Abstract
Intense interest has been focused on cell-based therapy for the infarcted heart given that stem cells have exhibited the ability to reduce infarct size and mitigate cardiac dysfunction. Despite this, it is unknown whether mesenchymal stem cell (MSC) therapy can prevent metabolic remodeling following a myocardial infarction (MI). This study examines the ability of MSCs to rescue the infarcted heart from perturbed substrate uptake in vivo. C57BL/6 mice underwent chronic ligation of the left anterior descending coronary artery to induce a MI. Echocardiography was performed on conscious mice at baseline as well as 7 and 23 days post-MI. Twenty-eight days following the ligation procedure, hyperinsulinemic euglycemic clamps assessed in vivo insulin sensitivity. Isotopic tracer administration evaluated whole body, peripheral tissue, and cardiac-specific glucose and fatty acid utilization. To gain insight into the mechanisms by which MSCs modulate metabolism, mitochondrial function was assessed by high-resolution respirometry using permeabilized cardiac fibers. Data show that MSC transplantation preserves insulin-stimulated fatty acid uptake in the peri-infarct region (4.25 ± 0.64 vs. 2.57 ± 0.34 vs. 3.89 ± 0.54 μmol·100 g−1·min−1, SHAM vs. MI + PBS vs. MI + MSC; P < 0.05) and prevents increases in glucose uptake in the remote left ventricle (3.11 ± 0.43 vs. 3.81 ± 0.79 vs. 6.36 ± 1.08 μmol·100 g−1·min−1, SHAM vs. MI + PBS vs. MI + MSC; P < 0.05). This was associated with an enhanced efficiency of mitochondrial oxidative phosphorylation with a respiratory control ratio of 3.36 ± 0.18 in MSC-treated cardiac fibers vs. 2.57 ± 0.14 in the infarct-only fibers (P < 0.05). In conclusion, MSC therapy exhibits the potential to rescue the heart from metabolic aberrations following a MI. Restoration of metabolic flexibility is important given the metabolic demands of the heart and the role of energetics in the progression to heart failure.
Keywords: substrate uptake, mitochondria, hyperinsulinemic euglycemic clamp, skeletal muscle, peroxisome proliferator-activated receptor-γ coactivator-1α
to satisfy the continuous energetic demand of contractile function, fatty acids (FA) and to a lesser extent glucose are catabolized by mitochondria to synthesize ATP via oxidative phosphorylation (OXPHOS) (28). In response to physiological stressors, the heart must maintain an efficient means of energy provision. This is accomplished by the heart possessing metabolic flexibility, the ability to switch to the most appropriate substrate. Following a myocardial infarction (MI), the heart undergoes energetic alterations in which this adaptability is lost (14). This metabolic remodeling is characterized by a reduction in cardiac FA utilization and an increased reliance on glucose. Although the switch to glucose is initially adaptive, the prolonged dependence on a single substrate limits the heart from using the most appropriate substrate milieu in response to physiological stressors, which compromises the heart's capacity for energy provision and promotes substrate toxicity. Shifts in substrate balance are often accompanied by mitochondrial insult. Mitochondrial content is reduced, and existing mitochondria exhibit OXPHOS dysfunction (18). Consequently, satisfying cardiac energetic requirements becomes exceedingly difficult, and contractile dysfunction is perpetuated.
Research into the efficacy of stem cell therapy as a treatment post-MI is receiving substantial attention. Cell-based therapy promotes cellular viability and neovascularization and improves cardiac contractile performance following a MI (4, 9). Given the dynamic relationship between the aforementioned and energetics, manipulation of metabolism via stem cell therapy may represent an additional mechanism through which transplanted cells minimize myocardial contractile impairment. A limited number of existing reports indicate bone marrow-derived mesenchymal stem cell (MSC) therapy may modulate myocardial metabolism. Ex vivo, MSC transplantation blunts increases in basal cardiac glucose uptake 2 wk post-MI in a rat model (8). Additionally, MSC transplantation into the infarcted swine heart maintained the peri-infarct phosphocreatine-to-ATP (PCr/ATP) ratio, a ratio reflecting OXPHOS regulation, energy efficiency, and ventricular function in cardiomyopathies (7, 33). In contrast, Eun et. al (5) reported that MSC therapy does not restore cardiac glycolytic-related metabolites 7–11 days following a MI. The present study builds upon these initial findings characterizing the influence of MSC transplantation on ex vivo basal cardiac glucose uptake by assessing the in vivo effects of intramyocardial MSC injection on insulin sensitivity, insulin-stimulated substrate utilization, and mitochondrial function following a MI in the conscious, unrestrained mouse. It was hypothesized MSC transplantation would prevent metabolic inflexibility and mitochondrial impairment.
MATERIALS AND METHODS
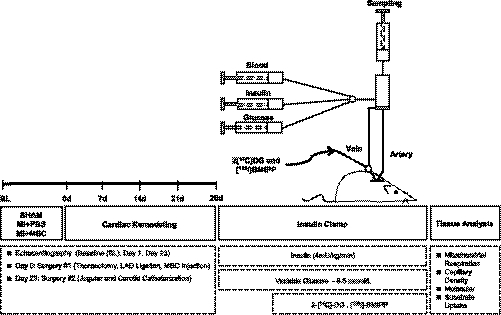
See Fig. 1 for a schematic of the experimental outline.
Fig. 1.
Schematic representation of experimental procedures and timeline. Echocardiography on conscious mice was performed at baseline (BL), 7 and 23 days following ligation of the left anterior descending (LAD) coronary artery. Arterial and jugular catheterization were performed 23 days following the LAD ligation for the sampling and infusion protocols of the hyperinsulinemic euglycemic (insulin) clamp. Insulin clamps were performed following 5 days of recovery from the catheterization surgeries (28 days post-LAD ligation) to assess insulin action in the conscious, unrestrained mouse. Isotopic tracer {2-[14C]deoxyglucose (2-[14C]DG) and [125I]β-methyl-p-iodophenylpentadecanoic acid ([125I]-BMIPP)} administration during the insulin clamps allowed for whole body and tissue-specific substrate uptake to be assessed in vivo. Additional experiments included evaluation of mitochondrial respiration in permeabilized cardiac tissue, capillary density indicated by platelet endothelial cell adhesion molecule 1 (PECAM-1)-positive area, and key molecular indexes of metabolism and myocardial remodeling by immunoblotting and RT-PCR. MI, myocradial infarction; MSC, mesenchymal stem cell.
Animal characteristics and MI.
Procedures were approved by the Vanderbilt University Animal Care and Use Committee and performed according to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, revised 1996, A3227-01). Mice were randomly separated into three groups: SHAM, MI + phosphate-buffered saline (MI + PBS), and MI + mesenchymal stem cells (MI + MSC). A left anterior descending (LAD) coronary artery ligation model of MI was used. Briefly, at 3 mo of age mice of each group were anesthetized with 50 mg/kg pentobarbital sodium via intraperitoneal injection. All animals underwent a thoracotomy, and a 7-0 silk suture was placed through the myocardium into the anterolateral left ventricle wall, followed by ligation of the LAD coronary artery of C57BL/6 mice in the MI + PBS and MI + MSC groups. Following the LAD ligation and visualization of the infarct manifestation via blanching, 15 μl of PBS, pH 7.2, was injected into the peri-infarct area of the MI + PBS group; 2.5 × 105 MSCs in 15 μl of PBS, pH 7.2, was injected into the peri-infarct area of the MI + MSC group. The experimental protocol of a single administration of 2.5 × 105 cells was employed as a result of previous work by Alfaro et al. (1) showing a modest reduction in infarct size with this experimental cell number. Intraprocedural anesthesia adequacy was ensured by evaluating the respiratory and/or heart rate as well as the absence of withdrawal reflex for all surgical procedures.
MSCs.
Primary MSCs were generated and expanded in DMEM-LG (Biowhittaker), 10% defined FBS (HyClone, Mediatech; Cellgro), antibiotics, fungizone, and 20 μg/ml PDGF, as described previously (1, 29). The immunophenotype of MSCs was identified by FACS analysis, as reported previously (1). MSCs were CD44+ and LY6A/E/ScaI+. The immunofluorescent staining confirmed the cells to be CD45−, CD14−, CD11b−, CD16/32−, CD144−, and CD146−. Confirmation of MSC phenotype (3, 29) was supported by identifying capacity for trilineage (osteoblast, adipocyte, and chondrocyte) differentiation. Differentiation was performed as reported previously (1).
Cardiac performance.
M-mode echocardiography was performed at baseline, 7 and 23 days following the ligation of the LAD coronary artery in conscious mice, as described previously (6, 22).
Catheterization procedures.
The catheterization procedures were performed 23 days post-MI, as described previously (25). Briefly, mice were anesthetized with isoflurane, and the carotid artery and jugular vein were catheterized for arterial sampling and venous infusions, respectively. The free ends of the catheters were tunneled under the skin to the back of the neck, where they were attached via stainless-steel connectors to lines made of Micro-Renathane. These lines were exteriorized, flushed with saline containing 200 U/ml heparin and 5 mg/ml ampicillin, and then sealed with stainless-steel plugs. Postsurgery, the mice were housed individually and given 5–7 days of postoperative recovery prior to hyperinsulinemic euglycemic (insulin) clamps. The postoperative recovery period ensured mice were within 10% of presurgical weight.
Hyperinsulinemic euglycemic clamps.
The insulin clamps were performed, ensuring that previously described technical considerations (2) were employed. Mice were fasted at 5 AM, 5 h prior to commencement of the insulin clamps at 10 AM. Approximately 1 h prior to the experiment, the Micro-Renathane tubing was connected to catheter leads and infusion syringes to allow the mice to acclimate to their environment. A baseline arterial blood sample (∼100 μl) was taken to measure arterial levels of plasma glucose, insulin, nonesterified fatty acids (NEFA) and hematocrit. The insulin clamp was initiated with a constant, continuous infusion of human insulin at 4 mU·kg−1·min−1 maintained throughout the experiment (Humulin R; Eli Lilly). In the 5-h-fasted mouse, the insulin dose of 4 mU·kg−1·min−1 and the corresponding plasma insulin concentration result in complete suppression of hepatic glucose production and increase the rate of glucose disposal approximately threefold over basal conditions (2). This methodology allows assessment of insulin action on peripheral tissues at high physiological insulin levels. Stable euglycemia (∼8.5 mmol/l) was maintained during the insulin clamps by measuring blood glucose. Mice received saline-washed erythrocytes from donors for the duration of the insulin clamps (5 μl/min of saline-washed erythrocytes) to prevent a fall of >5% hematocrit. In all mice, at t = 0 min, ∼50 μl of arterial blood was sampled to determine arterial plasma glucose and NEFA. Immediately after sampling, a bolus containing 2-[14C]deoxyglucose (2-[14C]DG; 13 μCi) and [125I]BMIPP ([125I]β-methyl-p-iodophenylpentadecanoic acid; 20 μCi) was administered into the jugular vein to provide an index of tissue-specific glucose and long-chain fatty acid (LCFA) uptake and clearance. At t = 2, 5, 10, 15, and 20 min, ∼50 μl of arterial blood was sampled to determine arterial plasma glucose, NEFA, 2-[14C]DG and [125I]BMIPP. Hematocrit was also measured at t = 20 min. At t = 30 min, ∼200 μl of arterial blood was taken for the measurement of arterial glucose, insulin, NEFA, 2-[14C]DG, and [125I]BMIPP. Plasma was stored at −20°C until analysis. Mice were then immobilized by cervical dislocation, and tissues were rapidly excised for immediate analysis or flash-frozen in liquid nitrogen and stored at −80°C for future analysis. Tissues collected included the heart, gastrocnemius, superficial vastus lateralis, and white adipose tissue from the epididymal deposits.
Plasma and tissue analyses.
Plasma and tissue [125I]BMIPP radioactivity were determined directly using a γ-counter (Beckman Instruments). Plasma 2-[14C]DG was assessed following deproteinization (24). Tissue-phosphorylated 2-[14C]DG was measured using a liquid scintillation analyzer (PerkinElmer). Plasma insulin was assayed via double-antibody method (19), and plasma NEFA were measured using a colorimetric assay (Wako Chemicals). Plasma glucose was determined from the same deproteinized sample used to determine 2-[14C]DG radioactivity. Briefly, plasma glucose was determined spectrophotometrically (Molecular Devices) in a two-reaction process measuring the arising NADPH at 340 nm. The reaction mixture included 2.84 mg/ml ATP, 200 mM Tris·HCl (pH 7.4), 10 mM MgCl2, 2.03mg/ml NADP, 1.73 U/ml hexokinase, and 0.86 U/ml glucose-6-phoshate dehydrogenase. The relationship between γ-radioactivity and β-emissions was established in the experimental counters.
Mitochondrial respiration and enzymatic measurements.
Peri-infarct permeabilized fibers were prepared as described (15). In brief, cardiac samples were excised following insulin clamps and permeabilized in buffer X [7.23 mM K2EGTA, 2.77 mM CaK2EGTA, 20 mM imidazole, 0.5 mM DTT, 20 mM taurine, 5.7 mM ATP, 14.3 mM PCr, 6.56 mM MgCl2, 50 mM K-MES, and 50 μg/ml saponin (pH 7.1)]. Fiber bundles were washed in buffer Z [105 mM K-MES, 30 mM KCl, 10 mM K2HPO4, 5 mM MgCl2, 5 g/l BSA, and 1 mM EGTA (pH 7.1)]. High-resolution respirometry (Oroboros Instruments) was performed in duplicate at 37°C in buffer Z. Substrates included 10 mM glutamate plus 2 mM malate, 5 mM ADP, and 10 mM succinate.
Peri-infarct citrate synthase activity was determined through adaptation of previous methods (11) at 25°C. Following slight modifications, peri-infarct NADH-cytochrome c oxidoreductase activity (CI + III) was determined as reported previously (16). Protein was determined using the Bradford method (Bio-Rad).
Immunoblotting and immunostaining.
Muscle samples were homogenized in a lysis buffer containing 50 mM HEPES (pH 7.5), 2 mM EDTA, 10% glycerol, 1% NP-40, 150 mM NaCl, 2 mM PMSF, 10 mM β-glycerophosphate, 1 mM CaCl2, 1 mM MgCl2, 2 mM Na3VO4, 10 mg/l aprotinin, 10 mg/l leupeptin, 3 mM benzamidine, 10 mM NaF, and 20 mM NaP. Tissue homogenate was centrifuged (10 min at 1,000 g and 4°C), and protein determination in the supernatant was assessed using the Bradford method (Bio-Rad). Protein expression of glucose transporter 4 (GLUT4), hexokinase II (HKII), peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), glutathione peroxidase (GPx), mitochondrial oxidative phosphorylation complexes I–V (CI–CV), uncoupling protein 3 (UCP3), and adenine nucleotide translocase (ANT) in the peri-infarct region of cardiac tissue was determined. Proteins (20–30 μg) were resolved on NuPAGE 4–12% Bis-Tris gels (Invitrogen) and transferred to a PVDF membrane. The membranes were blocked and subsequently probed with anti-UCP3 (1:1,000; Abcam), anti-ANT (1:500; Santa Cruz Biotechnology), anti-GLUT4 (1:10,000; Abcam), anti-hexokinase (1:1,000; Chemicon), anti-PGC-1α (Santa Cruz Biotechnology; 1:1,000), anti-α-actin (1:2,000; Thermoscientific), and anti-voltage-dependent anion channel (VDAC) (1:1,000; Abcam). OXPHOS complexes of the electron transport chain were determined using MitoProfile Total OXPHOS Rodent WB Antibody Cocktail (1:500; MitoSciences). Following incubation with horseradish peroxidase-conjugated secondary antibodies, the membranes were exposed to a chemiluminescent horseradish peroxidase substrate (Millipore), and imaging was performed using the Chemigenius2 BioImaging System (SynGene). Densitometry was completed using Gene Tools software (Syngene). Protein expression was normalized to VDAC expression, which has routinely been used as a mitochondrial loading control. α-Actin was used as the control for GLUT4, HKII, and PGC-1α.
Capillary density was assessed in 8-μm sections of formalin-fixed, paraffin-embedded cardiac tissue following immunofluorescence detection of platelet endothelial cell adhesion molecule 1 (PECAM-1). For immunofluorescence, sections were washed with PBS and then incubated with a PBS-blocking buffer (1% BSA and 10% goat serum) for 30 min. The slides were then incubated with rabbit anti-PECAM-1 (1:250; Santa Cruz Biotechnology) overnight at 4°C. The sections were washed three times and incubated with the fluorescently labeled secondary antibody (Alexa Fluor 546-labeled anti-rabbit IgG; Invitrogen) at room temperature for 1 h. Sections were washed three times with PBS and mounted with Prolong Gold Antifade Reagent (Invitrogen). Quantification of vascularity (PECAM-1) in the heart was evaluated on nine to 10 randomly chosen fields (objective ×40/0.75) from tissues obtained from nine to 10 mice by fluorescence microscopy. For confocal microscopy analysis, an Olympus BX61 W confocal microscope was used, and images were generated using Fluorview 1000 software. The PECAM-1-positive area of tissue for each field was quantified using Image J by outlining tissue and calculating total area per field. Areas are expressed relative to the SHAM group for the infarct and remote left ventricle.
Quantitative real-time PCR.
Total RNA was extracted from freeze-clamped heart tissue with Trizol reagent (Invitrogen). Reverse transcription, real-time PCR, and data analysis were performed as described previously (26). Real-time PCR reactions were employed with Quantitect mouse atrial natriuretic peptide primer pairs (Qiagen) and the following mouse β-actin primers: forward (TGTTACCAACTGGGACGACA) and reverse (GGGGTGTTGAAGGTCTCAAA). β-Actin was used as a housekeeping gene because it did not change between treatment groups. Relative gene expression levels are presented as the fold change compared with the SHAM group, for which all levels were designated as 1.
Whole body and tissue-specific substrate kinetics.
The metabolic index of glucose (Rg) and FA (Rf) uptake was calculated (25) and expressed (21) as described previously. Whole body glucose (MCRg) and FA (MCRf) clearance were determined as reported previously (27). Rg for cardiac and peripheral tissues was expressed relative to Rg of the brain, which represents the constant reservoir of glucose uptake under various physiological conditions (21). As a result of no tissue exhibiting constant fatty acid uptake, [125I]BMIPP accumulation in the tissue was expressed as absolute values.
Statistical analyses.
One-way ANOVAs were performed to detect statistical differences (P < 0.05). Differences within the ANOVA were determined using Tukey's post hoc test. All data are reported as means ± SE.
RESULTS
Metabolic characteristics.
Prior to the initiation of the insulin clamps, baseline metabolic parameters were evaluated (Table 1). Fasting concentrations of arterial plasma glucose, NEFA, and insulin were similar between groups. Additionally, the experimental plasma insulin concentration in response to the constant insulin infusion was comparable for animals of each treatment group.
Table 1.
Metabolic characteristics of C57BL/6 mice
| SHAM | MI + PBS | MI + MSC | |
|---|---|---|---|
| Fasting plasma glucose, mM | 6.2 ± 0.8 | 6.2 ± 0.3 | 5.8 ± 0.5 |
| Fasting plasma NEFA, mM | 0.98 ± 0.09 | 1.12 ± 0.11 | 1.13 ± 0.22 |
| Fasting plasma insulin, μU/ml | 33.2 ± 4.7 | 24.7 ± 2.9 | 22.5 ± 3.3 |
| Experimental plasma insulin, μU/ml | 79.8 ± 8.4 | 80.8 ± 11.1 | 99.9 ± 13.0 |
Data are means ± SE; n = 8-12 mice/group for plasma glucose, n = 7-8 for plasma nonesterified fatty acids (NEFA), n = 11-15 for fasting plasma insulin, and n = 9-16 for experimental plasma insulin. MI, myocardial infarction; MSC, mesenchymal stem cell.
Cardiovascular parameters.
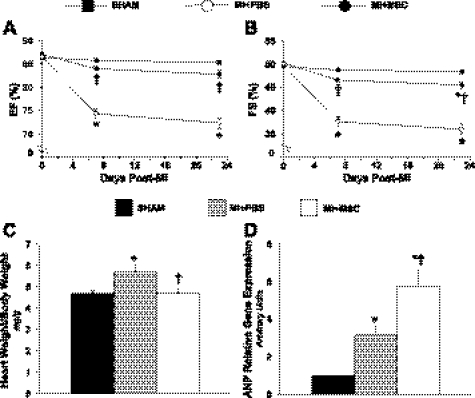
To gain insight into the impact of MSC therapy on left ventricle contractile performance, echocardiography was performed on conscious mice at baseline and 7 and 23 days post-MI. Functional abnormalities (Table 2) were observed in the MI + PBS group, which is indicative of left ventricle systolic impairment with depressed ejection fraction (Fig. 2A) and fractional shortening (Fig. 2B). The effects of MSC transplantation were manifested 7 days postsurgery with preservation of fractional shortening (Fig. 2B). At 23 days, the MSC transplantation blunted abnormalities in ventricular function. MI + PBS mice had a lower fractional shortening than the SHAM and MI + MSC animals (Fig. 2B). However, fractional shortening was reduced in MSC-treated mice (Fig. 2B). The data demonstrate that MSC injection post-MI preserves left ventricle contractile performance. Indices of alterations in cardiac structure and geometry were evaluated. Post-MI hearts exhibited pathological hypertrophy as the heart weight-to-body weight ratio was increased (Fig. 2C). MSC treatment normalized heart-to-body weight ratio (Fig. 2C). Additionally, cardiac atrial natriuretic peptide (ANP) gene expression was elevated in the MI + PBS mice (Fig. 2D). Unexpectedly, ANP was further elevated in the MI + MSC animals (Fig. 2D).
Table 2.
Cardiovascular parameters in the conscious C57BL/6 mice
| Baseline |
7 Days |
23 Days |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| SHAM | MI + PBS | MI + MSC | SHAM | MI + PBS | MI + MSC | SHAM | MI + PBS | MI + MSC | |
| HR, beats/min | 694 ± 4 | 673 ± 7 | 676 ± 10 | 706 ± 13 | 685 ± 22 | 697 ± 6 | 700 ± 10 | 673 ± 18 | 692 ± 9 |
| FS | 0.500 ± 0.005 | 0.495 ± 0.002 | 0.503 ± 0.004 | 0.488 ± 0.005 | 0.376 ± 0.008* | 0.466 ± 0.005† | 0.484 ± 0.004 | 0.360 ± 0.009* | 0.455 ± 0.007*† |
| EF | 0.864 ± 0.004 | 0.866 ± 0.002 | 0.871 ± 0.003 | 0.858 ± 0.004 | 0.743 ± 0.01* | 0.840 ± 0.004† | 0.853 ± 0.004 | 0.723 ± 0.01* | 0.828 ± 0.008† |
| IVSd, cm | 0.067 ± 0.001 | 0.066 ± 0.001 | 0.063 ± 0.001*† | 0.071 ± 0.002 | 0.068 ± 0.002 | 0.068 ± 0.001 | 0.072 ± 0.002 | 0.073 ± 0.002 | 0.069 ± 0.002 |
| LVIDd, cm | 0.287 ± 0.004 | 0.289 ± 0.002 | 0.293 ± 0.004 | 0.300 ± 0.005 | 0.340 ± 0.01* | 0.301 ± 0.007† | 0.290 ± 0.006 | 0.354 ± 0.009* | 0.310 ± 0.008† |
| LVPWd, cm | 0.052 ± 0.001 | 0.054 ± 0.001 | 0.054 ± 0.001 | 0.060 ± 0.002 | 0.066 ± 0.002 | 0.064 ± 0.002 | 0.060 ± 0.002 | 0.062 ± 0.002 | 0.064 ± 0.002 |
| IVSs, cm | 0.119 ± 0.002 | 0.116 ± 0.002 | 0.110 ± 0.002* | 0.120 ± 0.001 | 0.106 ± 0.003* | 0.115 ± 0.003 | 0.120 ± 0.002 | 0.114 ± 0.002 | 0.117 ± 0.004 |
| LVIDs, cm | 0.145 ± 0.003 | 0.144 ± 0.001 | 0.146 ± 0.002 | 0.154 ± 0.003 | 0.213 ± 0.008* | 0.16 ± 0.004† | 0.150 ± 0.003 | 0.227 ± 0.008* | 0.169 ± 0.006† |
| LVPWs, cm | 0.084 ± 0.001 | 0.081 ± 0.001 | 0.085 ± 0.002 | 0.085 ± 0.001 | 0.095 ± 0.003* | 0.095 ± 0.002* | 0.088 ± 0.002 | 0.090 ± 0.003 | 0.091 ± 0.002 |
Data are means ± SE for n = 15–19 mice/group. HR, heart rate; FS, fractional shortening; EF, ejection fraction; IVSd, interventricular septal thickness in diastole; LVIDd, left ventricle end-diastolic dimension; LVPWd, left ventricle posterior wall thickness in diastole; IVSs, interventricular septal thickness in systole; LVIDs, left ventricle end-systolic dimension; LVPWs, left ventricle posterior wall thickness in systole.
P < 0.05 vs. SHAM at specified time point;
P < 0.05 vs. MI + PBS at specified time point.
Fig. 2.
Cardiac functional and structural indices. A: cardiac ejection fraction (EF) at BL, 7 and 23 days following a MI. B: fractional shortening (FS) at BL, 7 and 23 days following a MI; n = 15–19 mice/group. C: heart weight/body weight ratio 28 days following a MI; n = 12–18 mice/group. D: cardiac atrial natriuretic peptide (ANP) gene expression 28 days following a MI relative to the SHAM mice; n = 6–7 mice/group. Data are means ± SE. *P < 0.05 vs. SHAM; †P < 0.05 vs. MI + PBS.
Metabolic indices of cardiac-specific glucose and FA metabolism.
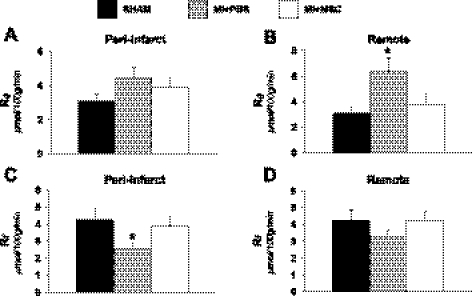
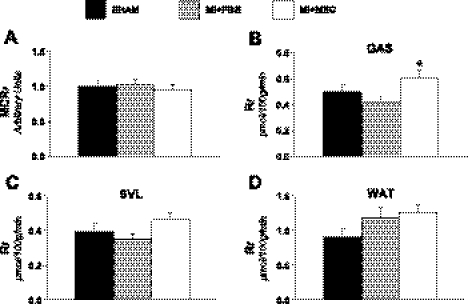
Isotopic tracers were administered during insulin clamps in the conscious, unrestrained mouse to evaluate glucose and FA uptake at high physiological insulin levels. Cardiac glucose uptake (Rg) exhibited regional differences in the infarcted heart. MI + PBS mice were found to have a similar peri-infarct glucose uptake compared with SHAM mice (Fig. 3A). However, the remote left ventricle glucose utilization was elevated (Fig. 3B). MSC therapy inhibited the increase in remote left ventricle glucose metabolism (Fig. 3B). Similarly, insulin-stimulated cardiac Rf displayed site-specific differences following a MI. The peri-infarct FA uptake was depressed, and the remote left ventricle was unaltered in the MI + PBS animals (Fig. 3, C and D). More importantly, this is the first report to demonstrate that MSC transplantation maintains peri-infarct FA uptake post-MI (Fig. 3C).
Fig. 3.
Cardiac metabolic indices. A: metabolic index of glucose uptake (Rg) in the peri-infarct. B: Rg in the remote left ventricle. C: metabolic index of long-chain fatty acid (LCFA) uptake (Rf) in the peri-infarct. D: metabolic index of LCFA uptake in the remote left ventricle. Cardiac Rg values are relative to brain Rg; n = 8–12 mice/group. Data are means ± SE. *P < 0.05 vs. SHAM.
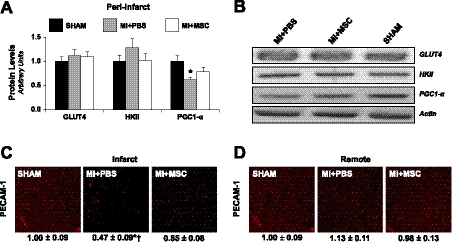
Given the alterations in substrate kinetics, protein levels of key regulators of substrate metabolism were evaluated. GLUT4 and HKII, key mediators in glucose transport and phosphorylation, were comparable (Fig. 4, A and B). However, PGC-1α, a transcriptional cofactor exhibiting a central role in the regulation of FA oxidation, mitochondrial biogenesis, and OXPHOS, was depressed post-MI (Fig. 4, A and B). Substrate uptake is also influenced by substrate availability, muscle blood flow, and capillary recruitment (31). The reduced peri-infarct FA utilization may be influenced by differences in vascularity given that infarct PECAM-1-positive area was lower in MI + PBS mice (Fig. 4C). However, aberrations in substrate utilization in the remote left ventricle were independent of vascular density because PECAM-1-positive area was similar between groups (Fig. 4D).
Fig. 4.
Metabolic regulators of substrate metabolism and capillary density. A: glucose transporter 4 (GLUT4), hexokinase II (HKII), and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) from the peri-infarct region, as determined by immunoblotting. B: representative immunoblotting performed to measure GLUT4, HKII, and PGC-1α. Protein levels are normalized to actin content and are relative to the SHAM group; n = 6–8 mice/group. C: PECAM-1 immunofluorescence staining of the infarct. D: PECAM-1 immunofluorescence staining of the remote left ventricle. Vascular density determined as the %immunopositive PECAM-1 area/total tissue area. Values are relative to SHAM left ventricle; n = 9–10 mice/group. Scale bar, 50 μm. Data are means ± SE. *P < 0.05 vs. SHAM. Of note, the order presentation of the groups in B is different from the order presentation of the groups in A, C, and D. The SHAM group presented in C and D is the same data set.
Metabolic indices of whole body and peripheral tissue glucose metabolism.
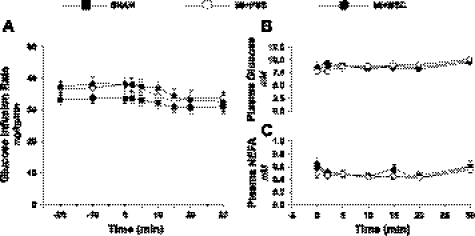
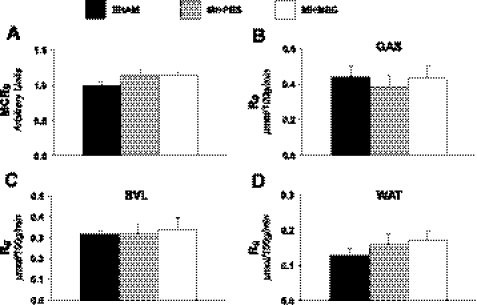
To assess insulin sensitivity, insulin clamps were performed in conscious animals. Glucose infusion rate (Fig. 5A) was not different between treatments, indicating no difference in whole body insulin sensitivity with MSC administration. Figure 5, B and C, represents a time course of arterial plasma glucose and NEFA concentration during the insulin clamps and confirms that stable euglycemia was achieved. Additionally, systemic glucose uptake was examined to evaluate whole body metabolic alterations following a MI and/or MSC therapy. Whole body clearance of glucose from the blood (MCRg; Fig. 6A) was comparable between groups. Peripheral tissue glucose uptake was assessed. Specifically, gastrocnemius (Fig. 6B), superficial vastus lateralis (Fig. 6C), and white adipose tissue (Fig. 6D) substrate utilization was determined. Similar to whole body glucose clearance, peripheral tissue glucose utilization was similar between groups.
Fig. 5.
Insulin sensitivity and plasma substrates during the hyperinsulinemic euglycemic (insulin) clamp. A: glucose infusion rate (GIR) during the insulin clamp. The GIR is equivalent to glucose disposal rate in response to insulin stimulation. The GIR is presented as a time course starting 20 min prior to administration of 2-[14C]DG and [125I]BMIPP (−20-min time point) until 30 min following 2-[14C]DG and [125I]BMIPP infusion (30-min time point). B: arterial plasma glucose concentration following 2-[14C]DG and [125I]BMIPP injection. C: arterial plasma nonesterified fatty acids (NEFA) in mice following 2-[14C]DG and [125I]BMIPP injection. Data are means ± SE for n = 8–12 mice/group.
Fig. 6.
Whole body and peripheral tissue glucose utilization. A: index of whole body glucose clearance rate (MCRg). MCRg values are relative to the SHAM group. SHAM values are set to an arbitrary value of 1. B: Rg in the gastrocnemius (GAS). C: Rg in the superficial vastus lateralis (SVL). D: Rg in white adipose tissue (WAT). Tissue Rg values are relative to brain Rg; n = 8–12 mice/group.
Metabolic indices of whole body and peripheral tissue long-chain FA metabolism.
Peripheral FA metabolism was also evaluated following a MI and/or MSC transplantation. Whole body LCFA clearance from the circulation (MCRf; Fig. 7A) was similar between groups. Identical to glucose utilization, peripheral tissue FA uptake was assessed. Gastrocnemius (Fig. 7B), superficial vastus lateralis (Fig. 7C), and white adipose tissue (Fig. 7D) FA uptake kinetics were determined. Unexpectedly, gastrocnemius FA uptake was significantly lower in the MI + PBS mice (Fig. 7B).
Fig. 7.
Whole body and peripheral tissue LCFA utilization. A: index of whole body LCFA clearance rate (MCRf). MCRf values are relative to the SHAM group. SHAM values are set to an arbitrary value of 1. B: metabolic index of LCFA uptake (Rf) in the GAS. C: Rf in the superficial vastus lateralis. D: Rf in WAT; n = 8–11 mice/group. Data are means ± SE. *P < 0.05 vs. MI + PBS.
Cardiac mitochondrial function.
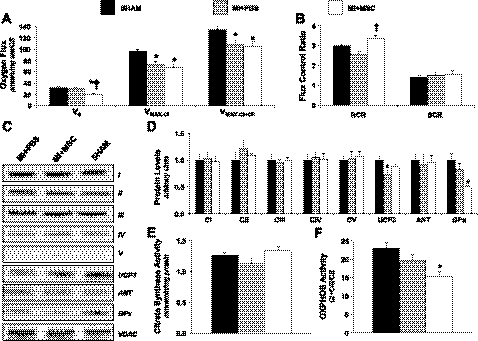
Because contractile function and substrate utilization are affected by energy metabolism, polarographic oxygen consumption measurements were performed using permeabilized cardiac fibers to evaluate the integrative function of OXPHOS. MSC therapy lowered basal oxygen consumption through complex I (V0; Fig. 8A). Adenosine diphosphate (ADP)-stimulated oxygen consumption through complex I was reduced in MI + PBS and MI + MSC animals (Vmax-CI; Fig. 8A). Similarly, ADP-stimulated oxygen consumption via convergent electron flux was reduced in both treatment groups (Vmax-CI + CII; Fig. 8A). The succinate control ratio was unchanged, suggesting that complex II is not responsible for the lower respiratory capacity (Fig. 8B). Interestingly, the respiratory control ratio (RCR) was depressed in the MI + PBS mice (Fig. 8B) compared with the MI + MSC mice. This indicates reduced coupling of ADP phosphorylation and oxygen consumption and translates into a decreased efficiency of mitochondrial OXPHOS in the infarcted mice. Citrate synthase activity was assessed to determine the influence of mitochondrial heterogeneity on the reduced oxygen flux. Citrate synthase activity was similar between groups, excluding any effect of mitochondrial content on OXPHOS (Fig. 8E). This prompted the evaluation of mitochondrial protein levels. Protein expression of mitochondrial OXPHOS CI–CV and ANT were unchanged (Fig. 8, C and D). The absence of differences in many of the mitochondrial protein levels did not account for the reduced oxygen flux following MSC therapy. However, a reduced CI + CIII activity (Fig. 8F) provides a mechanism, in part, for the depressed oxygen flux through CI in MI + MSC mice. Peri-infarct UCP3 was lower in the MI + PBS mice (Fig. 8, C and D), consistent with reduced FA uptake. The lower UCP3 may be an adaptive response to depressed FA utilization since this protein has been speculated to function as a FA anion transporter. In addition to generating ATP, mitochondria are a source of reactive oxygen species (ROS). The alterations in mitochondrial function and efficiency provided the rationale for cardiac antioxidant defense assessment. GPx, a ROS scavenger, exhibited lower protein levels in the MI + MSC mice (Fig. 8, C and D).
Fig. 8.
Peri-infarct mitochondria. A: peri-infarct permeabilized cardiac fiber basal oxygen consumption supported by glutamate and malate (V0), maximal oxygen consumption supported by glutamate and malate through complex I (Vmax-CI), and maximal convergent oxygen consumption supported by glutamate, malate, and succinate (Vmax-CI + CII). B: respiratory control ratio (RCR; defined as Vmax-CI/V0) and succinate control ratio (SCR; defined as Vmax-CI + CII/Vmax-CI); n = 8 mice/group. C: mitochondrial oxidative phosphorylation (OXPHOS) complexes, uncoupling protein 3 (UCP3), adenine nucleotide translocase (ANT), and glutathione peroxidase (GPx) from the peri-infarct region, as determined by immunoblotting. D: representative immunoblotting of OXPHOS complexes, UCP3, ANT, GPx, and voltage-dependent anion channel (VDAC). Protein levels were normalized to VDAC content and are relative to the SHAM group; n = 6 mice/group. Of note, the order presentation of the groups in D is different from the order presentation of the groups in C. E: citrate synthase (CS) activity (mmol·min−1·mg protein−1). F: relative CI + CIII activity (mol·min−1·mg protein) normalized to CS; n = 6–8 mice/group. Data are means ± SE. *P < 0.05 vs. SHAM; †P < 0.05 vs. MI + PBS.
DISCUSSION
Metabolic remodeling is a hallmark characteristic post-MI (20). This study uniquely combines a series of technically challenging experiments to address the therapeutic value of MSCs as a metabolic modulator to rescue the infarcted heart from contractile and energetic dysfunction in the conscious mouse. We report regional differences in insulin-stimulated cardiac glucose uptake post-MI. The peri-infarct glucose uptake was comparable, and the remote left ventricle glucose uptake was increased. Novel findings show that MSC transplantation normalized the deranged insulin-stimulated cardiac glucose uptake in vivo. This extends a recent report that MSC transplantation prevents increases in basal glucose uptake ex vivo (8). Additionally, insulin-stimulated FA utilization exhibited site-specific alterations in the infarcted heart. A depression in the peri-infarct FA uptake was observed; however, the remote left ventricle was unaffected. In agreement, a reduced utilization of FA is an oft-reported alteration under basal conditions following a MI (10). Moreover, this is the first study to show that MSC treatment preserves in vivo peri-infarct FA uptake. A growing number of reports postulate that FAs are required for optimal left ventricle function (30, 32). We found that MSC therapy minimized left ventricle systolic dysfunction and pathological hypertrophy, as indicated by improved fractional shortening and reduced heart-to-body weight ratio, respectively. Our findings suggest that in the early stages post-MI the maintenance of a metabolic network that can utilize both FAs and glucose has positive associations with improved cardiac structure and function.
The switch away from FA utilization toward a glucose preference may be in response to alterations in substrate availability. Following a MI, capillary density is lost in the insulted and surrounding cardiac regions (33). Our findings show that the infarcted region of the MI + PBS mice has a lower PECAM-1-positive area, suggesting a reduction in capillary supply to the insulted region of the heart. The decline in infarct capillary density likely contributes to the reduced peri-infarct FA utilization in the untreated mice. In contrast, the transplantation of MSCs preserved capillary density in the infarcted mouse heart and would assist in the maintenance of cardiac FA uptake.
Alterations in substrate utilization may also be the result of abnormal molecular regulation. Of note, PGC-1α is a transcription factor coactivator that promotes the expression of target genes involved in FA uptake and oxidation (17). Our results indicate that PGC-1α protein levels were reduced in the peri-infarct region following a MI; however, levels were maintained in the peri-infarct region of MSC-treated mice. These results suggest that MSC therapy inhibits the decline in PGC-1α. This would maintain activation of genes involved in FA metabolism and assist in preserving FA utilization in the peri-infarct region of the MI + MSC mice. The decline in PGC-1α following a MI may also link abnormalities in cardiac FA metabolism and mitochondrial function.
PGC-1α promotes mitochondrial biogenesis, respiration, and OXPHOS coupling in addition to its role in FA metabolism (17). Upon evaluation, maximal oxygen flux was reduced in the peri-infarct bundles of the infarct-only hearts. Our findings indicate that the decline in ADP-stimulated respiration was unrelated to alterations in electron transport. Protein levels of mitochondrial CI–CIV as well as the enzymatic activity were unchanged in the MI + PBS mice. The reduction in maximal respiration in the absence of electron transport chain alterations suggests that the phosphorylation system is dysfunctional. Further support for alterations in the phosphorylation system is the reduced RCR in the MI + PBS mice. This ratio represents coupling between ADP phosphorylation and oxygen consumption and may be related to lower PGC-1α levels.
Of particular interest, cell-based therapy appears to elicit changes in cardiac mitochondria that do not completely normalize energetics. Recently, Eun et al. (5) reported that MSC transplantation into infarcted rat hearts produces ATP synthesis-related metabolite profiles that are significantly different from those of control and infarct-only animals. MSC transplantation has also been shown to reduce CI subunit gene expression in the peri-infarct region 4 mo post-MI (12). We did not identify differences in the protein levels of the OXPHOS complexes, which may be due to the current study determining alterations in CI at 4 wk post-MI rather than 4 mo. Additionally, the contrasting findings may arise from the evaluation of different CI subunits. However, in agreement with the previous report that MSC therapy augments cardiac CI characteristics, our data show a reduced CI + CIII activity induced by the MSC treatment. This catalytic reduction in OXPHOS components contributes to the depressed oxygen flux in the peri-infarct fiber bundles following MSC injection. The causation for the lowered OXPHOS enzymatic activity is currently unknown. Jameel et al. (12) hypothesized that a reduction in regional wall stress and energetic demands following the cell-based therapy may result in the differential CI subunit gene expression and may contribute to OXPHOS enzymatic alterations. In support, we observed cardiac ANP gene expression to be increased in the MI + MSC mice above that of the MI + PBS and SHAM animals. The biological functions of ANP include dieresis, natriuresis, and hypotensive promotion as well as antihypertrophic and -fibrotic actions (23). In speculation, the large increase in ANP expression mediated by the MSC transplantation may have augmented wall stresses, leading to CI alterations. Additionally, previous work evaluating MSC administration into the infarcted swine heart has been reported to blunt the peri-infarct PCr/ATP ratio decline (7, 33). We found the RCR to be higher in MSC-treated mice compared with MI + PBS mice. This suggests that MSCs preserve mitochondrial efficiency and ADP responsiveness for adequate high-energy phosphate provision to assist in the maintenance of the PCr/ATP ratio.
To date, few data are available concerning the influence of MSC transplantation on cardiac oxidative stress and antioxidant status. Of interest, Kim et al. (13) reported that human dermal fibroblasts treated with the conditioned culture medium of adipose-derived MSCs augment the activity of fibroblast GPx and superoxide dismutase. In agreement, our findings also show that MSC treatment influences the antioxidant defense systems of the heart. Specifically, MSC transplantation depressed GPx protein levels in the peri-infarct region 4 wk post-MI. Although preliminary, this may indicate that cell-based therapies induce alterations in the antioxidant defense systems. Future work is warranted to identify the role of MSC transplantation on ROS production and whether MSCs minimize oxidative stress as a means of cardioprotection following a MI.
The therapeutic influence of intramyocardial administration of MSCs also extends beyond the heart. FA uptake by the gastrocnemius was lower in the MI + PBS mice compared with the MI + MSC animals. To our knowledge, this is the first study to address in vivo skeletal muscle substrate utilization post-MI. As such, mechanisms for the depressed FA uptake observed are currently unknown. Previous reports indicate that PGC-1α expression is reduced in the gastrocnemius of a rat MI model (34). A reduction in PGC-1α would explain, in part, the observed depression in gastrocnemius FA uptake.
CONCLUSION
These experiments are the first to combine a comprehensive in vivo approach for evaluating insulin sensitivity, substrate uptake, and cardiac function with stem cell therapy following a standardized MI in the conscious mouse. The experimental approach required for these studies is technically demanding but can be applied to a variety of murine models to further address mechanisms. Our findings show that MSCs exhibit therapeutic potential as a metabolic intervention that attenuates post-MI energetic abnormalities. Specifically, the MSC therapy inhibited a shift in substrate reliance toward glucose and increased mitochondrial ADP responsiveness for improved energetic efficiency. This preserves metabolic flexibility and improves the heart's ability to meet the energy demands of cardiac contraction.
GRANTS
This work was supported by the Canadian Institutes of Health Research, the Alberta Heritage Foundation of Medical Research, the Canadian Diabetes Association, the Heart and Stroke Foundation, and the National Institutes of Health (Grant nos. R01-HL-088424, DK-054902, and U24-DK-059637).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.C.H., P.P.Y., D.H.W., J.N.R., D.S.H., and J.S. did the conception and design of the research; C.C.H., V.L.J., L.M., F.D.J., P.P.Y., and J.S. performed the experiments; C.C.H., V.L.J., D.H.W., and J.N.R. analyzed the data; C.C.H. interpreted the results of the experiments; C.C.H. prepared the figures; C.C.H. drafted the manuscript; C.C.H., V.L.J., P.P.Y., D.H.W., J.N.R., D.S.H., and J.S. edited and revised the manuscript; C.C.H., V.L.J., L.M., F.D.J., P.P.Y., D.H.W., J.N.R., D.S.H., and J.S. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We acknowledge the Vanderbilt Mouse Metabolic Phenotypying Center, Cardiovascular Pathophysiology and Complications Core, Metabolic Pathophysiology Core, and Analytical Resources Core. We thank Deanna Bracy for assistance during catheterization surgeries, Dr. Russ Knapp (Oak Ridge International Laboratories) for generously providing BMIPP, and Dr. Jeff Clanton for performing BMIPP radioionization.
REFERENCES
- 1. Alfaro MP, Pagni M, Vincent A, Atkinson J, Hill MF, Cates J, Davidson JM, Rottman J, Lee E, Young PP. The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc Natl Acad Sci USA 105: 18366–18371, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55: 390–397, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem 89: 1235–1249, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Dinsmore JH, Dib N. Stem cell therapy for the treatment of acute myocardial infarction. Cardiol Clin 28: 127–138, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Eun LY, Song H, Choi E, Lee TG, Moon DW, Hwang D, Byun KH, Sul JH, Hwang KC. Implanted bone marrow-derived mesenchymal stem cells fail to metabolically stabilize or recover electromechanical function in infarcted hearts. Tissue Cell 43: 238–245, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Exil VJ, Roberts RL, Sims H, McLaughlin JE, Malkin RA, Gardner CD, Ni G, Rottman JN, Strauss AW. Very-long-chain acyl-coenzyme a dehydrogenase deficiency in mice. Circ Res 93: 448–455, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Feygin J, Mansoor A, Eckman P, Swingen C, Zhang J. Functional and bioenergetic modulations in the infarct border zone following autologous mesenchymal stem cell transplantation. Am J Physiol Heart Circ Physiol 293: H1772–H1780, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Gnecchi M, He H, Melo LG, Noiseaux N, Morello F, de Boer RA, Zhang L, Pratt RE, Dzau VJ, Ingwall JS. Early beneficial effects of bone marrow-derived mesenchymal stem cells overexpressing Akt on cardiac metabolism after myocardial infarction. Stem Cells 27: 971–979, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 103: 1204–1219, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heather LC, Cole MA, Lygate CA, Evans RD, Stuckey DJ, Murray AJ, Neubauer S, Clarke K. Fatty acid transporter levels and palmitate oxidation rate correlate with ejection fraction in the infarcted rat heart. Cardiovasc Res 72: 430–437, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Hepple RT, Baker DJ, Kaczor JJ, Krause DJ. Long-term caloric restriction abrogates the age-related decline in skeletal muscle aerobic function. FASEB J 19: 1320–1322, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Jameel MN, Li Q, Mansoor A, Qiang X, Sarver A, Wang X, Swingen C, Zhang J. Long-term functional improvement and gene expression changes after bone marrow-derived multipotent progenitor cell transplantation in myocardial infarction. Am J Physiol Heart Circ Physiol 298: H1348–H1356, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim WS, Park BS, Kim HK, Park JS, Kim KJ, Choi JS, Chung SJ, Kim DD, Sung JH. Evidence supporting antioxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. J Dermatol Sci 49: 133–142, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Kolwicz SC, Jr, Tian R. Metabolic therapy at the crossroad: how to optimize myocardial substrate utilization? Trends Cardiovasc Med 19: 201–207, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc 3: 965–976, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Lee-Young RS, Griffee SR, Lynes SE, Bracy DP, Ayala JE, McGuinness OP, Wasserman DH. Skeletal muscle AMP-activated protein kinase is essential for the metabolic response to exercise in vivo. J Biol Chem 284: 23925–23934, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 106: 847–856, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lemieux H, Hoppel CL. Mitochondria in the human heart. J Bioenerg Biomembr 41: 99–106, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Morgan CR, Lazarow A. Immunoassay of pancreatic and plasma insulin following alloxan injection of rats. Diabetes 14: 669–671, 1965 [DOI] [PubMed] [Google Scholar]
- 20. Neubauer S. The failing heart—an engine out of fuel. N Engl J Med 356: 1140–1151, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Rottman JN, Bracy D, Malabanan C, Yue Z, Clanton J, Wasserman DH. Contrasting effects of exercise and NOS inhibition on tissue-specific fatty acid and glucose uptake in mice. Am J Physiol Endocrinol Metab 283: E116–E123, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Rottman JN, Ni G, Khoo M, Wang Z, Zhang W, Anderson ME, Madu EC. Temporal changes in ventricular function assessed echocardiographically in conscious and anesthetized mice. J Am Soc Echocardiogr 16: 1150–1157, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Saito Y. Roles of atrial natriuretic peptide and its therapeutic use. J Cardiol 56: 262–270, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Shearer J, Fueger PT, Bracy DP, Wasserman DH, Rottman JN. Partial gene deletion of heart-type fatty acid-binding protein limits the severity of dietary-induced insulin resistance. Diabetes 54: 3133–3139, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Shearer J, Fueger PT, Wang Z, Bracy DP, Wasserman DH, Rottman JN. Metabolic implications of reduced heart-type fatty acid binding protein in insulin resistant cardiac muscle. Biochim Biophys Acta 1782: 586–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shearer J, Ross KD, Hughey CC, Johnsen VL, Hittel DS, Severson DL. Exercise training does not correct abnormal cardiac glycogen accumulation in the db/db mouse model of type 2 diabetes. Am J Physiol Endocrinol Metab 301: E31–E39, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Shearer J, Severson DL, Su L, Belardinelli L, Dhalla AK. Partial A1 adenosine receptor agonist regulates cardiac substrate utilization in insulin-resistant rats in vivo. J Pharmacol Exp Ther 328: 306–311, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Taegtmeyer H. Energy metabolism of the heart: from basic concepts to clinical applications. Curr Probl Cardiol 19: 59–113, 1994 [DOI] [PubMed] [Google Scholar]
- 29. Tropel P, Noel D, Platet N, Legrand P, Benabid AL, Berger F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res 295: 395–406, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Tuunanen H, Engblom E, Naum A, Scheinin M, Nagren K, Airaksinen J, Nuutila P, Iozzo P, Ukkonen H, Knuuti J. Decreased myocardial free fatty acid uptake in patients with idiopathic dilated cardiomyopathy: evidence of relationship with insulin resistance and left ventricular dysfunction. J Card Fail 12: 644–652, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Wasserman DH. Four grams of glucose. Am J Physiol Endocrinol Metab 296: E11–E21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yan J, Young ME, Cui L, Lopaschuk GD, Liao R, Tian R. Increased glucose uptake and oxidation in mouse hearts prevent high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation 119: 2818–2828, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zeng L, Hu Q, Wang X, Mansoor A, Lee J, Feygin J, Zhang G, Suntharalingam P, Boozer S, Mhashilkar A, Panetta CJ, Swingen C, Deans R, From AH, Bache RJ, Verfaillie CM, Zhang J. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation 115: 1866–1875, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Zoll J, Monassier L, Garnier A, N'Guessan B, Mettauer B, Veksler V, Piquard F, Ventura-Clapier R, Geny B. ACE inhibition prevents myocardial infarction-induced skeletal muscle mitochondrial dysfunction. J Appl Physiol 101: 385–391, 2006 [DOI] [PubMed] [Google Scholar]