Abstract
Testosterone (T) plays a permissive role in the development of benign prostatic hyperplasia (BPH), and phosphodiesterase 5 inhibitors (PDE5is) have been found to be effective for BPH and lower urinary tract symptoms (LUTS) in clinical trials. This study investigated the effect of T on smooth muscle (SM) contractile and regulatory signaling pathways, including PDE5 expression and functional activity in prostate in male rats (sham-operated, surgically castrated, and castrated with T supplementation). In vitro organ bath studies, real-time RT-PCR, Western blot analysis, and immunohistochemistry were performed. Castration heavily attenuated contractility, including sensitivity to phenylephrine with SM myosin immunostaining revealing a disrupted SM cell arrangement in the stroma. PDE5 was immunolocalized exclusively in the prostate stroma, and orchiectomy signficantly reduced PDE5 immunopositivity, mRNA, and protein expression, along with nNOS and ROKβ mRNA, whereas it increased eNOS plus α1a and α1b adrenoreceptor expression in castrated animals. The PDE5i zaprinast significantly increased prostate strip relaxation to the nitric oxide donor sodium nitroprusside (SNP) in control but not castrated rats. But SNP alone was more effective on castrated rats, comparable with sham treated with SNP plus zaprinast. T supplementation prevented or restored all above changes, including SNP and zaprinast in vitro responsiveness. In conclusion, our data show that T positively regulates PDE5 expression and functional activities in prostate, and T ablation not only suppresses prostate size but also reduces prostatic SM contractility, with several potential SM contraction/relaxation pathways implicated. Zaprinast findings strongly suggest a major role for PDE5/cGMP in this signaling cascade. PDE5 inhibition may represent a novel mechanism for treatment of BPH.
Keywords: benign prostatic hyperplasia, contraction, lower urinary tract symptoms, phosphodiesterase 5, phosphodiesterase 5 inhibitors, smooth muscle myosin, nitric oxide
benign prostatic hyperplasia (BPH), a cause of lower urinary tract symptoms (LUTS) and erectile dysfunction (ED) in aging men, is a common pathological process with a histological prevalence of ∼50–60% for men in their 60s and 80–90% for men in their 70s and 80s (54). Although the etiology of prostatic hyperplasia is not well understood, androgens and aging are necessary for the development of BPH (55) containing two physiological components, static (increased prostate size) (40) and dynamic [increased prostatic smooth muscle (SM) tone] (11).
The dynamic tone of prostatic SM is regulated mainly by the α1-adrenoreceptor (72), with up to 50% of the total urethral pressure in BPH patients being attributed to α-adrenoreceptor-mediated muscle tone (18), and α1-blockers are the first-line therapy for BPH. Three major subtypes of adrenoreceptors have been identified to exist in the prostate at the molecular level (α1a, α1b, and α1d) (15). Eckert et al. (14), using a patch clamp combined with the fura-2 fluorescence calcium labeling technique, demonstrated that prostatic SM cells from BPH patients exhibited coupling of α1-adrenoceptors to phospholipase C converting phosphoinositol-4,5-bisphosphate into diacylglycerol (DAG). DAG is an endogenous activator of protein kinase C and inositol trisphosphate, which releases Ca2+ from intracellular stores stimulating L-type Ca2+-channel current via Ca2+/calmodulin-dependent protein kinase induced phosphorylation of voltage-dependent Ca2+ channels (14). However, the SM cells in the prostate have not been characterized extensively, but presumably the molecular basis for prostatic SM contraction is similar to other SMs involving SM myosin (SMM) phosphorylation and dephosphorylation resulting from increases and decreases, respectively, in cytosolic free Ca2+ ([Ca2+]i) (1).
Generally, stimulation of the adrenergic nerve system increases [Ca2+]i (23). The [Ca2+]i binds to calmodulin, which activates myosin light-chain kinase, which then phosphorylates the 20-kDa regulatory myosin light chains and induces the development of force (1, 13). When the [Ca2+]i returns to basal levels, the calcium sensitivity increases, preventing myosin dephosphorylation, which involves the RhoA/Rho kinase (ROK) mechanism, thus maintaining force (61). Indeed, both isoforms of ROK (ROKα and ROKβ) were found to be expressed in human prostate, and ROK was shown to mediate endothelin and norepinephrine contraction of prostatic SM (62). Most recently, Saito et al. (57) showed that rat prostate contains RhoA, RhoB, RhoC, ROKα, and ROKβ. Moreover, the ROK inhibitor fasudil significantly inhibited carbachol- or norepinephrine-induced prostatic contractions in a dose-dependent manner, suggesting that the RhoA/ROK pathway plays an important role in the autonomic nerve-mediated contractile responses in the prostate.
On the other hand, decrease of free Ca2+ in the sarcoplasm is caused mainly by the nitric oxide (NO)/cyclic guanosine monophosphate (cGMP)/cGMP-dependent protein kinase-1 (cGKI)/phosphodiesterase 5 (PDE5) pathway, followed by calmodulin dissociation from myosin light-chain kinase, leading to SMM dephosphorylation and subsequent SM relaxation (20). This signaling pathway has been indentified in human prostate and colocalization of α-actin with cGMP, and cGKI supports the role of this pathway in regulating prostatic SM contractility (71). Indeed, the PDE5 inhibitors (PDE5is) sildenafil and zaprinast have been shown to induce ∼50 and 70% relaxation, respectively, of the phenylephrine (PE)-precontracted prostate, further supporting a role for PDE5 in modulating prostate contractility (36). PDE5 inhibition can also potentiate tamsulosin (a selective α1a-blocker)-induced prostate relaxation, demonstrating an interaction between adrenergic and NO/cGMP signaling (36).
The current standard therapy for bothersome BPH/LUTS is α1-adrenergic antagonist (α-blocker) and antiandrogenic 5α-reductase inhibitor, targeting the dynamic and static components, respectively (52, 55). Although effective, these drugs may be associated with unwanted side effects, including dizziness, hypotension, and sexual dysfunction, which may be exacerbated by combination therapy (41). PDE5is such as tadalafil, sildenafil, and vardenafil, the first-line treatment for ED, have been explored for treating BPH/LUTS because a link between BPH/LUTS because and ED is well supported by studies of their epidemiology, pathophysiology, and treatment (3, 31, 43). Increasing evidence has demonstrated that PDE5is significantly improved multiple measurements of LUTS and related quality of life, except for overall peak urinary flow, when compared with placebo (44, 45, 56, 59). Although the clinical importance of the relatively limited improvements in flow rates remains a subject of debate, the mechanisms by which PDE5is might influence LUTS and the relationship between improvements in LUTS and erectile function are intriguing areas to be investigated.
NO/cGMP/PDE5 is the major signaling pathway mediating penile erection. It is believed that a similar mechanism exists in the prostate. To date, at least 21 PDE types have been identified with PDE4, -5 (67), and -11 (69) expressed mainly in the prostate. Most recently, PDE9 was also detected in human prostate but mainly in the prostatic urethral urothelium (49). We demonstrated that PDE5 is expressed in the rat lower urogenital tract, with the higher mRNA levels in the corpus cavernosum, vas deferens, and prostate compared with the epididymis and testis (77). However, this study did not examine bladder. More recently, an apparently even higher expression of PDE5 in bladder compared with prostate, testis, and epididymis was reported (48). Examination of human tissues revealed similar findings (16, 47). In addition to modulating SM tone by cGMP degradation, PDE5 also regulates cell proliferation (2, 74). Zenzmaier et al. (74) have reported that tadalafil reduces proliferation of primary prostate stromal cells and to a lesser extent primary prostate basal epithelial cells in a dose-dependent manner. Moreover, tadalafil strongly attenuated TGFβ1-induced fibroblast-to-myofibroblast trans-differentiation, suggesting another effect on the static component of BPH in vivo. Recently, PDE5i was demonstrated to regulate prostate blood flow. Tadalafil increased spontaneous hypertensive rat prostate tissue oxygenation, suggesting a possible mechanism through which PDE5is exert beneficial effects on LUTS (47).
We have demonstrated that testosterone (T) positively regulates PDE5 expression and tadalafil in vivo activity in rat corpus cavernosum (76, 77). This was also confirmed by others (46, 65). Interestingly, PDE5 in the urinary bladder and vas deferens is also androgen dependent (17, 38). These animal studies are also in line with clinical reports that a lack of response to PDE5is in hypogonadal patients, including diabetes, can be reversed by an adequate T supplementation, demonstrating a null effect of hypogonadism on penile erection, most probably because androgen reduction produces both decreased cGMP formation (effect on NO) and degradation (effect on PDE5) (10, 22). On the other hand, chronic treatment with PDE5is increased serum T level and Leydig cells' steroidogenic capacity (4, 21). As an accessory sexual organ, testicular androgens are required for prostate development, puberty, and aging. The aim of the current study was to investigate T effects on prostatic PDE5 at both molecular and functional activity levels, whereas SM contractile and regulatory pathways, including α-adrenergic, ROK, and NO synthase (NOS) signaling pathways, were also examined.
MATERIALS AND METHODS
Animals and tissues.
A total of 46 male Sprague-Dawley rats weighing 300–350 g were used in this study. Rats that underwent just a perineal incision served as sham. Others that underwent bilateral orchiectomy were divided into two subgroups. Immediately after orchiectomy, one subgroup was injected subcutaneously with 30 mg/kg T (T propionate; Sigma-Aldrich, St. Louis, MO) according to a previous report (17), and another subgroup was treated with vehicle (sesame oil) only. Only one other injection was made exactly 1 wk postsurgery. Rats were euthanized 2 wk postsurgery; ventral prostates and seminal vesicles were harvested and weighed. Prostatic strips of ∼1 × 1 × 0.5 cm were prepared (the surrounding prostatic capsule along with excess fatty tissue was dissected free) for organ bath physiology studies and immediately placed in Krebs-Henseleit (Krebs) solution, with the rest of the tissue frozen in liquid nitrogen and saved at −80°C for subsequent molecular analyses or put into 10% neutral buffered formalin for histological examination. All surgical procedures were performed under anesthesia by intraperitoneal injection of pentobarbital sodium (35 mg/kg; Abbott Laboratory, Chicago, IL). All animal protocols are approved by the Animal Use Committees at the Albert Einstein College of Medicine and Guangxi Medical University.
In vitro organ bath studies.
All chemicals used were from Sigma-Aldrich. A stock solution of zaprinast (20 μM) was made in dimethyl sulfoxide (DMSO); the other substances were dissolved daily in double-distilled water. Control experiments showed that the final concentration of 1/1,000 (vol/vol) DMSO used in these studies did not significantly modify the relaxation response. As described previously (75), rat prostatic strips were mounted longitudinally in a 4-ml organ bath (Multi-Myograph Model 810MS; Danish Myo Technology, Aarhus, Denmark). The myograph was connected in line to a PowerLab 4/30 Data Acquisition System (ADInstruments, Colorado Springs, CO) and in turn to a Dual-Core processor Pentium computer for real-time monitoring of physiological force. Strips were equilibrated for ≥1 h in Krebs buffer at 37°C with continuous bubbling of 95% O2 and 5% CO2. The buffer had the following mM composition, and it was changed every 15 min: 110 NaCl, 4.8 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, and 11 dextrose. Strips were continuously adjusted to 500–800 mg of resting tension (64). After equilibration, prostate tissue was contracted with 60 mM KCl, washed, and then contracted with increasing concentrations (10−8–10−4 M) of PE. Force produced by the above stimuli was normalized to strip weight. Next, strips were precontracted with 10 μM PE (a dose that was determined to induce maximal contraction) and allowed to reach a stable tension, and then the relaxant effects of increasing doses (10−8–10−4 M) of sodium nitroprusside (SNP) with or without 20 nM zaprinast preincubaution were evaluated.
Total RNA extraction and real-time RT-PCR.
As described previously (8), total RNA was isolated from the frozen tissues using TRIzol reagent (Invitrogen, Carlsbad, CA). One microgram of RNA was converted to complementary DNA (cDNA) using reverse transcriptase via the SuperScript II First-Strand Synthesis System according to the manufacturer's instructions (Invitrogen). Primer pairs were designed using published cDNA sequences obtained via Entrez Nucleotide of the National Center for Biotechnology Information and the Primer Express program (Invitrogen). Amplicons were deliberately kept between 50 and 100 base pairs for all primer pairs to enable equal transcriptional efficiency. RT products then were amplified in a 96-well plate in a 25-μl reaction volume, with all samples run in triplicate, using a model 7300 Real-Time thermocycler (Applied Biosystems, Foster City, CA). The experimental protocol utilized was denaturation (95°C for 10 min to activate the polymerase) followed by an amplification program repeated for 40 cycles (95°C for 15 s, then 60°C for 60 s), using a single fluorescence measurement. The following targets were amplified, using SYBR Green for amplicon detection: PDE5, neuronal NOS (nNOS), endothelial NOS (eNOS), and α1-andrenoreceptors, including α1a, α1b, and α1d subtypes and ROKα/ROKβ isoforms. Primer sequences are shown in Table 1. For relative quantification, the efficiency of amplification for each individual primer pair was determined using cDNA target and the 2−ΔΔCT method (37) in conjunction with the RQ Study Software version 1.2.3 (Applied Biosystems). Gene expression was normalized to expression of the RPL19 ribosomal protein housekeeping gene. In our previous study, we compared the expression of RPL19, β-actin, and GAPDH by real-time RT-PCR. RPL19 was determined to be more stable than either β-actin or GAPDH in a rat model of partial bladder outlet obstruction. However, we did not find any effect of aging, diabetes, or castration on these three housekeeping genes (8).
Table 1.
Primer sequences used to amplify rat target genes by real-time RT-PCR
| Target Gene | Primer Sequence |
|---|---|
| PDE5 | |
| Forward | 5′-TTGGAGAGCCCTTGAACATCA-3′ |
| Reverse | 5′-GTAGCCTGTAATTTGGTCAACTTCTG-3′ |
| nNOS | |
| Forward | 5′-GGCAAACATGACTTCCGAGTGT-3′ |
| Reverse | 5′-CCCCAAGGTAGAGCCATCTG-3′ |
| eNOS | |
| Forward | 5′-CACCCACTGAGCAGTATTGG-3′ |
| Reverse | 5′-CCTGGGAACCACTCCTTTTG-3′ |
| α1a | |
| Forward | 5′-GCCCTTCTCTGCCATCTTG-3′ |
| Reverse | 5′-GGCCGCCCAGATATTGC-3′ |
| α1b | |
| Forward | 5′-CCAGGAGTTCCATAGCTGTCAAAC-3′ |
| Reverse | 5′-CCGACTACAATGCCCAAGGT-3′ |
| α1d | |
| Forward | 5′-TGCGCCACTCGCTCAA-3′ |
| Reverse | 5′-CCAAAGCAGAGCCAGAATGG-3′ |
| ROKα | |
| Forward | 5′-TTTATCAATTCTTCAGTCAGCTCTAGAATC-3′ |
| Reverse | 5′-TCCAACCCAGATATTCTACCTTGTAA-3′ |
| ROKβ | |
| Forward | 5′-AGGCCTGTGCCAAACCTT-3′ |
| Reverse | 5′-TCCCTGTGGGACTTAACATGG-3′ |
| RPL19 | |
| Forward | 5′-GCGTCCTCCGCTGTGGTA-3′ |
| Reverse | 5′-CATTGGCGATTTCGTTGGT-3′ |
PDE5, phosphodiesterase 5; nNOS, neuronal nitric oxide synthase; eNOS, endothelial nitric oxide synthase; ROKα and -β, Rho kinase-α and -β, respectively; RPL19, ribosomal protein L19.
SDS-PAGE and Western blot analysis.
As described previously (77), proteins were extracted from frozen tissue samples using the CelLytic NuCLEAR Extraction kit (Amsbio, Abingdon, UK), and 30 μg of each sample was eletrophoresed on a 10% SDS-polyacrylamide gel and transferred to nitrocellulose membrane (Amersham Pharmacia, Piscataway, NJ) by semidry electroblotting for 1 h. The membrane was blocked for 2 h at room temperature with 5% nonfat dried milk dissolved in phosphate-buffered saline (PBS) solution. The membrane was incubated for 2 h with primary PDE5 antibody (1:3,000). After washing several times with PBS, the membrane was incubated with anti-rabbit immunoglobulin G (IgG) linked with horseradish peroxidase at a 1:10,000 dilution (Thermo Scientific Fisher, Rockford, IL) for 1 h at room temperature. Detection of reaction antigen was performed with an enhanced chemiluminescence kit (Thermo Scientific Fisher). The bands were quantified by densitometry using Quantity One software (Bio-Rad, Hercules, CA). A monoclonal mouse antibody against β-actin (1:5,000; Sigma-Aldrich) was used as a control to ascertain equivalent loading.
Histology.
As described previously (27), rat ventral prostate was fixed overnight and processed into paraffin, and 5-μm sections were prepared. Sections were stained with hematoxylin and eosin (H & E) or Gomori's trichrome to evaluate collagen (not shown). Histological evaluation was blinded and performed by a trained veterinary pathologist.
Immunohistochemistry.
Tissues fixed in 10% neutral buffered formalin for 24–36 h were processed routinely for paraffin embedding. Samples for immunohistochemistry were sectioned at 5 μm and deparaffinized in xylene followed by graded alcohols. Antigen retrieval was performed in 10 mM sodium citrate buffer at pH 6.0 and heated to 96°C for 30 min, followed by proteinase K treatment for 10 min. Endogenous peroxidase activity was quenched by using 3% hydrogen peroxide in PBS for 15 min. Blocking was performed by incubating sections in 5% normal donkey serum with 2% BSA for 1 h. The sections were stained by routine immunohistochemistry methods, using horseradish peroxidase polymer conjugate (Invitrogen) to localize the antibody bound to antigen, with diaminobenzidine as the final chromogen. All immunostained sections were lightly counterstained with hematoxylin. The primary antibodies to myosin heavy chain (MHC; cat. no. sc-6956, 1:40; Santa Cruz Biotechnology, Santa Cruz, CA) or PDE5 (cat. no. 611498, 1:50; BD Transduction Laboratories, Lexington, KY) were incubated for 1 h at room temperature. Primary species (mouse IgG1) was substituted for the primary antibody to serve as a negative control. Lung tissue was used as a positive control for PDE5 staining. Slides were evaluated for immunostaining by light microscopy.
Statistical analysis.
Results are expressed as means ± SE for n experiments. Statistical analysis used either the Student's t-test with Excel software (2 sample treatments compared) or ANOVA and Bonferroni posttests with GraphPad Prism 5.0 (multiple means compared). P < 0.05 was considered significant. GraphPad Prism was also used for the analysis of PE-induced dose-dependent contractions that resulted in sigmoid (S-shaped) curves after logarithmic transformation, and the constrained four-parameter logistic model was used to analyze sigmoid curves, to obtain estimates of ED50 values, and to compare them using an F-test and tests of randomness of the residuals around the fitted curves.
RESULTS
After a 2-wk surgical castration, the ventral prostate was profoundly shrunken (Fig. 1, B and C), with weight decreased by approximately sevenfold. Interestingly, the mass of the seminal vesicles was similarly lessened approximately sevenfold (Fig. 1D). T supplementation completely maintained or reinstated the weight of both androgen-sensitive organs. In contrast, orchiectomy had no significant effect on body weight gain during this experimental period (Fig. 1A). Histological examination further showed that the atrophy of the prostate occurred mainly at the epithelial compartment (Fig. 2), whereas the stroma component, which is composed mainly of fibromuscular cells, was relatively increased. As shown in Fig. 2, bottom, prostatic SM in control rats was immunolocalized with the SM-specific marker MHC and displayed an orderly spatial arrangement confined to the outer stroma layer, as indicated by the arrows with scanty PDE5 immunostaining localizing to the same area as SMM in the fibromuscular stroma (Fig. 3, top). But prostatic SM per se generated significant force in response to KCl depolarization (Fig. 4A) and PE-mediated adrenergic stimulation in a dose-dependent manner (Fig. 4B). However, in response to castration, SMM staining revealed a disordered arrangement of the SM cells in the stroma (Fig. 2). Castration attenuated not only PE-induced maximal contraction but also the sensitivity to adrenergic agonist, which is depicted in Fig. 4B. Isolated prostatic strips from sham rats almost reached maximal force generation (∼28 mg force/mg tissue) at 10−6 M PE, whereas castration generated only ∼16 mg force/mg tissue maximal contraction at 10−5 M PE, a 10-fold higher concentration. The EC50 of intact rats (0.27 μM) was around four times lower than that of castrated ones (1.09 μM). T replacement also maintained or restored the orderly SM spatial arrangement in the stroma (Fig. 2) as well as both prostatic SM contractility and sensitivity (Fig. 4).
Fig. 1.
Effect of testosterone (T) on body weight, ventral prostate weight, and seminal vesicle weight. A: bar graph of the average initial and final body weights of all rats used. B: photograph of a typical ventral prostate from a sham (a), castrated (b), and castrated + T rat (c). C: bar graph of the average weight of the ventral prostates from all experimental groups. D: bar graph of the average weight of the seminal vesicles from all experimental groups. **P < 0.001 vs. castration (n = total of 46 rats).
Fig. 2.
Hematoxylin and eosin (H & E) staining and immunolocalization of smooth muscle (SM) myosin of ventral prostate. Representative sections of rat ventral prostate from sham (left), castrated (middle), and castrated + T (right). Top: H & E sections with all magnification ×100. Bottom: immunodistribution of SM myosin in prostatic stroma (magnification ×400). Black arrows indicate intense SM mysoin heavy chain staining in the stroma, whereas weak cross-reactivity is noted in the epithelium.
Fig. 3.
Immunolocalization of phosphodiesterase 5 (PDE5) in rat ventral prostate. A, top: PDE5 positive immunoreactivity in the prostatic stroma from sham-operated rats with magnification ×100 (left) and ×400 (right). A, bottom left: positive PDE5 control (rat lung, magnification ×400); A, bottom right: negative IgG control staining (rat lung, magnification ×400). B: PDE5 immunopositivity in the prostatic stroma from sham (top left), castrated (top right), and castrated + T (bottom left) rats with magnification ×400.
Fig. 4.
Rat ventral prostate SM in vitro contractility. A: summary graph of KCl-induced contraction. Responses to KCl were normalized to strip weight. Values are expressed as means ± SE (n = strips obtained from 46 different animals). B: curves of phenylephrine (PE)-induced dose response contraction. Responses to PE were normalized to strip weight. Values are expressed as means ± SE (n = strips obtained from 46 different animals). **P < 0.01 vs. castration.
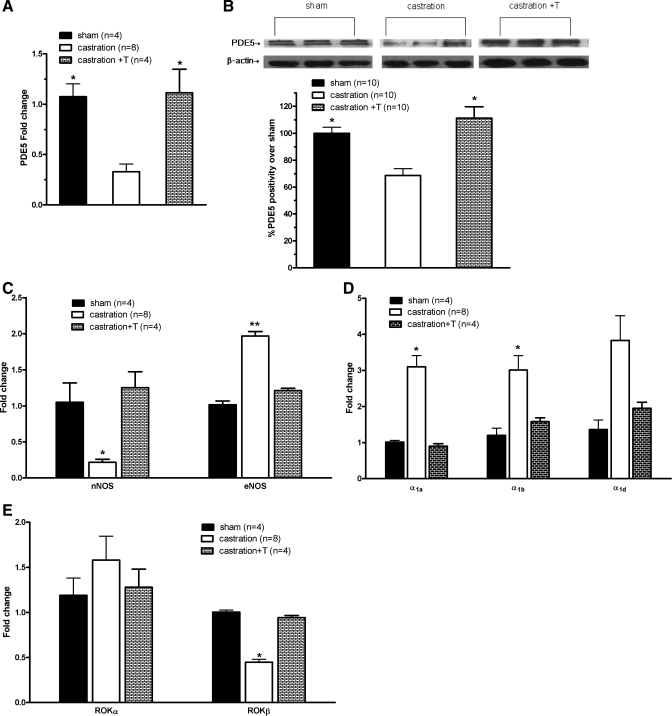
Subsequently, the PDE5/NOS pathways mediating SM tone in the prostate were examined. As demonstrated in Fig. 5, A and B, there was abundant PDE5 in the prostate, in line with our previous study. In particular, two intense bands of the expected molecular weights (∼85 and 95 kDa) were found in the prostate (Fig. 5B). Castration significantly decreased PDE5 immunopositivity (Fig. 3B). The specificity of immunostaining for PDE5 was ascertained by mouse IgG substituting for the primary antibody in a control section, whereas positive immunostaining was demonstrated in lung tissue (Fig. 3A). Orchiectomy also downregulated PDE5 expression threefold at the transcriptional level (Fig. 5A). The analysis of PDE5 protein expression by Western blotting demonstrated similar reduction by castration (Fig. 5B). Additionally, the expressions of several other major molecules linked to SM regulatory and contraction pathways were determined by real-time RT-PCR, including α1-adrenergic receptors and the NOS and ROK systems. As demonstrated in Fig. 5, C–E, nNOS and ROKβ mRNA were found to be significantly decreased, but eNOS, α1a, and α1b receptors were significantly increased, with no change in α1d (Fig. 5D) or ROKα (Fig. 5E) in the castrated animals. Altered expression of all of these molecules was maintained or recovered to control levels when T was given back.
Fig. 5.
Expression of PDE5 and other major SM contractility-associated molecules in rat ventral prostrate. The expression of all studied molecules was quantified by real-time RT-PCR. Gene expression was normalized to expression of the ribosomal protein L19 (RPL19) housekeeping gene. PDE5 expression was also determined by Western blot. A: PDE5 mRNA level. *P < 0.05 vs. castration (n = 4–8 different rats for each group). B: PDE5 protein level. Top: representative Western blot bands. Two specific major bands of the expected sizes (85 and 95 kDa) are evident in all lanes. Bottom: densitometric evaluation of PDE5 protein expression, calculated as %band intensity over the control, taken as 100%. *P < 0.05 vs. castration (n = 10 different rats for each group). C: neuronal (nNOS) and endothelial nitric oxide synthase (eNOS) mRNA level. *P < 0.05 vs. sham or castration + T; **P < 0.01 vs. sham or castration + T (n = 4–8 different rats for each group). D: α1-adrenergic receptor mRNA level. *P < 0.05 vs. sham or castration + T (n = 4–8 different rats for each group). E: Rho kinase (ROK)α and ROKβ mRNA level. *P < 0.05 vs. sham or castration + T (n = 4–8 different rats for each group).
To further determine the PDE5 functional activity and its modulation by androgens, in vitro organ bath relaxation studies with the specific PDE5 inhibitor zaprinast were performed. As shown in Fig. 6, isolated prostatic strips precontracted with maximal stimulation (10 μM PE) can be potently and dose-dependently relaxed by the NO donor SNP for all preparations with complete relaxation (even below baseline) at 10−4 M. Zaprinast significantly potentiated the SNP-relaxing effect at lower (10−8–10−6 M) but not higher doses (10−5–10−4 M) for the sham group. Interestingly, prostatic SM from castrated rats is more sensitive to SNP than that from sham rats, comparable with sham treated with zaprinast plus SNP. However, zaprinast did not amplify SNP-induced relaxation for castrated rats. T replacement reinstated both SNP and zaprinast responsiveness.
Fig. 6.
PDE5 inhibitor zaprinast potentiating effect on sodium nitroprusside (SNP) in relaxing prostatic SM. Prostate strips were precontracted with 10 μM PE and allowed to reach a stable tension and then relaxed by increasing doses (10−8–10−4 M) of SNP with or without 20 nM zaprinast preincubaution. The maximal response to PE was taken as 100%, whereas the relaxant effect of SNP with or without zaprinast was evaluated as a percentage of this response. Values are expressed as means ± SE. *P < 0.05 vs. sham or castration + T; **P < 0.01 vs. sham; oP < 0.05 vs. sham or castration + T (n = 4–7 different animals).
DISCUSSION
The current study demonstrates for the first time that T positively regulates PDE5 expression and functional activity in the prostate. Our data also clearly show that T ablation not only decreases prostate size (static component), affecting primarily the epithelium rather than the stroma, but also reduces the prostatic tone (dynamic component).
Our surgically castrated rat model was validated by the significant atrophy of the androgen-sensitive organs (prostate and seminal vesicle), with weight decreased approximately sevenfold and maintenance/recovery with T supplementation. Surgical castration was observed to cause maximal lowering of T levels within 24 h and resulted in a significant prostate volume reduction in a short period (19, 29). In contrast, prostate regression was maximal at 6 mo after initiation of a 5α-reductase inhibitor such as finasteride, which is a selective inhibitor of the type 2 isozyme of 5α-reductase and does not reduce dihydrotestosterone levels to castrate levels because circulating T is converted to dihydrotestosterone by type 1 isozymes that exist in skin and liver (42). Moreover, finasteride reduces prostate volume ∼20% (42). In parallel with previous observations (6, 26, 34), 2-wk castration led mainly to a marked involution of the glandular compartment (Fig. 2) and corresponded mostly to the loss of epithelial cells. In contrast, the relative volume of the stroma increased rather than decreased. The prostatic SM represents a significant component of the stroma. In the present study it was identified by using the SM-specific marker MHC, and its spatial arrangement in the stroma was uncovered. It is assumed that this arrangement is not optimal for force generation; however, we observed that it did produce significant force in response to either KCl or PE (Fig. 4). Although the contractile properties of prostatic SM have not been well characterized until now, based upon our current in vitro contractility studies, it seems similar to other SM organs. Thus, the active forces in the prostatic tissue may play an important role in the pathophysiology of BPH.
Despite a decreased stroma response to androgen withdrawal, the contractility and sensitivity changes of prostatic SM to PE were heavily blunted or reversed by T deprivation. For the sham, maximal contraction induced by PE was reached at 10−6 M, whereas 10−5 M was required for maximal contraction of prostatic SM from castrated rats. Moreover, the highest force produced from castrated animals was around only one-half of that generated by prostatic SM from intact rats. To date, there are few published investigations on the interaction between prostate contractility and castration. Our observation opposes two of these studies. Lin et al. (34) found heightened α-adrenergic contractility in dogs castrated for 1 mo without discernible changes in the in vivo measurement of prostatic urethral pressure. These authors attributed the increased contractility to the elevation of the relative proportion of stromal tissue and concluded that hormonal intervention had no influence on prostate tension. Another study from Japan used the same species and 2-wk castration protocol as ours (25). They also found that the contractile response to PE increased but with lowered contraction potency and downregulation of α1a-adrenoreceptor in castrated rats. Again, a relative increase in the stromal component in these animals was linked to the heightened contraction. However, similarly to our study, these authors concluded that androgen deprivation suppressed α1-adrenergic contractility of rat prostate strips. The significant weakness of both of these previous studies is that there was such a small maximal contraction (≈3 mg force/mg tissue) induced with PE that it seems hard physiologically to justify the results.
However, supporting our current observation, Hib and Magarinos (24) have reported that, at the in vivo level, bilateral castration of the rat caused a progressive declination of norepinephrine-induced prostate tone. Also, correlating with our current study, Thiyagarajan et al. (63) found that normal male rats receiving subcutaneous injection of T 5 days/wk for 15 days shifted the dose-response curve for various α-adrenoreceptor agonists (including PE) to the left, indicating increased sensitivity, the opposite of our castration findings.
The attenuated prostatic SM contractility for tissue from castrated rats observed in our study could be the result of altered contractile proteins or neuroreceptors or a change in SM phenotype. It has been reported that prostatic SM cells progressively dedifferentiate after castration, switching from a contractile to a synthetic phenotype (5, 6, 51, 78). As shown in Fig. 2, castration was associated with a disordering of the spatial arrangement of the SMM staining cells in the stroma and which clearly could decrease force development. For example, Sarma et al. (58) reported that the alignment of SM cells in a tissue has a significant role in determining its length tension behavior and the isometric forces that it could generate and that off-axis alignments can diminish shortening capacity.
In the current study, we found that PDE5 may contribute to castration-attenuated prostate SM contraction. In rat ventral prostate both the PDE5 gene and protein are highly expressed, and it was immunodistributed exclusively in the stroma, in agreement with Fibbi et al. (16) that PDE5 was immunolocalized only in the muscular fibers and blood vessels in the human prostate with negative immunoreactivity in the glandular area but in contrast to the findings by Uckert et al. (69) that the epithelium of human prostate also expressed PDE5. Prostatic tissues were obtained from BPH patients in the Fibbi et al. (16) study, whereas they were obtained from prostate cancer patients in the other investigation. Additionally, the patients' ages were different, which could contribute to the contrasting results. Consistent with the penis (77) and bladder as well as vas deferens (17, 38), we demonstrated that both the PDE5 mRNA and protein expression in the prostate are strictly T regulated, being significantly reduced by surgical castration and completely reinstated by T supplementation. Actually, since we showed a clear relative increase in the stroma layer (where SM would be; H & E staining in Fig. 2), this would then make the downregulation of PDE5 even more significant because there would be even less PDE5 per area of stroma. This is also supported by the SMM immunostaining in Fig. 2 as well. It is plausible that the suppressed hydrolytic PDE5 in the castrated animal can increase the cGMP level by inhibiting its degradation and thus lead to prostatic SM favoring relaxation instead of contraction.
Additionally, other major signaling pathways associated with prostatic SM contraction were examined. It was found in the present study that T differentially modulates these molecules. nNOS and ROKβ mRNA were significantly deceased, but eNOS, α1a, and α1b receptors were increased, with no change of α1d and ROKα in the castrated animals. Lacey et al. (32) also found an increase in the α1-adrenergic receptor density. In the present study, on the surface, the upregulation of α1-adrenergic receptor in the face of decreased prostatic SM contractility seems conflicting. However, in line with previous reports, the elevation of α1-adrenoreceptors was due possibly to the relative increase of stroma volume (32, 34). Thus T might have no effect on these receptors. Moreover, Auger-Pourmarin et al. (7) found that exogenous T given to normal male rats did not seem to influence the expression of α1a-, α1b-, or α1d-adrenoreceptors, suggesting a maximum effect of endogenous T on α1-adrenergic receptors in normal rats. As mentioned above, switching from a contractile to a synthetic phenotype and disordered arrangement of the SMM (Fig. 2) after T ablation could also blunt the SM response to PE. Additionally, we showed that EC50s for PE-induced contraction of prostate tissue from castrated rats was significantly different (∼4-fold higher) from that of tissues from either sham- or T-treated animals, indicating a decreased sensitivity to PE by castration. This could result in less force generation in castrated rats. Consistently, Passmore et al. (53) reported that, although expression of the α1a, α1b, or α1d receptors was not reduced during aging in renal blood vessels of men or women, the binding of prozosin (an α1-agonist) decreased with aging in both men and women. Thus there is a dissociation between receptor expression and binding that could help explain our difference in expression vs. contraction of the prostatic SM. Alternatively, the increased expression of the α1a- and α1b-adrenoreceptors found in our study in response to castration may represent a compensatory mechanism to the altered SMM organization and decreased PDE5 expression in an attempt to maintain prostatic SM tone and contractility (60). The fact that altered MHC gene expression has been reported previously in normal and hyperplastic tissue (35) makes further investigation into this area in relation to T levels clearly warranted.
Also consistent with another previous report (12), nNOS expression could be androgen dependent. It is known that the nNOS is the primary NOS isoform in the rat prostate and is involved mainly in the control of SM tone and glandular function, including proliferation of epithelial and subepithelial cells, whereas eNOS is related to the maintenance of local vascular perfusion (30). Hence, downregulation of nNOS expression cannot decrease prostatic SM contractility, and it most probably contributes to prostate weight loss after orchiectomy. Interestingly, eNOS expression was increased by castration, which could be hypothesized to compensate for the decreased nNOS, but the real mechanism is unknown. ROKβ was also T modulated, as observed in the penis (70, 73), and thus possibly plays a role in the decreased prostatic SM tone. However, like PDE5 and α-adrenoreceptors mentioned above, the structural changes could have an effect on the expression of ROK and NOS. The downregulation of ROKβ may be due to heavy loss of epithelial cells after T withdrawal, because ROK was found localized in both the stroma and gland (57). With regard to NOS isoforms, their distribution in the prostate is inconsistently reported, and thus more studies are required.
Our in vitro relaxation studies demonstrated further a role for PDE5 in modulating prostatic SM tone and its regulation by androgens. In serial experimental scenarios, we showed that the NO donor SNP potently and completely attenuated PE-precontracted prostatic SM. This relaxing effect was amplified by the PDE5i zaprinast, suggesting PDE5 to modulate the tone of the prostate. Interestingly, the relaxing activity of SNP alone in the castrated group was even higher than that in the sham, comparable with that of sham treated with SNP plus zaprinast. However, zaprinast did not potentiate SNP vasodilatory ability in the castrated rats. This was most probably attributed to the downregulation of PDE5, the molecular target of zaprinast by androgen deprivation. Although the relaxation response to SNP for the prostate from castrated rats was increased compared with that from sham rats at all concentrations of SNP tested, the increase was only statistically significant at 1 μM and lower. However, similar observations have been made by Nakamura et al. (50), who found that, in contrast to the stimulatory effect of SNP at a lower dose (10 μM), a higher dose of SNP (1 mM) reduced channel activity, which was not restored by 8-BrcGMP. These authors suggested that it is likely that the suppressive effect of SNP on channel activity at a high dose involves accelerated formation of peroxynitrite. Also, Tschakovsky et al. (66) reported that the effect of SNP on forearm vascular conductance was greater during low-dose SNP infusion vs. rest (P < 0.05) but not different from rest at the high dose of SNP infusion (P = 0.507), suggesting saturation.
Despite modulating SM tone, PDE5, NOS, α-adrenoreceptor, and ROK are also involved in cell growth and death. Thus, PDE5 may be involved in the castration-induced prostate atrophy. PDE5is have been demonstrated to exert an antiproliferation effect on cultured prostatic SM with higher efficacy on stromal rather than epithelial cells (74). Similar to PDE5is inhibiting PDE5, surgical castration-mediated downregulation of PDE5 may prevent prostatic cell proliferation, resulting in prostate shrinkage. However, the present study and others showed that the stroma was resistant to androgen withdrawal (6, 26). Further studies, including the measurement of prostatic cGMP, are required and will be intriguing.
When translating our findings to humans, antiandrogen therapy, one of the major treatments for BPH, not only reduces the size of prostate but also may blunt PDE5 expression and decrease prostatic SM tension through the NO/cGMP/PDE5 signaling pathway. Thus, both passive and active force are relieved. Clinically, there is now good level 1 evidence of a beneficial effect of PDE5is on urinary symptoms, especially for men with LUTS and significant ED (42). To note, as mentioned previously, PDE5is provided only limited improvement in urinary flow rate, which gave rise to controversy regarding this therapy, although mounting and consistent evidence is showing their significant benefits in treating BPH/LUTS (39, 68). According to the American Urological Association guidelines, α1-adrenergic blockers combined with 5α-reductase inhibitors are considered the most effective therapy for the treatment of BPH, especially for larger prostates. Because of the strong association between BPH/LUTS and ED, the coprescription of PDE5is and α1-adrenergic blockers is more likely to result in greater improvements in BPH/LUTS and ED than either drug alone. In vitro studies demonstrated that the combination of both PDE5is and α-blockers resulted in a greater relaxant effect on human prostate SM compared with a single agent. Preclinical and clinical studies have provided promising evidence that the combination of PDE5is and α-blockers may be an effective and well-tolerated treatment option for BPH/LUTS (9, 28, 33). Because of the potential additive beneficial effect, combination therapy deserves further investigation. However, caution should be applied to combining PDE5is with 5α-reductase inhibitors, because our present study demonstrated that antiandrogen therapy would decrease PDE5 (PDE5i substrate) expression.
In conclusion, our molecular, physiological, and immunohistochemical data provide a rationale for the therapeutic use of PDE5is for BPH. We also provide novel data that T positively regulates prostatic PDE5 expression at the molecular level and PDE5 functional activity through modulating prostatic SM contractility. Therefore, castration not only suppressed the prostate size but also blunted prostatic SM tone at least partially through PDE5 signaling. Furthermore, in addition to the traditional concept of prostatic involution, it suggests that antiandrogen treatment with 5α-reductase for BPH patients may have a new therapeutic mechanism via targeting at PDE5 to relieve the dynamic component of BPH.
GRANTS
This study was supported by National Natural Science Foundation of China Grant no. 30760249 to X. Zhang and National Institute of Diabetes and Digestive and Kidney Diseases Grant no. 5-R01-DK-077116 to M. E. DiSanto.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.Z. and M.E.D. did the conception and design of the research; X.Z., N.Z., Y.W., J.Y., and R.T. performed the experiments; X.Z., N.Z., and M.E.D. analyzed the data; X.Z., N.Z., A.S., and M.E.D. interpreted the results of the experiments; X.Z., N.Z., Y.W., and M.E.D. prepared the figures; X.Z. and M.E.D. drafted the manuscript; X.Z., A.S., and M.E.D. edited and revised the manuscript; M.E.D. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Rani S. Sellers, Dr. Barbara Cannella, and Hong Zhang for performing histology and immunochemistry studies (Histopathology Shared Resource at Albert Einstein College of Medicine).
REFERENCES
- 1. Adelstein RS, Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem 49: 921–956, 1980 [DOI] [PubMed] [Google Scholar]
- 2. Adolfsson PI, Ahlstrand C, Varenhorst E, Svensson SP. Lysophosphatidic acid stimulates proliferation of cultured smooth muscle cells from human BPH tissue: sildenafil and papaverin generate inhibition. Prostate 51: 50–58, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Andersson KE, de Groat WC, McVary KT, Lue TF, Maggi M, Roehrborn CG, Wyndaele JJ, Melby T, Viktrup L. Tadalafil for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: pathophysiology and mechanism(s) of action. Neurourol Urodyn 30: 292–301, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Andric SA, Janjic MM, Stojkov NJ, Kostic TS. Sildenafil treatment in vivo stimulates Leydig cell steroidogenesis via the cAMP/cGMP signaling pathway. Am J Physiol Endocrinol Metab 299: E544–E550, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Antonioli E, Cardoso AB, Carvalho HF. Effects of long-term castration on the smooth muscle cell phenotype of the rat ventral prostate. J Androl 28: 777–783, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Antonioli E, Della-Colleta HH, Carvalho HF. Smooth muscle cell behavior in the ventral prostate of castrated rats. J Androl 25: 50–56, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Auger-Pourmarin L, Roubert P, Chabrier PE. Alpha1-adrenoceptors in testosterone-induced prostatic hypertrophy. Eur J Pharmacol 341: 119–126, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Aydin M, Downing K, Villegas G, Zhang X, Chua R, Melman A, Disanto ME. The sphingosine-1-phosphate pathway is upregulated in response to partial urethral obstruction in male rats and activates RhoA/Rho-kinase signalling. BJU Int 106: 562–571, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Bechara A, Romano S, Casabé A, Haime S, Dedola P, Hernández C, Rey H. Comparative efficacy assessment of tamsulosin vs. tamsulosin plus tadalafil in the treatment of LUTS/BPH. Pilot study. J Sex Med 5: 2170–2178, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Buvat J, Montorsi F, Maggi M, Porst H, Kaipia A, Colson MH, Cuzin B, Moncada I, Martin-Morales A, Yassin A, Meuleman E, Eardley I, Dean JD, Shabsigh R. Hypogonadal men nonresponders to the PDE5 inhibitor tadalafil benefit from normalization of testosterone levels with a 1% hydroalcoholic testosterone gel in the treatment of erectile dysfunction (TADTEST study). J Sex Med 8: 284–293, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Caine M. Alpha-adrenergic mechanisms in dynamics of benign prostatic hypertrophy. Urology 32: 16–20, 1988 [PubMed] [Google Scholar]
- 12. Crone JK, Burnett AL, Chamness SL, Strandberg JD, Chang TS. Neuronal nitric oxide synthase in the canine prostate: aging, sex steroid, and pathology correlations. J Androl 19: 358–364, 1998 [PubMed] [Google Scholar]
- 13. DiSanto ME, Wang Z, Menon C, Zheng Y, Chacko T, Hypolite J, Broderick G, Wein AJ, Chacko S. Expression of myosin isoforms in smooth muscle cells in the corpus cavernosum penis. Am J Physiol Cell Physiol 275: C976–C987, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Eckert RE, Schreier U, Drescher P, Madsen PO, Derouet H, Becht E, Steffens J, Ziegler M. Regulation of prostatic smooth muscle contractility by intracellular second messengers: implications for the conservative treatment of benign prostatic hyperplasia. Urol Int 54: 6–21, 1995 [DOI] [PubMed] [Google Scholar]
- 15. Faure C, Pimoule C, Vallancien G, Langer SZ, Graham D. Identification of alpha 1-adrenoceptor subtypes present in the human prostate. Life Sci 54: 1595–1605, 1994 [DOI] [PubMed] [Google Scholar]
- 16. Fibbi B, Morelli A, Vignozzi L, Filippi S, Chavalmane A, De Vita G, Marini M, Gacci M, Vannelli GB, Sandner P, Maggi M. Characterization of phosphodiesterase type 5 expression and functional activity in the human male lower urinary tract. J Sex Med 7: 59–69, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Filippi S, Morelli A, Sandner P, Fibbi B, Mancina R, Marini M, Gacci M, Vignozzi L, Vannelli GB, Carini M, Forti G, Maggi M. Characterization and functional role of androgen-dependent PDE5 activity in the bladder. Endocrinology 148: 1019–1029, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Furuya S, Kumamoto Y, Yokoyama E, Tsukamoto T, Izumi T, Abiko Y. Alpha-adrenergic activity and urethral pressure in prostatic zone in benign prostatic hypertrophy. J Urol 128: 836–839, 1982 [DOI] [PubMed] [Google Scholar]
- 19. Gao F, Yang M, Luo CL, Wu XH. Local insulin-like growth factor-I of ventral prostate was upregulated during long-term castration and may function through the autocrine system. Prostate Cancer Prostatic Dis 14: 136–142, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Ghalayini IF. Nitric oxide-cyclic GMP pathway with some emphasis on cavernosal contractility. Int J Impot Res 16: 459–469, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Greco EA, Pili M, Bruzziches R, Corona G, Spera G, Aversa A. Testosterone:estradiol ratio changes associated with long-term tadalafil administration: a pilot study. J Sex Med 3: 716–722, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Greco EA, Spera G, Aversa A. Combining testosterone and PDE5 inhibitors in erectile dysfunction: basic rationale and clinical evidences. Eur Urol 50: 940–947, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Gu H, Martin H, Barsotti RJ, Labelle EF. Rapid increase in inositol phosphate levels in norepinephrine-stimulated vascular smooth muscle. Am J Physiol Cell Physiol 261: C17–C22, 1991 [DOI] [PubMed] [Google Scholar]
- 24. Hib J, Magarinos GA. Effects of castration, androgen replacement and flutamide treatment on the contractile function of the rat prostate. Acta Physiol Pharmacol Latinoam 39: 281–287, 1989 [PubMed] [Google Scholar]
- 25. Homma Y, Hamada K, Nakayama Y, Tsujimoto G, Kawabe K. Effects of castration on contraction and alpha(1)-adrenoceptor expression in rat prostate. Br J Pharmacol 131: 1454–1460, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huttunen E, Romppanen T, Helminen HJ. A histoquantitative study on the effects of castration on the rat ventral prostate lobe. J Anat 132: 357–370, 1981 [PMC free article] [PubMed] [Google Scholar]
- 27. Kanika ND, Tar M, Tong Y, Kuppam DS, Melman A, Davies KP. The mechanism of opiorphin-induced experimental priapism in rats involves activation of the polyamine synthetic pathway. Am J Physiol Cell Physiol 297: C916–C927, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaplan SA, Gonzalez RR, Te AE. Combination of alfuzosin and sildenafil is superior to monotherapy in treating lower urinary tract symptoms and erectile dysfunction. Eur Urol 51: 1717–1723, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Kashiwagi B, Shibata Y, Ono Y, Suzuki R, Honma S, Suzuki K. Changes in testosterone and dihydrotestosterone levels in male rat accessory sex organs, serum, and seminal fluid after castration: establishment of a new highly sensitive simultaneous androgen measurement method. J Androl 26: 586–591, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Kedia GT, Uckert S, Jonas U, Kuczyk MA, Burchardt M. The nitric oxide pathway in the human prostate: clinical implications in men with lower urinary tract symptoms. World J Urol 26: 603–609, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Kohler TS, McVary KT. The relationship between erectile dysfunction and lower urinary tract symptoms and the role of phosphodiesterase type 5 inhibitors. Eur Urol 55: 38–48, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Lacey JP, Donatucci CF, Price DT, Page SO, Bennett SA, Tenniswood MP, Schwinn DA. Effects of androgen deprivation on prostate alpha 1-adrenergic receptors. Urology 48: 335–341, 1996 [DOI] [PubMed] [Google Scholar]
- 33. Liguori G, Trombetta C, De Giorgi G, Pomara G, Maio G, Vecchio D, Ocello G, Ollandini G, Bucci S, Belgrano E. Efficacy and safety of combined oral therapy with tadalafil and alfuzosin: an integrated approach to the management of patients with lower urinary tract symptoms and erectile dysfunction. Preliminary report. J Sex Med 6: 544–552, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Lin AT, Chen MT, Chiang H, Yang CH, Chang LS. Effect of orchiectomy on the alpha adrenergic contractile response of dog prostate. J Urol 154: 1930–1933, 1995 [DOI] [PubMed] [Google Scholar]
- 35. Lin VK, Wang D, Lee IL, Vasquez D, Fagelson JE, McConnell JD. Myosin heavy chain gene expression in normal and hyperplastic human prostate tissue. Prostate 44: 193–203, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Liu CM, Lo YC, Wu BN, Wu WJ, Chou YH, Huang CH, An LM, Chen IJ. cGMP-enhancing- and alpha1A/alpha1D-adrenoceptor blockade-derived inhibition of Rho-kinase by KMUP-1 provides optimal prostate relaxation and epithelial cell anti-proliferation efficacy. Prostate 67: 1397–1410, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[−Delta Delta C(T)] Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Mancina R, Filippi S, Marini M, Morelli A, Vignozzi L, Salonia A, Montorsi F, Mondaini N, Vannelli GB, Donati S, Lotti F, Forti G, Maggi M. Expression and functional activity of phosphodiesterase type 5 in human and rabbit vas deferens. Mol Hum Reprod 11: 107–115, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Martínez-Salamanca JI, Carballido J, Eardley I, Giuliano F, Gratzke C, Rosen R, Salonia A, Stief C. Phosphodiesterase type 5 inhibitors in the management of non-neurogenic male lower urinary tract symptoms: critical analysis of current evidence. Eur Urol 60: 527–535, 2011 [DOI] [PubMed] [Google Scholar]
- 40. McConnell JD. Medical management of benign prostatic hyperplasia with androgen suppression. Prostate Suppl 3: 49–59, 1990 [DOI] [PubMed] [Google Scholar]
- 41. McConnell JD, Roehrborn CG, Bautista OM, Andriole GL, Jr, Dixon CM, Kusek JW, Lepor H, McVary KT, Nyberg LM, Jr, Clarke HS, Crawford ED, Diokno A, Foley JP, Foster HE, Jacobs SC, Kaplan SA, Kreder KJ, Lieber MM, Lucia MS, Miller GJ, Menon M, Milam DF, Ramsdell JW, Schenkman NS, Slawin KM, Smith JA; Medical Therapy of Prostatic Symptoms (MTOPS) Research Group The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 349: 2387–2398, 2003 [DOI] [PubMed] [Google Scholar]
- 42. McNicholas TA, Kirby RS, Lepor H. Evaluation and nonsurgical management of benign prostatic hyperplasia. In: Campbell-Walsh Urology, edited by Wein A, Kavoussi LR, Novick A, Partin AW, Peters CA. Philadelphia, PA: Saunders, 2011, p. 2611–2654 [Google Scholar]
- 43. McVary K. Lower urinary tract symptoms and sexual dysfunction: epidemiology and pathophysiology. BJU Int 97, Suppl 2: 23–28, 2006 [DOI] [PubMed] [Google Scholar]
- 44. McVary KT, Monnig W, Camps JL, Jr, Young JM, Tseng LJ, van den Ende G. Sildenafil citrate improves erectile function and urinary symptoms in men with erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized, double-blind trial. J Urol 177: 1071–1077, 2007 [DOI] [PubMed] [Google Scholar]
- 45. McVary KT, Roehrborn CG, Kaminetsky JC, Auerbach SM, Wachs B, Young JM, Esler A, Sides GD, Denes BS. Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol 177: 1401–1407, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Morelli A, Filippi S, Mancina R, Luconi M, Vignozzi L, Marini M, Orlando C, Vannelli GB, Aversa A, Natali A, Forti G, Giorgi M, Jannini EA, Ledda F, Maggi M. Androgens regulate phosphodiesterase type 5 expression and functional activity in corpora cavernosa. Endocrinology 145: 2253–2263, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Morelli A, Sarchielli E, Comeglio P, Filippi S, Mancina R, Gacci M, Vignozzi L, Carini M, Vannelli GB, Maggi M. Phosphodiesterase type 5 expression in human and rat lower urinary tract tissues and the effect of tadalafil on prostate gland oxygenation in spontaneously hypertensive rats. J Sex Med 8: 2746–2760, 2011 [DOI] [PubMed] [Google Scholar]
- 48. Muller D, Mukhopadhyay AK, Davidoff MS, Middendorff R. Cyclic GMP signaling in rat urinary bladder, prostate, and epididymis: tissue-specific changes with aging and in response to Leydig cell depletion. Reproduction 142: 333–343, 2011 [DOI] [PubMed] [Google Scholar]
- 49. Nagasaki S, Nakano Y, Masuda M, Ono K, Miki Y, Shibahara Y, Sasano H. Phosphodiesterase type 9 (PDE9) in the human lower urinary tract: an immunohistochemical study. BJU Int. In press [DOI] [PubMed] [Google Scholar]
- 50. Nakamura K, Hirano J, Kubokawa M. Regulation of an inwardly rectifying K+ channel by nitric oxide in cultured human proximal tubule cells. Am J Physiol Renal Physiol 287: F411–F417, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Niu Y, Xu Y, Zhang J, Bai J, Yang H, Ma T. Proliferation and differentiation of prostatic stromal cells. BJU Int 87: 386–393, 2001 [DOI] [PubMed] [Google Scholar]
- 52. O'Leary MP, Roehrborn CG, Black L. Dutasteride significantly improves quality of life measures in patients with enlarged prostate. Prostate Cancer Prostatic Dis 11: 129–133, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Passmore JC, Joshua IG, Rowell PP, Tyagi SC, Falcone JC. Reduced alpha adrenergic mediated contraction of renal preglomerular blood vessels as a function of gender and aging. J Cell Biochem 96: 672–681, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Roehrborn CG. Benign prostatic hyperplasia: etiology, pathophysiology, epidemiology and natural history. In: Campbell-Walsh Urology, edited by Wein A, Kavoussi LR, Novick A, Partin AW, Peters CA. Philadelphia, PA: Saunders, 2011, p. 2570–2610 [Google Scholar]
- 55. Roehrborn CG. Benign prostatic hyperplasia: an overview. Rev Urol 7, Suppl 9: S3–S14, 2005 [PMC free article] [PubMed] [Google Scholar]
- 56. Roehrborn CG, McVary KT, Elion-Mboussa A, Viktrup L. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a dose finding study. J Urol 180: 1228–1234, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Saito M, Ohmasa F, Shomori K, Dimitriadis F, Ohiwa H, Shimizu S, Tsounapi P, Kinoshita Y, Satoh K. Rhos and Rho kinases in the rat prostate: their possible functional roles and distributions. Mol Cell Biochem 358: 207–213, 2011 [DOI] [PubMed] [Google Scholar]
- 58. Sarma AP, Pidaparti RM, Meiss RA. Effect of off-axis cell orientation on mechanical properties in smooth muscle tissue. J Biomed Sci Eng 4: 10–17, 2011 [Google Scholar]
- 59. Stief CG, Porst H, Neuser D, Beneke M, Ulbrich E. A randomised, placebo-controlled study to assess the efficacy of twice-daily vardenafil in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur Urol 53: 1236–1244, 2008 [DOI] [PubMed] [Google Scholar]
- 60. Suematsu N, Satoh S, Kinugawa S, Tsutsui H, Hayashidani S, Nakamura R, Egashira K, Makino N, Takeshita A. α1-Adrenoceptor-Gq-RhoA signaling is upregulated to increase myofibrillar Ca2+ sensitivity in failing hearts. Am J Physiol Heart Circ Physiol 281: H637–H646, 2001 [DOI] [PubMed] [Google Scholar]
- 61. Sward K, Mita M, Wilson DP, Deng JT, Susnjar M, Walsh MP. The role of RhoA and Rho-associated kinase in vascular smooth muscle contraction. Curr Hypertens Rep 5: 66–72, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Takahashi R, Nishimura J, Seki N, Yunoki T, Tomoda T, Kanaide H, Naito S. RhoA/Rho kinase-mediated Ca2+ sensitization in the contraction of human prostate. Neurourol Urodyn 26: 547–551, 2007 [DOI] [PubMed] [Google Scholar]
- 63. Thiyagarajan M, Kaul CL, Ramarao P. Enhancement of alpha-adrenoceptor-mediated responses in prostate of testosterone-treated rat. Eur J Pharmacol 453: 335–344, 2002 [DOI] [PubMed] [Google Scholar]
- 64. Tokanovic S, Malone DT, Ventura S. Stimulation of epithelial CB1 receptors inhibits contractions of the rat prostate gland. Br J Pharmacol 150: 227–234, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Traish A, Kim N. The physiological role of androgens in penile erection: regulation of corpus cavernosum structure and function. J Sex Med 2: 759–770, 2005 [DOI] [PubMed] [Google Scholar]
- 66. Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol 541: 623–635, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Uckert S, Kuthe A, Jonas U, Stief CG. Characterization and functional relevance of cyclic nucleotide phosphodiesterase isoenzymes of the human prostate. J Urol 166: 2484–2490, 2001 [PubMed] [Google Scholar]
- 68. Uckert S, Oelke M. Phosphodiesterase (PDE) inhibitors in the treatment of lower urinary tract dysfunction. Br J Clin Pharmacol 72: 197–204, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Uckert S, Oelke M, Stief CG, Andersson KE, Jonas U, Hedlund P. Immunohistochemical distribution of cAMP- and cGMP-phosphodiesterase (PDE) isoenzymes in the human prostate. Eur Urol 49: 740–745, 2006 [DOI] [PubMed] [Google Scholar]
- 70. Vignozzi L, Morelli A, Filippi S, Ambrosini S, Mancina R, Luconi M, Mungai S, Vannelli GB, Zhang XH, Forti G, Maggi M. Testosterone regulates RhoA/Rho-kinase signaling in two distinct animal models of chemical diabetes. J Sex Med 4: 620–630, 2007 [DOI] [PubMed] [Google Scholar]
- 71. Waldkirch ES, Uckert S, Langnase K, Richter K, Jonas U, Wolf G, Andersson KE, Stief CG, Hedlund P. Immunohistochemical distribution of cyclic GMP-dependent protein kinase-1 in human prostate tissue. Eur Urol 52: 495–501, 2007 [DOI] [PubMed] [Google Scholar]
- 72. Wang JM, McKenna KE, Lee C. Determination of prostatic secretion in rats: effect of neurotransmitters and testosterone. Prostate 18: 289–301, 1991 [DOI] [PubMed] [Google Scholar]
- 73. Wingard CJ, Johnson JA, Holmes A, Prikosh A. Improved erectile function after Rho-kinase inhibition in a rat castrate model of erectile dysfunction. Am J Physiol Regul Integr Comp Physiol 284: R1572–R1579, 2003 [DOI] [PubMed] [Google Scholar]
- 74. Zenzmaier C, Sampson N, Pernkopf D, Plas E, Untergasser G, Berger P. Attenuated proliferation and trans-differentiation of prostatic stromal cells indicate suitability of phosphodiesterase type 5 inhibitors for prevention and treatment of benign prostatic hyperplasia. Endocrinology 151: 3975–3984, 2010 [DOI] [PubMed] [Google Scholar]
- 75. Zhang X, Kanika ND, Melman A, Disanto ME. Smooth Muscle Myosin Expression, Isoform Composition and Functional Activities in Rat Corpus Cavernosum Altered by the Streptozotocin-Induced Type 1 Diabetes. Am J Physiol Endocrinol Metab. First published September 13, 2011; doi:10.1152/ajpendo.00231.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhang XH, Filippi S, Morelli A, Vignozzi L, Luconi M, Donati S, Forti G, Maggi M. Testosterone restores diabetes-induced erectile dysfunction and sildenafil responsiveness in two distinct animal models of chemical diabetes. J Sex Med 3: 253–264, 2006 [DOI] [PubMed] [Google Scholar]
- 77. Zhang XH, Morelli A, Luconi M, Vignozzi L, Filippi S, Marini M, Vannelli GB, Mancina R, Forti G, Maggi M. Testosterone regulates PDE5 expression and in vivo responsiveness to tadalafil in rat corpus cavernosum. Eur Urol 47: 409–416, 2005 [DOI] [PubMed] [Google Scholar]
- 78. Zhao GQ, Holterhus PM, Dammshauser I, Hoffbauer G, Aumuller G. Estrogen-induced morphological and immunohistochemical changes in stroma and epithelium of rat ventral prostate. Prostate 21: 183–199, 1992 [DOI] [PubMed] [Google Scholar]