Abstract
Elevated tissue levels of angiotensin II (ANG II) are associated with impairment of insulin actions in metabolic and cardiovascular tissues. ANG II-stimulated activation of mammalian target of rapamycin (mTOR)/p70 S6 kinase (p70S6K) in cardiovascular tissues is implicated in cardiac hypertrophy and vascular remodeling. However, the role of ANG II-stimulated mTOR/p70S6K in vascular endothelium is poorly understood. In the present study, we observed that ANG II stimulated p70S6K in bovine aortic endothelial cells. ANG II increased phosphorylation of insulin receptor substrate-1 (IRS-1) at Ser636/639 and inhibited the insulin-stimulated phosphorylation of endothelial nitric oxide synthase (eNOS). An inhibitor of mTOR, rapamycin, attenuated the ANG II-stimulated phosphorylation of p70S6K and phosphorylation of IRS-1 (Ser636/639) and blocked the ability of ANG II to impair insulin-stimulated phosphorylation of eNOS, nitric oxide production, and mesenteric-arteriole vasodilation. Moreover, point mutations of IRS-1 at Ser636/639 to Ala prevented the ANG II-mediated inhibition of insulin signaling. From these results, we conclude that activation of mTOR/p70S6K by ANG II in vascular endothelium may contribute to impairment of insulin-stimulated vasodilation through phosphorylation of IRS-1 at Ser636/639. This ANG II-mediated impairment of vascular actions of insulin may help explain the role of ANG II as a link between insulin resistance and hypertension.
Keywords: angiotensin II, hypertension, insulin resistance, endothelial dysfunction, mammalian target of rapamycin, 70-kDa ribosomal S6 kinase, nitric oxide
the renin-angiotensin system (RAS) plays a crucial role in maintenance of vascular tone as well as other cardiovascular functions (3). Elevated levels of angiotensin II (ANG II) in the plasma and/or tissues contribute to various pathological conditions, including hypertension, coronary artery disease, and metabolic disorders, including insulin resistance and diabetes (14, 17, 26, 48). ANG II is a potent vasoconstrictor that transmits signals through a G protein-coupled receptor, ANG II receptor 1 (AT1R) (40). Activation of AT1R is associated with impairment of insulin signaling, which may contribute to the pathophysiology of a number of cardiometabolic conditions, including hypertension, insulin resistance, and cardiomyopathy (41, 49, 51). However, the molecular mechanism for the AT1R-associated impairment of insulin signaling in vascular tissue is not completely understood.
A number of the critical metabolic and vascular actions of insulin, including glucose uptake and nitric oxide (NO) production, occur through an insulin receptor (IR)/insulin receptor substrate-1 (IRS-1)/phosphoinositide 3-kinase (PI 3-kinase)/protein kinase B (Akt) pathway (25, 26, 33). Insulin resistance in the vasculature is characterized by an endothelial dysfunction that is often described as a lack of vasodilatory responses to insulin due to a reduced bioavailability of endothelium-derived NO in response to insulin stimulation (26). One of the molecular mechanisms for insulin resistance involves increased phosphorylation of IRS-1 at inhibitory serine (Ser) residues. A number of inhibitory Ser residues on IRS-1 and the kinases responsible for their phosphorylation have been implicated as indicators of insulin resistance (1, 8, 19, 46, 53). The mammalian targets of rapamycin (mTOR)/70-kDa ribosomal S6 kinase (p70S6K) are Ser kinases that play a crucial role in growth, proliferation, and cellular metabolism. The mTOR/p70S6K signaling pathway is activated through the IR/PI 3-kinase/Akt. Interestingly, activation of Akt leads to activation of mTOR/p70S6K, which phosphorylates IRS-1 at Ser residues to regulate insulin signaling as a negative feedback mechanism (19). Hormonal and nutritional activation of mTOR/p70S6K causes insulin resistance in high-fat diet-induced insulin-resistant animal models (24, 53). Furthermore, p70S6K knockout mice are protected from high-fat diet-induced obesity and insulin resistance (47). ANG II activates mTOR/p70S6K in cardiomyocytes and coronary vascular smooth muscle cells, leading to cardiac and vascular hypertrophy and remodeling (21, 43). This suggests that p70S6K may play an important role in ANG II-mediated insulin resistance in cardiovascular tissues. Therefore, we examined the role of mTOR/p70S6K in the ANG II-mediated endothelial dysfunction and vascular insulin resistance.
MATERIALS AND METHODS
Reagents.
Rapamycin, 4,5-diaminofluorescein diacetate (DAF-2 DA), and NG-nitro-l-arginine methyl ester (l-NAME) were acquired from EMD Biosciences (Gibbstown, NJ). Angiotensin II, pertussis toxin, and other chemicals were acquired from Sigma-Aldrich (St. Louis, MO), antibodies to IRS-1, phospho-IRS-1 (Ser636/639), phospho-endothelial NO synthase (eNOS; Ser1179), phospho-Akt (Ser473), Akt, phospho-p70S6 kinase (Thr389), and p70S6 kinase were from Cell Signaling Technology (Danvers, MA), and the antibody to eNOS was from BD Biosciences (San Jose, CA), AG1478 and PD-123319 were from Tocris Biosciences (Ellisville, MO).
Animals.
All animal procedures were performed in accordance with the Animal Use and Care Committee at the University of Missouri-Columbia. Male Sprague-Dawley rats (200–300 g) were obtained from Charles River (Wingmington, MA). Rats were provided with standard rat chow and water for ad libitum consumption while housed in pairs under a 12-h/day illumination regimen.
Functional assessment for isolated mesentery arterioles.
Rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (100 mg/kg). Mesenteric arterioles were excised from the animal and placed in a cooled (4°C) chamber containing dissection buffer [145 mmol/l NaCl, 4.7 mmol/l KCl, 2 mmol/l CaCl, 1.2 mmol/l MgSO4, 1 mmol/l NaH2PO4, 5 mmol/l glucose, 3 mmol/l 3-(N-morpholino)propansulfonic acid (MOPS) buffer, 2 mmol/l pyruvate, 0.02 mmol/l EDTA, 1% bovine serum albumin, pH 7.4]. The isolated arterioles were then cannulated onto glass micropipettes with 10-0 monofilament suture and mounted in a custom-designed tissue chamber (Living System Instrumentation, Burlington, VT). The arterioles were pressurized to 70 mmHg intraluminally with the same buffer without flow and superfused with buffer without albumin. The vessel preparations were positioned on the stage of an inverted microscope. The vessel segments were gradually warmed to 37°C during a 30-min equilibration period. After baseline diameter was established, arterioles were exposed to phenylephrine (1 μmol/l) until a maximal contraction was achieved (∼5 min). The vessels were subsequently incubated without and with ANG II (100 nmol/l) for 4 h. Then insulin was applied abluminally at incremental concentrations (10−9∼10−5 mol/l, 3 min/concentration). Dilator responses to insulin were observed and recorded. Measurement of vessel diameter (in μm) was performed with an electronic video caliper. In some experiments, rapamycin (10 nmol/l) or the NOS inhibitor l-NAME (100 μmol/l) was incubated 30 min prior to and during the exposure to ANG II.
The data are expressed as means ± SE. The vasodilator responses to insulin were calculated as percent relaxation from the phenylephrine constriction according to the following equation:
where IDtreat is the diameter observed obtained as the diameter when the vessel was treated with insulin, IDPE is the obtained diameter when the vessel was constricted with penylephrine, and IDw/oCa++ is the maximal passive diameter observed when the vessel was fully dilated in buffer containing 2 mmol/l EGTA and 100 μmol/l adenosine without Ca++.
Cell culture and transfection.
Bovine aortic endothelial cells (BAECs) in primary culture were obtained from Cell Applications (San Diego, CA) and maintained in F-12K medium containing 5% fetal bovine serum (FBS), endothelial cell growth supplement (15 μmol/l; BD Biosciences), heparin sulfate (50 μg/ml), penicillin (100 U/ml), and streptomycin (100 μg/ml). All experiments were conducted on BAECs between three and six passages. NIH-3T3IR cells were maintained in Dulbecco's MEM supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml). NIH-3T3IR cells were transfected with plasmids containing cDNA constructs using polyethyleneimine (27). Briefly, cells were plated onto 60-mm dishes, and transfection was conducted when the cells reached 70% confluency. Cells were replenished with new medium containing FBS (complete medium) before the transfection. Isolated plasmid DNA (3 μg) was diluted with 200 ml of OPTI-MEM (Invitrogen, Carlsbad, CA), and then 6 μl (1 mg/ml) of polyethyleneimine was added to DNA. The DNA mixture was incubated for 15 min at room temperature, and 1 ml of complete medium was added to the DNA mixture and sprinkled onto the cells.
Plasmid constructs and mutagenesis.
HA-human IRS-1 was a kind gift from Dr. Michael Quon (26). Point mutants of IRS-1-WT were created using the Quick Change site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. Human IRS-1 cDNA template was amplified by polymerase chain reaction (PCR) using primers 5′-AGA CTA TAT GCC CAT GGC CCC CAA GGC CGT ATC TGC CCC ACA G-3′ and 5′-CTG TGG GGCAGATAC GGC CTT GGG GGC CAT GGG CAT ATA GTC T-3′.
Preparation of cell lysate and immunoblotting.
Before lysis, cells were briefly washed with ice-cold PBS. Cells were then scraped in lysis buffer containing 50 mM Tris (pH 7.2), 125 mmol/l NaCl, 1% Triton X-100, 0.5% NP-40, 1 mmol/l EDTA, 1 mmol/l Na3VO4, 20 mmol/l NaF, 1 mmol/l Na pyrophosphate, and complete protease inhibitor cocktail (Roche Applied Science). Cell debris was pelleted by centrifugation of samples at 17,000 g for 10 min at 4°C. Supernatants were then boiled with Laemmli sample buffer for 5 min, and proteins were resolved by 10% SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with specific antibodies as described in the figures using standard methods. Immunoblots were quantified by Image Analyzer and Quantity One (Bio-Rad, Hercules, CA).
Measurement of NO production.
BAECs were grown to 95% confluence in 24-well black plates (Denville, Metuchen, NJ) and serum-starved overnight. The next day, the cells were treated without or with ANG II (100 nmol/l) for 4 h. Cells were then loaded with DAF-2 DA (1 μmol/l) for 10 min at 37°C, rinsed three times with F-12K, and kept in the dark. Production of NO was assessed using the NO-specific fluorescent dye DAF-2 DA (EMD Biosciences), as described previously (27). Cells were then treated without or with insulin, as indicated in the figure legends. In some experiments, l-NAME (100 μmol/l) or rapamycin (25 nmol/l) was added 30 min before ANG II treatment. After stimulation, cells were fixed in 2% paraformaldehyde for 5 min at 4°C. Fixed cells were visualized with a Zeiss inverted epifluorescence microscope (Axio Observer A1) using appropriate filters for a peak excitation wavelength of 480 nm and a peak emission wavelength of 510 nm. Images were captured and analyzed by using AV Rel 4.7 software with multichannel modules.
Statistics.
Values are presented as means ± SE. Western blots were analyzed by one-way analysis of variance (ANOVA) combined with Dunnett's post hoc test. For the comparisons of vasodilatory responses between no pretreatment, ANG II, rapamycin, and ANG II plus rapamycin were analyzed by repeated measurements of two-way ANOVA combined with Bonferroni post hoc test.
RESULTS
Ang II inhibits insulin-stimulated phosphorylation of eNOS and Akt.
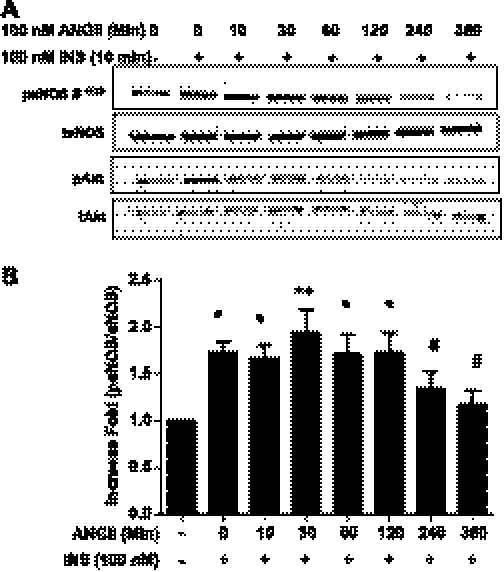
Insulin-signaling pathways in endothelial cells contribute to increased blood flow and vasodilation through the IR/IRS-1/PI 3-kinase/Akt signaling pathway, which leads to phosphorylation of eNOS at Ser1179 (1177 in human) (32). To explore the effect of ANG II on insulin signaling in vascular endothelial cells, we pretreated BAECs with ANG II (100 nmol/l) over various time periods and then stimulated with insulin (100 nmol/l) for 10 min. We observed that insulin-stimulated phosphorylation of eNOS and Akt was inhibited with pretreatment of ANG II (Fig. 1).
Fig. 1.
Angiotensin II (ANG II) inhibits insulin-stimulated phosphorylation of endothelial nitric oxide synathase (eNOS) and Akt. Bovine aortic endothelial cells (BAECs) were serum-starved overnight and then treated without or with ANG II (100 nmol/l) for the indicated times. Subsequently, cells were treated with insulin (100 nmol/l, 10 min) and harvested. The cell lysates were immunoblotted with antibodies against eNOS, Akt, phospho-eNOS (Ser1179), and phospho-Akt (Ser479). Pretreatment with ANG II inhibited the insulin-stimulated phosphorylation of eNOS and Akt. Pretreatment for 4 h and longer with ANG II inhibited insulin-stimulated phosphorylation of eNOS (means ± SE) *P ≤ 0.05; **P < 0.001; #P > 0.05 compared with no treatment, 1-way ANOVA with Dunnett's post hoc test.
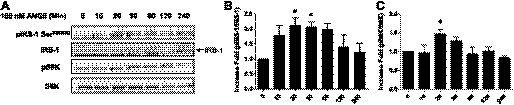
The mTOR/p70S6K signaling pathway plays a role in the ANG II-mediated inhibition of insulin-stimulated phosphorylation of eNOS.
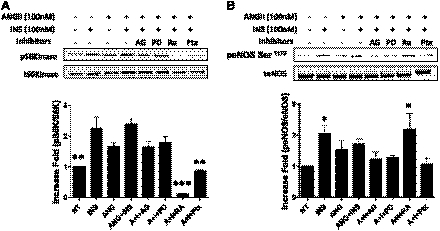
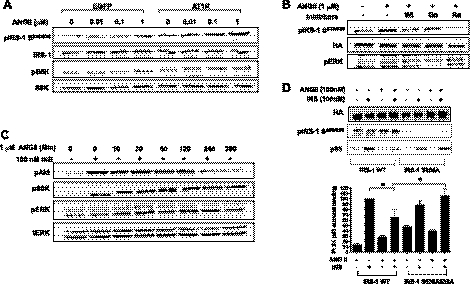
To examine the effect of ANG II alone on the phosphorylation of p70S6K in BAECs, we treated BAECs with ANG II for different time periods. ANG II alone stimulated the phosphorylation of p70S6K in a time-dependent manner (Fig. 2). Previously, it was shown that the mTOR/p70S6K pathway plays a role in the negative regulation of insulin signaling through increased phosphorylation of IRS-1 at Ser residues (47, 53). Therefore, we next examined whether ANG II promoted the phosphorylation of IRS-1 at Ser636/639, one of the well-known target sites for mTOR/p70S6K (47, 53). We observed that treatment with ANG II in BAECs stimulated the phosphorylation of IRS-1 at Ser636/639 in a time-dependent manner (Fig. 2A). These results suggest that the ANG II-mediated inhibition of insulin signaling may be through a mechanism that stimulates phosphorylation of IRS-1 by mTOR/p70S6K. To investigate the specific pathway by which ANG II activates mTOR/p70S6K, we explored previously known ANG II signaling pathways, including the transactivation of the epidermal growth factor receptor (EGFR) (31) and the activation of Gi associated with ANG II receptor 2 (AT2R). We pretreated BAECs with AG1478 (an inhibitor of EGFR), pertussis toxin (an inhibitor of Gi), PD-123319 (an inhibitor of AT2R), or rapamycin (an inhibitor of mTOR) and then performed the similar experiment, as described previously (Fig. 1). The ANG II-stimulated phosphorylation of p70S6K was inhibited significantly by pretreatment of rapamycin and pertussis toxin but not by AG1478 or PD-123319 (Fig. 3A). The insulin-stimulated phosphorylation of eNOS was restored by pretreatment with rapamycin but not by AG1478, pertussis toxin, or PD-123319 (Fig. 3B). These results suggest that activation of mTOR/p70S6K plays an important role in inhibition of insulin-stimulated phosphorylation of eNOS.
Fig. 2.
ANG II alone stimulates phophorylation of insulin receptor substrate-1 (IRS-1) and 70-kDa ribosomal S6 kinase (p70S6K). BAECs were serum-starved overnight and then treated without or with ANG II (100 nmol/l) for the indicated periods. A: cells were harvested, and the cell lysates were immunoblotted with antibodies against IRS-1, phospho-IRS-1 (Ser636/639), p70S6K, and phospho-p70S6K (Thr389). B and C: treatment with ANG II stimulates phosphorylation of IRS-1 and p70S6K in a time-dependent manner. Three independent experiments were analyzed and shown in bar graphs (means ± SE). *P < 0.05 compared with no treatment, 1-way ANOVA with Dunnett's post hoc test.
Fig. 3.
Pretreatment with rapamycin restores the insulin-stimulated phophorylation of eNOS that is inhibited by ANG II. BAECs were serum-starved overnight and treated without or with AG1478 (5 μmol/l), PD-123319, (10 μM), rapamycin (25 nmol/l), or pertussis toxin (100 ng/ml) for 30 min prior to ANG II (100 nmol/l, 4 h). Then the cells were stimulated without or with insulin (100 nmol/l) for 10 min. Cells were harvested, and the cell lysates were immunoblotted with antibodies against phospho-(Thr389) and total p70S6K (A) and phospho- (Ser1179) and total eNOS (B). Immunoblots shown are representative of those that were independently repeated at least 3 times, and the quantifications of 3 independent experiments are shown as bar graphs (means ± SE). *P < 0.05; **P < 0.01; ***P < 0.001 compared with no treatment (A) and insulin treatment (B), 1-way ANOVA with Dunnettt's post hoc test.
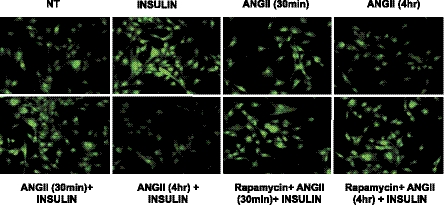
mTOR/p70S6K is involved in the ANG II-mediated inhibition of insulin-stimulated NO production.
Phosphorylation of eNOS stimulates the catalytic activity of eNOS to produce NO (12, 15). The effect of ANG II-mediated inhibition of insulin-stimulated NO production in BAECs was measured using the NO-specific fluorescent dye DAF-2 DA. Insulin-treated cells showed increased fluorescence, indicating that insulin stimulates NO production. Short time treatment (30 min) of ANG II did not affect insulin-stimulated NO production. However, prolonged treatment with ANG II (4 h) inhibited insulin-stimulated NO production. In addition, pretreatment with rapamycin restored the insulin-stimulated NO production that was inhibited by treatment with ANG II (Fig. 4). These results are consistent with our immunoblotting experiments shown in Fig. 3. Furthermore, insulin-stimulated NO production is dependent on eNOS activity because pretreatment with l-NAME abolished the insulin-stimulated production of NO (data not shown). These results indicate that ANG II inhibits the insulin-stimulated activity of eNOS through a mTOR/p70S6K-mediated mechanism.
Fig. 4.
Pretreatment with rapamycin restores the insulin-stimulated nitric oxide (NO) production that is inhibited by treatment with ANG II. BAECs were serum-starved overnight and treated without or with ANG II (100 nmol/l, 4 h). Then the cells were loaded with 4,5-diaminofluorescein diacetate, as described in materials and methods. Cells were then stimulated without or with insulin (100 nmol/l, 10 min). After insulin treatment, cells were fixed in 2% paraformaldehyde and visualized with an epifluorescent microscope, as described in materials and methods. Emission of green fluorescence is indicative of NO production. Experiments shown are representative of those that were repeated independently at least 3 times.
mTOR/p70S6K plays a role in the ANG II-mediated inhibition of insulin-stimulated vasodilation.
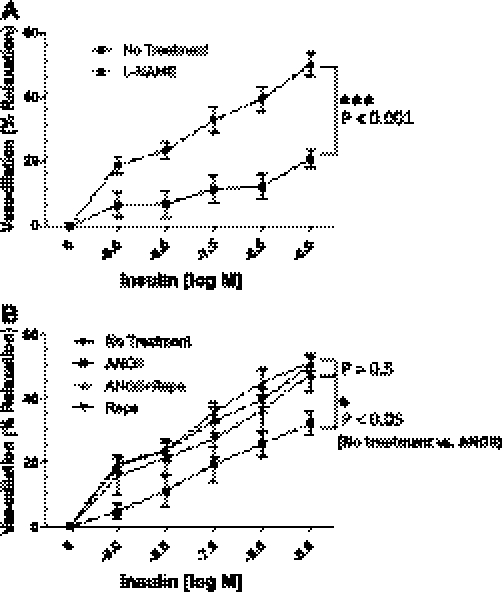
To examine the potential physiological relevance of the ANG II-mediated inhibitory effects on insulin-stimulated NO production, we evaluated the vasodilatory responses of isolated mesenteric arterioles to insulin after a 4-h incubation without or with ANG II. Mesenteric arterioles were dilated in response to insulin in a concentration-dependent manner (Fig. 5A). The insulin-stimulated vasodilatory responses were significantly blunted by pretreatment with l-NAME (100 nmol/l), indicating that the insulin-stimulated vasodilatory response in these arterioles is due mainly to the activation of eNOS via phosphorylation at Ser1179. Next, we incubated the arterioles with ANG II for 4 h and then examined the vasodilatory responses to insulin without or with rapamycin. Treatment with ANG II inhibited insulin-stimulated vasodilatory responses compared with the response to insulin alone (P < 0.05; Fig. 5B). Pretreatment with rapamycin restored the insulin-stimulated vasodilatory responses. Rapamycin alone did not affect the insulin-stimulated vasodilatory responses (P > 0.5; Fig. 5B). Consistent with our in vitro experiments in cultured endothelial cells (Figs. 3 and 4), these ex vivo results suggest that ANG II inhibits the insulin-stimulated vasodilatory responses through a mTOR/p70S6K-mediated mechanism.
Fig. 5.
Pretreatment with rapamycin (Rapa) restores the insulin-stimulated vasodilation that is inhibited by treatment with ANG II. Mesenteric arterioles were isolated from Sprague-Dawley rats. The arterioles were cannulated onto glass micropipettes and pressurized to 70 mmHg using a Pressure Servo System and warmed to 37°C over 30 min for stabilization. The arterioles were treated without or with rapamycin (10 nmol/l, 30 min), and arterioles were treated without or with ANG II (100 nmol/l, 4 h). Arterioles were constricted with penylephrine (1 mmol/l) and then exposed to increasing concentrations of insulin. The vasodilatory activity of insulin on the arterioles was observed and recorded using a data acquisition system. In some experiments, NG-nitro-l-arginine methyl ester (l-NAME; 100 μmol/l, 30 min) was applied prior to insulin exposure. A: mesenteric arterioles were dilated in response to insulin in a concentration-dependent manner, and the insulin-stimulated dilation was inhibited by pretreatment of l-NAME. B: insulin-stimulated vasodilation was inhibited by ANG II and restored by pretreatment with rapamycin. No treatment (n = 6), ANG II (100 nmol/l for 4 h; n = 5), Rapa (10 nmol/l; n = 6), Rapa + Ang II (n = 6), and l-NAME (n = 11). %Vasorelaxation was calculated as described materials and methods. Means ± SE. *P < 0.05, ***P < 0.001; the data were analyzed by repeated measurements of 2-way ANOVA combined with Bonferroni post hoc test.
ANG II inhibits insulin signaling through AT1R-mediated activation of the mTOR/p70S6K pathway.
To examine the involvement of AT1R, we transiently transfected a fibroblast cell line (NIH-3T3IR) that normally does not express AT1R with plasmid containing AT1R cDNA and then treated the cells without or with ANG II. The cells overexpressing AT1R were able to stimulate p70S6K and phosphorylate IRS-1 at Ser636/639 in response to ANG II (Fig. 6A). The phosphorylation of p70S6K and IRS-1 at Ser636/639 was inhibited by pretreatment with wortmanin (PI 3-kinase inhibitor) or rapamycin but not by Go6976 (calcium-dependent PKC inhibitor). However, pretreatment with Go6976 was able to inhibit the ANG II-stimulated phosphorylation of ERK (Fig. 6B). Next, we investigated whether insulin signaling is inhibited by ANG II in the cells overexpresing AT1R. Insulin-stimulated phosphorylation of Akt was inhibited by treatment with ANG II, whereas phosphorylation of ERK was not affected (Fig. 6C). We then examined the role of phosphrylation of IRS-1 Ser636/639 in ANG II-mediated inhibition of insulin signaling. To address this, we constructed a point mutation of IRS-1 at Ser636/639 to Ala and then transiently cotransfected NIH-3T3IR cells with plasmids containing IRS-1 WT or IRS-1 S636/639A mutant with AT1R. The cells overexpressing IRS-1 WT showed reduced phosphorylation of IRS-1-associated p85 subunit of PI 3-kinase in response to ANG II. In contrast, in the cells overexpressing the IRS-1 Ser636/639A mutant, association of p85 subunit of PI 3-kinase with IRS-1 was not inhibited by ANG II treatment (Fig. 6).
Fig. 6.
ANG II stimulates phosphorylation of IRS-1 at Ser636/639 and p70S6K in cells overexpressing ANG II receptor 1 (AT1R). NIH-3T3IR cells were transiently transfected with plasmid (pcDNA 3.1) containing AT1R cDNA. One day after transfection, the cells were serum-starved overnight and treated without or with indicated concentration of ANG II for 30 min. Cells were harvested, and the cell lysates were immunoblotted with antibodies as indicated. A: ANG II stimulated the phosphorylation of IRS-1 at Ser636/639 and p70S6K in the cells overexpressing AT1R. B: the ANG II-stimulated phosphorylation of IRS-1 at Ser636/639 was inhibited by wortmanin (100 nmol/l) or Rapa (25 nmol/l) but not by Go6976 (1 μmol/l). C: insulin (INS)-stimulated phosphorylation of Akt was attenuated by treatment with ANG II. D: point mutation of IRS-1 at Ser636/639A is protective from ANG II-induced inhibition of insulin-stimulated phosphorylation of Akt (means ± SE). *P < 0.05 compared with wild-type IRS-1, 2-way ANOVA with Bonferroni post hoc test.
DISCUSSION
ANG II plays an important role in the pathophysiology of cardiovascular diseases and metabolic disorders, including insulin resistance and diabetes (11, 34, 35). Evidence from clinical trials, animal models, and cellular studies indicates that RAS inhibitors, including inhibitors of renin, AT1R blockers, and angiotensin-converting enzyme inhibitors, improve insulin sensitivity and ameliorate endothelial dysfunction (9, 14, 20, 28–30, 36). This suggests that ANG II plays an important role in insulin resistance and endothelial dysfunction. eNOS is one of the crucial molecules that link the reciprocal relationship between endothelial dysfunction and insulin resistance (26). In the present study, we observed that ANG II activates mTOR/p70S6K, leading to phosphorylation of IRS-1 at Ser636/639 and inhibition of insulin-stimulated phosphorylation of eNOS, reducing the production of NO, and impairing vasodilation. This ANG II-mediated vascular insulin resistance may be one of the mechanisms for the link between metabolic disorders and cardiovascular complications.
Insulin resistance vs. endothelial dysfunction.
Insulin signaling is inhibited by an acute treatment with ANG II (25–30 min) in both hepatocytes and human umbilical vascular endothelial cells (4). We observed that the inhibitory effect of ANG II on insulin-stimulated phosphorylation of eNOS and NO production occurred after 2–4 h of ANG II treatment (Fig. 1). The discrepancies in the pattern of responses to ANG II may be due to the cell type specificity that includes expression levels of AT1R, cross-talk between AT1R and AT2R, cross-talk between G proteins, involvement of unknown molecules with regard to the ANG II signaling pathway, etc.
Differential effects of AT1R and AT2R stimulation on insulin-mediated NO production.
Both AT1R and AT2R are expressed in vascular endothelial cells, whereas AT1R is expressed exclusively in vascular smooth muscle cells (37, 39, 42). The expression levels of AT1R and AT2R are differentially regulated by various conditions, including developmental stage, pathological conditions, tissue specificity, etc. (6, 7, 39). ANG II inhibits insulin-mediated NO production and blood flow through AT1R (10, 37), whereas AT2R is required for insulin-stimulated blood flow (10). ANG II stimulates NADPH oxidase and produces excess reactive oxygen species (ROS), which is implicated as a mechanism for ANG II-mediated pathophysiology of hypertension, cardiac dysfunction, and endothelial dysfunction as well as insulin resistance (11, 38, 50). We cannot exclude the possibility that NADPH oxidase and ROS are involved in ANG II-mediated inhibition of vasodilation. We observed that NADPH oxidase inhibitors, including apocynin and diphenylene iodonium, were able to inhibit the phosphorylation of IRS-1 at Ser636/639 in the presence of ANG II (data not shown). Thus, ANG II-mediated ROS can contribute to activation of mTOR/p70S6K as well as directly reduce NO availability that leads to impairment of insulin-stimulated vasodilation.
ANG II activates EGFR.
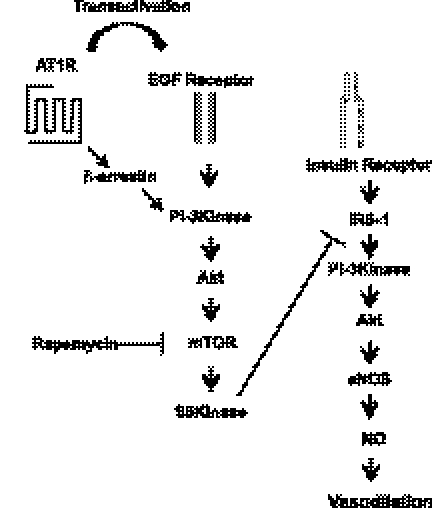
Treatment of ANG II alone acutely activates the PI 3-kinase/Akt pathway through AT1R (Fig. 2). AT1R stimulates the PI 3-kinase/Akt/mTOR/p70S6K pathway through transactivation of EGFR or the β-arrestin-mediated mechanism (Fig. 7) (2, 5, 13, 23, 44, 52). We observed that the inhibitor of EGFR (AG1478) was able to inhibit the phosphorylation of IRS-1 at Ser636/639. However, unlike rapamycin, AG1478 was not able to restore the responses to insulin. It has been suggested that EGFR is involved in ANG II-mediated insulin resistance in hepatocytes through a MAPK-dependent mechanism (5). It is possible that EGFR is involved in ANG II-mediated insulin resistance, but the pharmacological reagent may have nonspecific activity that could inhibit a molecule that is required for the insulin-signaling pathway to activate eNOS.
Fig. 7.
Schematic diagram of ANG II signaling pathway in vascular endothelium. ANG II stimulates mammalian target of rapamycin (mTOR)/S6K through transactivation of epidermal growth factor (EGF) receptor activating mTOR/S6K that contributes to inhibition of insulin-stimulated phosphorylation of eNOS, production of NO, and vasodilation.
The role of mTOR/p70S6K.
In the present study, we observed that rapamycin was able to restore the insulin-stimulated phosphorylation of eNOS, production of NO, and vasodilation that was inhibited by treatment of ANG II. Thus, it is clear that inhibition of the mTOR/p70S6K pathway prevents the ANG II-mediated endothelial dysfunction. It is possible that both kinases contribute to ANG II-mediated phosphorylation of IRS-1 at Ser636/639. Further studies are required to clarify this issue. Overexpression of the IRS-1 Ser636/639A construct was resistant to ANG II-mediated insulin resistance. Phosphorylation of IRS-1 at Ser636/639 sites was inhibited by pretreatment with rapamycin. Since p70S6K phosphorylates multiple serine sites on IRS-1 (4, 53), it is possible that not only Ser636/639 but also the other sites may be responsible for the ANG II-mediated insulin resistance and endothelial dysfunction. It would be interesting to determine whether knockout of mTOR or p70S6K can prevent ANG II-mediated hypertension and insulin resistance.
Phosphorylation of IRS-1 at Ser636/639.
Increased serine phosphorylation of IRS-1 is implicated as one of the mechanisms for insulin resistance (8). Although there are a few serine sites that promote insulin signaling (18, 22), most of these serine sites are negatively regulating insulin signaling by inhibition of interactions with phospholipid, IR, and PI 3-kinase (8). We observed that ANG II stimulated the phosphorylation of IRS-1 at Ser636/639 at the early time point of 20 min. However, the attenuated response to insulin was not observed concurrently with the phosphorylation event. One possible scenario is that the phosphorylation event leads to ubiquitin-dependent degradation of IRS-1 that in turn contributes to insulin resistance (5, 53). In fact, ANG II causes the degradation of IRS-1 in hepatocyte and vascular smooth muscle cells (5, 45). Thus, it is possible that the phosphorylation of IRS-1 can lead to an acceleration of the degradation process. Phosphorylation of IRS-1 at Ser636/639 by Rho kinase enhanced insulin sensitivity in skeletal muscle cells (16), whereas it was increased in the adipose tissue from ob/ob mice that is insulin resistant (53). Thus, each kinase may differentially phosphorylate more than one Ser site on IRS-1, and the overall outcome of positive and negative modifications may determine the activity of IRS-1 that is closely related to insulin sensitivity. In conclusion, mTOR/p70S6K plays an important role in ANG II-mediated vascular insulin resistance in vascular endothelium that may contribute to cardiovascular and metabolic complications. Thus, mTOR/p70S6K may be a therapeutic target for ANG II-associated hypertension and insulin resistance.
GRANTS
This study was supported by the American Diabetes Association (1-09-JK-33; to J. Kim), a University of Alabama at Birmingham Diabetes Research Training Center-sponsored pilot and feasibility program (P60 DK-079626), the National Heart, Lung, and Blood Institute (R01-HL-73101-01A1 to J. R. Sowers and RO1-HL-088105-01A1 to L. A. Martinez-Lemus), Veterans Affairs Merit System Grant 0018 (to J. R. Sowers), and the American Heart Association (0530031N; to L. A. Martinez-Lemus).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.-a.K. did the conception and design of the research; J.-a.K., H.-J.J., and L.A.M.-L. performed the experiments; J.-a.K., H.-J.J., and L.A.M.-L. analyzed the data; J.-a.K., H.-J.J., L.A.M.-L., and J.R.S. interpreted the results of the experiments; J.-a.K. and H.-J.J. prepared the figures; J.-a.K. drafted the manuscript; J.-a.K., H.-J.J., L.A.M.-L., and J.R.S. edited and revised the manuscript; J.-a.K., H.-J.J., L.A.M.-L., and J.R.S. approved the final version of the manuscript.
REFERENCES
- 1. Adochio R, Leitner JW, Hedlund R, Draznin B. Rescuing 3T3-L1 adipocytes from insulin resistance induced by stimulation of Akt-mammalian target of rapamycin/p70 S6 kinase (S6K1) pathway and serine phosphorylation of insulin receptor substrate-1: effect of reduced expression of p85alpha subunit of phosphatidylinositol 3-kinase and S6K1 kinase. Endocrinology 150: 1165–1173, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Ahn S, Kim J, Hara MR, Ren XR, Lefkowitz RJ. {beta}-Arrestin-2 Mediates Anti-apoptotic Signaling through Regulation of BAD Phosphorylation. J Biol Chem 284: 8855–8865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ali MS, Schieffer B, Delafontaine P, Bernstein KE, Ling BN, Marrero MB. Angiotensin II stimulates tyrosine phosphorylation and activation of insulin receptor substrate 1 and protein-tyrosine phosphatase 1D in vascular smooth muscle cells. J Biol Chem 272: 12373–12379, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Andreozzi F, Laratta E, Sciacqua A, Perticone F, Sesti G. Angiotensin II impairs the insulin signaling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on Ser312 and Ser616 in human umbilical vein endothelial cells. Circ Res 94: 1211–1218, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Arellano-Plancarte A, Hernandez-Aranda J, Catt KJ, Olivares-Reyes JA. Angiotensin-induced EGF receptor transactivation inhibits insulin signaling in C9 hepatic cells. Biochem Pharmacol 79: 733–745, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bagby SP, LeBard LS, Luo Z, Ogden BE, Corless C, McPherson ED, Speth RC. ANG II AT1 and AT2 receptors in developing kidney of normal microswine. Am J Physiol Renal Physiol 283: F755–F764, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Bagby SP, LeBard LS, Luo Z, Speth RC, Ogden BE, Corless CL. Angiotensin II type 1 and 2 receptors in conduit arteries of normal developing microswine. Arterioscler Thromb Vasc Biol 22: 1113–1121, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Boura-Halfon S, Zick Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab 296: E581–E591, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Carvalheira JB, Calegari VC, Zecchin HG, Nadruz W, Jr, Guimarães RB, Ribeiro EB, Franchini KG, Velloso LA, Saad MJ. The cross-talk between angiotensin and insulin differentially affects phosphatidylinositol 3-kinase- and mitogen-activated protein kinase-mediated signaling in rat heart: implications for insulin resistance. Endocrinology 144: 5604–5614, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Chai W, Wang W, Dong Z, Cao W, Liu Z. Angiotensin II Receptors Modulate Muscle Microvascular and Metabolic Responses to Insulin In Vivo. Diabetes 60: 2939–2946, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, Stas S, Sowers JR. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol 293: H2009–H2023, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Eguchi S, Iwasaki H, Ueno H, Frank GD, Motley ED, Eguchi K, Marumo F, Hirata Y, Inagami T. Intracellular signaling of angiotensin II-induced p70 S6 kinase phosphorylation at Ser(411) in vascular smooth muscle cells. Possible requirement of epidermal growth factor receptor, Ras, extracellular signal-regulated kinase, and Akt. J Biol Chem 274: 36843–36851, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Folli F, Kahn CR, Hansen H, Bouchie JL, Feener EP. Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J Clin Invest 100: 2158–2169, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597–601, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Furukawa N, Ongusaha P, Jahng WJ, Araki K, Choi CS, Kim HJ, Lee YH, Kaibuchi K, Kahn BB, Masuzaki H, Kim JK, Lee SW, Kim YB. Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab 2: 119–129, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Giacchetti G, Sechi LA, Rilli S, Carey RM. The renin-angiotensin-aldosterone system, glucose metabolism and diabetes. Trends Endocrinol Metab 16: 120–126, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Giraud J, Leshan R, Lee YH, White MF. Nutrient-dependent and insulin-stimulated phosphorylation of insulin receptor substrate-1 on serine 302 correlates with increased insulin signaling. J Biol Chem 279: 3447–3454, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Gual P, Le Marchand-Brustel Y, Tanti JF. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 87: 99–109, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Habibi J, Whaley-Connell A, Hayden MR, DeMarco VG, Schneider R, Sowers SD, Karuparthi P, Ferrario CM, Sowers JR. Renin inhibition attenuates insulin resistance, oxidative stress, and pancreatic remodeling in the transgenic Ren2 rat. Endocrinology 149: 5643–5653, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hafizi S, Wang X, Chester AH, Yacoub MH, Proud CG. ANG II activates effectors of mTOR via PI3-K signaling in human coronary smooth muscle cells. Am J Physiol Heart Circ Physiol 287: H1232–H1238, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE. 5′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J Biol Chem 276: 46912–46916, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Kendall RT, Strungs EG, Rachidi SM, Lee MH, El-Shewy HM, Luttrell DK, Janech MG, Luttrell LM. The beta-arrestin pathway-selective type 1A angiotensin receptor (AT1A) agonist [Sar1,Ile4,Ile8]angiotensin II regulates a robust G protein-independent signaling network. J Biol Chem 286: 19880–19891, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology 146: 1473–1481, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Kim JA, Formoso G, Li Y, Potenza MA, Marasciulo FL, Montagnani M, Quon MJ. Epigallocatechin gallate, a green tea polyphenol, mediates NO-dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and Fyn. J Biol Chem 282: 13736–13745, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113: 1888–1904, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Kneuer C, Ehrhardt C, Bakowsky H, Kumar MN, Oberle V, Lehr CM, Hoekstra D, Bakowsky U. The influence of physicochemical parameters on the efficacy of non-viral DNA transfection complexes: a comparative study. J Nanosci Nanotechnol 6: 2776–2782, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Koh KK, Quon MJ, Han SH, Chung WJ, Ahn JY, Seo YH, Kang MH, Ahn TH, Choi IS, Shin EK. Additive beneficial effects of losartan combined with simvastatin in the treatment of hypercholesterolemic, hypertensive patients. Circulation 110: 3687–3692, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Koh KK, Quon MJ, Lee Y, Han SH, Ahn JY, Chung WJ, Kim JA, Shin EK. Additive beneficial cardiovascular and metabolic effects of combination therapy with ramipril and candesartan in hypertensive patients. Eur Heart J 28: 1440–1447, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Lastra G, Whaley-Connell A, Manrique C, Habibi J, Gutweiler AA, Appesh L, Hayden MR, Wei Y, Ferrario C, Sowers JR. Low-dose spironolactone reduces reactive oxygen species generation and improves insulin-stimulated glucose transport in skeletal muscle in the TG(mRen2)27 rat. Am J Physiol Endocrinol Metab 295: E110–E116, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu HW, Cheng B, Yu WL, Sun RX, Zeng D, Wang J, Liao YX, Fu XB. Angiotensin II regulates phosphoinositide 3 kinase/Akt cascade via a negative crosstalk between AT1 and AT2 receptors in skin fibroblasts of human hypertrophic scars. Life Sci 79: 475–483, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179). J Biol Chem 276: 30392–30398, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Montagnani M, Golovchenko I, Kim I, Koh GY, Goalstone ML, Mundhekar AN, Johansen M, Kucik DF, Quon MJ, Draznin B. Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. J Biol Chem 277: 1794–1799, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Nakashima H, Suzuki H, Ohtsu H, Chao JY, Utsunomiya H, Frank GD, Eguchi S. Angiotensin II regulates vascular and endothelial dysfunction: recent topics of Angiotensin II type-1 receptor signaling in the vasculature. Curr Vasc Pharmacol 4: 67–78, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Olivares-Reyes JA, Arellano-Plancarte A, Castillo-Hernandez JR. Angiotensin II and the development of insulin resistance: implications for diabetes. Mol Cell Endocrinol 302: 128–139, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Potenza MA, Marasciulo FL, Tarquinio M, Quon MJ, Montagnani M. Treatment of spontaneously hypertensive rats with rosiglitazone and/or enalapril restores balance between vasodilator and vasoconstrictor actions of insulin with simultaneous improvement in hypertension and insulin resistance. Diabetes 55: 3594–3603, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Presta I, Tassone EJ, Andreozzi F, Perticone M, Sciacqua A, Laino I, Musca D, Martino F, Sesti G, Perticone F. Angiotensin II type 1 receptor, but no type 2 receptor, interferes with the insulin-induced nitric oxide production in HUVECs. Atherosclerosis. In press [DOI] [PubMed] [Google Scholar]
- 38. Privratsky JR, Wold LE, Sowers JR, Quinn MT, Ren J. AT1 blockade prevents glucose-induced cardiac dysfunction in ventricular myocytes: role of the AT1 receptor and NADPH oxidase. Hypertension 42: 206–212, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Pueyo ME, Michel JB. Angiotensin II receptors in endothelial cells. Gen Pharmacol 29: 691–696, 1997 [DOI] [PubMed] [Google Scholar]
- 40. Sasaki K, Yamano Y, Bardhan S, Iwai N, Murray JJ, Hasegawa M, Matsuda Y, Inagami T. Cloning and expression of a complementary DNA encoding a bovine adrenal angiotensin II type-1 receptor. Nature 351: 230–233, 1991 [DOI] [PubMed] [Google Scholar]
- 41. Shiuchi T, Iwai M, Li HS, Wu L, Min LJ, Li JM, Okumura M, Cui TX, Horiuchi M. Angiotensin II type-1 receptor blocker valsartan enhances insulin sensitivity in skeletal muscles of diabetic mice. Hypertension 43: 1003–1010, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Stoll M, Steckelings UM, Paul M, Bottari SP, Metzger R, Unger T. The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J Clin Invest 95: 651–657, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stuck BJ, Lenski M, Bohm M, Laufs U. Metabolic switch and hypertrophy of cardiomyocytes following treatment with angiotensin II are prevented by AMP-activated protein kinase. J Biol Chem 283: 32562–32569, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Takahashi T, Taniguchi T, Konishi H, Kikkawa U, Ishikawa Y, Yokoyama M. Activation of Akt/protein kinase B after stimulation with angiotensin II in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 276: H1927–H1934, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Taniyama Y, Hitomi H, Shah A, Alexander RW, Griendling KK. Mechanisms of reactive oxygen species-dependent downregulation of insulin receptor substrate-1 by angiotensin II. Arterioscler Thromb Vasc Biol 25: 1142–1147, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem 276: 38052–38060, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431: 200–205, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Velloso LA, Folli F, Sun XJ, White MF, Saad MJ, Kahn CR. Cross-talk between the insulin and angiotensin signaling systems. Proc Natl Acad Sci USA 93: 12490–12495, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, Clark SE, Morris EM, Szary N, Manrique C, Stump CS. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem 281: 35137–35146, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Wei Y, Whaley-Connell AT, Chen K, Habibi J, Uptergrove GM, Clark SE, Stump CS, Ferrario CM, Sowers JR. NADPH oxidase contributes to vascular inflammation, insulin resistance, and remodeling in the transgenic (mRen2) rat. Hypertension 50: 384–391, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Whaley-Connell A, Govindarajan G, Habibi J, Hayden MR, Cooper SA, Wei Y, Ma L, Qazi M, Link D, Karuparthi PR, Stump C, Ferrario C, Sowers JR. Angiotensin II-mediated oxidative stress promotes myocardial tissue remodeling in the transgenic (mRen2) 27 Ren2 rat. Am J Physiol Endocrinol Metab 293: E355–E363, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Yano N, Suzuki D, Endoh M, Zhao TC, Padbury JF, Tseng YT. A novel phosphoinositide 3-kinase-dependent pathway for angiotensin II/AT-1 receptor-mediated induction of collagen synthesis in MES-13 mesangial cells. J Biol Chem 282: 18819–18830, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Zhang J, Gao Z, Yin J, Quon MJ, Ye J. S6K directly phosphorylates IRS-1 on Ser-270 to promote insulin resistance in response to TNF-(alpha) signaling through IKK2. J Biol Chem 283: 35375–35382, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]