. J Traum Stress. 1997;10:635–644. doi: 10.1023/a:1024849920494. [DOI] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL. The counting Stroop: an interference task specialized for functional neuroimaging--validation study with functional MRI. Hum Brain Mapp. 1998;6(4):270–282. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu PH, Holmes AJ, Pizzagalli DA. Dissociable recruitment of rostral anterior cingulate and inferior frontal cortex in emotional response inhibition. Neuroimage. 2008;42(2):988–997. doi: 10.1016/j.neuroimage.2008.04.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev. 2008;58(1):57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Dalgleish T, Taghavi R, Hamid N, Moradi A, Canterbury R, Yule W. Patterns of processing bias for emotional information across clinical disorders: a comparison of attention, memory, and prospective cognition in children and adolescents with depression, generalized anxiety, and Posttraumatic Stress Disorder. Journal of Clinical Child and Adolescent Psychology. 2003;32:10–21. doi: 10.1207/S15374424JCCP3201_02. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Vuilleumier P. Amygdala automaticity in emotional processing. Ann N Y Acad Sci. 2003;985:348–355. doi: 10.1111/j.1749-6632.2003.tb07093.x. [DOI] [PubMed] [Google Scholar]
- Domschke K, Dannlowski U. Imaging genetics of anxiety disorders. Neuroimage. 2010;53(3):822–831. doi: 10.1016/j.neuroimage.2009.11.042. [DOI] [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex. 2008;18(6):1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage. 2005;24(2):539–547. doi: 10.1016/j.neuroimage.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Ekman P, Oster H. Facial expressions of emotion. Annual Reviews in Psychology. 1979;30:527–554. [Google Scholar]
- Elsesser K, Sartory G, Tackenberg A. Attention, heart rate, and startle response during exposure to trauma-relevant pictures: a comparison of recent trauma victims and patients with posttraumatic stress disorder. Journal of Abnormal Psychology. 2004;113:289–301. doi: 10.1037/0021-843X.113.2.289. [DOI] [PubMed] [Google Scholar]
- Elsesser K, Sartory G, Tackenberg A. Initial symptoms and reactions to trauma-related stimuli and the development of posttraumatic stress disorder. Depression and Anxiety. 2005;21:61–70. doi: 10.1002/da.20047. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsetti SA, Resnick HS, Resick PA, Kilpatrick DG. The modified PTSD symptom scale: A brief self-report measure of posttraumatic stress disorder. Behav Ther. 1993;16:161–162. [Google Scholar]
- Fani N, Bradley RG, Ressler KJ, McClure-Tone EB. Attention bias in adult survivors of childhood maltreatment with and without posttraumatic stress disorder. Cognitive Therapy and Research. 2010;35(1):57–67. [Google Scholar]
- Fani N, Tone EB, Phifer J, Norrholm SD, Bradley B, Ressler KJ, et al. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol Med. 2011:1–11. doi: 10.1017/S0033291711001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham KL, Williams LM, Kemp AH, Rennie C, Gordon E, Bryant RA. Anterior cingulate activity to salient stimuli is modulated by autonomic arousal in posttraumatic stress disorder. Psychiatry Res. 2009;173(1):59–62. doi: 10.1016/j.pscychresns.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Foa EB, Huppert JD, Cahill SP. Emotional processing theory: An update. In: Rothbaum BO, editor. Pathological Anxiety: Emotional Processing in Etiology and Treatment. New York: Guilford Press; 2006. pp. 3–24. [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear: Exposure to corrective information. Psychological Bulletin. 1986;99:20–35. [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, et al. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry. 2009;31(6):505–514. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart SJ, Green SR, Casp M, Belger A. Emotional priming effects during Stroop task performance. Neuroimage. 2010;49(3):2662–2670. doi: 10.1016/j.neuroimage.2009.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper JW, Frewen PA, van der Kolk BA, Lanius RA. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J Trauma Stress. 2007;20(5):713–725. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, et al. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety. 2010;27(3):244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M, Neufeld RW, et al. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol Psychiatry. 2002;52(4):305–311. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, et al. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry. 2001;158(11):1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- Ledoux JE, Muller J. Emotional memory and psychopathology. Philos Trans R Soc Lond B Biol Sci. 1997;352(1362):1719–1726. doi: 10.1098/rstb.1997.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64(1):97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- Minear M, Park DC. A lifespan database of adult facial stimuli. Behav Res Methods Instrum Comput. 2004;36(4):630–633. doi: 10.3758/bf03206543. [DOI] [PubMed] [Google Scholar]
- Mitchell DG, Nakic M, Fridberg D, Kamel N, Pine DS, Blair RJ. The impact of processing load on emotion. Neuroimage. 2007;34(3):1299–1309. doi: 10.1016/j.neuroimage.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RL. Linear increases in BOLD response associated with increasing proportion of incongruent trials across time in a colour Stroop task. Exp Brain Res. 2010;203(1):193–204. doi: 10.1007/s00221-010-2225-3. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Some methodological issues in assessing attentional biases for threatening faces in anxiety: A replication study using a modified version of the probe detection task. Behav Res Ther. 1999;37:595–604. doi: 10.1016/s0005-7967(98)00158-2. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Engels AS, Herrington JD, Heller W, Ho MH, Banich MT, et al. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44(3):343–351. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163(6):1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65(5):568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Cooper DA, Labar KS, McCarthy G. Neural systems for executive and emotional processing are modulated by symptoms of posttraumatic stress disorder in Iraq War veterans. Psychiatry Res. 2008;162(1):59–72. doi: 10.1016/j.pscychresns.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Osuch EA, Benson B, Geraci M, Podell D, Herscovitch P, McCann UD, et al. Regional cerebral blood flow correlated with flashback intensity in patients with posttraumatic stress disorder. Biol Psychiatry. 2001;50(4):246–253. doi: 10.1016/s0006-3223(01)01107-6. [DOI] [PubMed] [Google Scholar]
- Pannu Hayes J, LaBar KS, Petty CM, McCarthy G, Morey RA. Alterations in the neural circuitry for emotion and attention associated with posttraumatic stress symptomatology. Psychiatry Research: Neuroimaging. 2009;172:7–15. doi: 10.1016/j.pscychresns.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci. 2002;16(2):291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Pine DS, Mogg K, Bradley BP, Montgomery L, Monk CS, McClure EB, et al. Attention bias to threat in maltreated children: Implications for vulnerability to stress-related psychopathology. American Journal of Psychiatry. 2005;162:291–296. doi: 10.1176/appi.ajp.162.2.291. [DOI] [PubMed] [Google Scholar]
- Rajeevan N, Papademetris X. MNI to Talairach coordinate converter. (Producer) retrieved from http://www.bioimagesuite.org/Mni2Tal/index.html.
- Robinson OJ, Charney DR, Overstreet C, Vytal K, Grillon C. The adaptive threat bias in anxiety: Amygdala-dorsomedial prefrontal cortex coupling and aversive amplification. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H, Fukuda R, Okuaki T, Rogers M, Kasai K, Machida T, et al. Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder: a functional MRI study. Neuroimage. 2005;26(3):813–821. doi: 10.1016/j.neuroimage.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Schwartz AC, Bradley RL, Sexton M, Sherry A, Ressler KJ. Posttraumatic stress disorder among African Americans in an inner city mental health clinic. Psychiatr Serv. 2005;56(2):212–215. doi: 10.1176/appi.ps.56.2.212. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: Three-dimensional proportional system. Stuttgart, Germany: Georg Thieme; 1988. [Google Scholar]
- Telzer EH, Mogg K, Bradley BP, Mai X, Ernst M, Pine DS, et al. Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biol Psychol. 2008;79(2):216–222. doi: 10.1016/j.biopsycho.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer N, de Ruiter MB, Elzinga BM, Sjoerds Z, et al. Increased anterior cingulate cortex and hippocampus activation in Complex PTSD during encoding of negative words. Soc Cogn Affect Neurosci. 2011 doi: 10.1093/scan/nsr084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermetten E, Bremner JD. Circuits and systems in stress. II. Applications to neurobiology and treatment in posttraumatic stress disorder. Depress Anxiety. 2002;16(1):14–38. doi: 10.1002/da.10017. [DOI] [PubMed] [Google Scholar]
- Weber DL. Information Processing Bias in Post-traumatic Stress Disorder. Open Neuroimag J. 2008;2:29–51. doi: 10.2174/1874440000802010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Giesbrecht B, Song AW, Mangun GR, Woldorff MG. Conflict monitoring in the human anterior cingulate cortex during selective attention to global and local object features. Neuroimage. 2003;19(4):1361–1368. doi: 10.1016/s1053-8119(03)00167-8. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, et al. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998;44(12):1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci U S A. 2002;99(17):11447–11451. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]