Abstract
Teenagers have an increased risk of delivering small-for-gestational-age (SGA) infants. Young maternal age and continued skeletal growth have been implicated as causal factors. In growing adolescent sheep, impaired placental development and nutrient transfer cause reduced birth weight. In human pregnancies, SGA is associated with reduced placental amino acid transport. Maternal growth has no effect on placental morphology or cell turnover, but growing teenagers have higher birth weight:placental weight ratios than nongrowing teenagers. We hypothesized that placental nutrient transporter activity would be affected by maternal age and/or growth status. Placentas from teenagers and adults were collected. Teenagers were defined as growing or nongrowing based on knee height measurements. System A amino acid transporter activity was quantified as sodium-dependent uptake of [14C]methylaminoisobutyric acid into placental fragments. Teenagers had lower placental system A activity than adults (P < 0.05). In adults, placental system A activity was lower in SGA infants than appropriate-for-gestational-age (AGA) infants (P < 0.05). In teenagers, AGA and SGA infants had lower placental system A activity than AGA infants born to adults (P < 0.05). Placental system A activity was higher in growing teenagers than in nongrowing teenagers (P < 0.001). Placental mRNA expression of system A transporter isoforms SLC38A1 and -2 was lower in teenagers than in adults (P < 0.05) but did not differ between growing and nongrowing teenagers. There was no difference in transporter protein expression/localization between cohorts. Teenagers have inherently reduced placental transport, which may underlie their susceptibility to delivering SGA infants. Growing teenagers appear to overcome this susceptibility by stimulating the activity, but not expression, of system A transporters.
Keywords: amino acid transport, fetal growth, syncytiotrophoblast, adolescent pregnancy
the UK has the highest incidence of teenage pregnancy in Western Europe (50). Despite a tendency for the rate and number of teenage pregnancies to decline over the last 10 years, 7% of all births in England and Wales in 2007 were to teenagers (41). Previous studies have shown that teenagers are susceptible to poor pregnancy outcomes, particularly for delivering low-birth weight, small-for-gestational-age (SGA), or growth-restricted infants (4, 13, 16, 38). Biological immaturity due to young maternal age and continued maternal growth have been implicated as causal factors of teenagers' susceptibility (12–14, 40, 45). In support of this, growing adolescent sheep delivered growth-restricted offspring (51). Importantly, a pivotal role for the placenta was defined in causing fetal growth restriction (FGR), as impaired placental development resulted in reduced placental weight and nutrient transfer to the fetus in growing sheep (52, 53).
However, not all studies of pregnant human teenagers have observed the same trends in pregnancy outcome (3, 27, 49). Indeed, our recent prospective study of 500 pregnant teenagers in the UK demonstrated that maternal growth was not detrimental to fetal growth in teenage pregnancies (29). In this study, the About Teenage Eating (ATE) study, growing teenagers were not at a greater risk of SGA birth; instead, they delivered infants with higher average birth weight and were more likely to deliver LGA infants than nongrowing teenagers. Nongrowing teenagers had an almost twofold increase in the incidence of SGA infants compared with that expected in adult pregnancies. The differences between the ATE study and previously published studies were attributed to our use of customized birth weight centiles in conjunction with a more accurate assessment of maternal growth (14, 45, 46). Furthermore, there were significant differences in the demographic and biophysical profiles of the study groups; the ATE study participants were predominantly Caucasian, primiparous 17–18 year olds, reflecting the majority of the contemporary pregnant teenage population (2).
Detailed morphometric analyses were used to assess the impact of maternal age and growth status on the growth and development of placentas collected from a subset of growing and nongrowing teenagers in the ATE study (22). Unexpectedly, young maternal age and continued growth during pregnancy had no major effect on placental weight, morphometry, or cell turnover and thus did not explain the susceptibility of teenagers to SGA birth nor the differences in pregnancy outcome between growing and nongrowing teenagers. However, growing teenagers had significantly higher birth weight:placental weight ratios compared with nongrowing teenagers, suggesting that growing teenagers have placentas that are more efficient. One possible explanation is that maternal growth promotes placental function, particularly nutrient transfer. However, placental nutrient transfer has not been investigated in human teenage pregnancies.
Normal fetal growth is dependent on an adequate supply of amino acids, which provide 20–40% of the fetus' energy and biosynthetic requirements (10). One of the best-characterized placental amino acid transporters is system A, a Na+-dependent transporter, which comprises three different transporter proteins: SNAT1, -2, and -4, encoded by SLC38A1, -2, and -4 genes (23). System A actively transfers small side-chain neutral amino acids, e.g., alanine and glycine, and the nonmetabolizable, synthetic methylaminoisobutyric acid (MeAIB) (5). There is substantial evidence that placental system A activity is important for normal fetal growth. Reduced system A activity has been consistently demonstrated in placentas of human pregnancies complicated by SGA or FGR (17, 21, 34), with the degree of reduction corresponding to the severity of the FGR (17). Interestingly, lower maternal prepregnancy upper arm muscle area has recently been shown to be associated with lower placental system A activity at term (33). This suggests that there is a relationship between maternal body composition and the activity of this amino acid transporter and provides further evidence for regulation of placental transport by the maternal endocrine/metabolic environment.
We hypothesized that placental amino acid transport activity is reduced in teenage compared with adult pregnancies, and, on the basis of the higher birth weight:placental weight ratio, growing teenagers will have higher placental amino acid transport than nongrowing teenagers. To test this hypothesis, the aims of our study were to investigate whether 1) placental transport is affected by maternal age, by comparing placental system A activity between pregnant teenagers and adults; 2) placental system A activity is influenced by maternal growth in teenage pregnancy; and 3) confounding variables contribute to effects observed in the first two aims.
METHODS
Ethical information.
This study was approved by the Central Manchester Research Ethics Committee (03/CM/32). Written informed consent was obtained from all participants. All participants were assessed for Gillick competency, a method used to determine whether a child/young person has the ability to understand and consent to the treatment/research study being proposed.
Participants and placental collection.
Placentas were collected following delivery (31 spontaneous vaginal deliveries and 2 Caesarean sections) from a subset of pregnant teenagers (15–18 yr, n = 33) participating in the ATE study (PIs Profs. P. N. Baker and L. Poston) (2, 29). Only teenagers with singleton pregnancies were recruited to the study; teenagers were excluded if they had preexisting medical or obstetric disorders, multiple gestations, or a history of three or more previous miscarriages. Through the ATE study, detailed sociodemographic and anthropometric data, including the assessment of maternal skeletal growth by a change in knee height during pregnancy, were obtained at two antenatal visits between 10 and 20 wk and 27 and 36 wk gestation. Growth data were normalized for a 90-day period between the two visits. Growing teenagers were defined as those with a gain in knee height of ≥2 mm/90 days, a threshold based on measurements obtained from a nongrowing pregnant adult population as described in our previous publication (29). Maternal height and weight were measured and used to calculate the body mass index (BMI) at booking. Teenagers were subdivided into underweight (BMI <18.5), normal weight (18.5–24.9), overweight (25–29.9), and obese (≥30). Data from teenagers were recorded by research midwives onto a secure internet-based database (MedSciNet, Stockholm, Sweden).
To make accurate comparisons between study groups, pregnancies were also subdivided into those with SGA, appropriate-for-gestational-age (AGA), and large-for-gestational-age (LGA) births based on individualized birth weight centiles (IBC) calculated with Centile Calculator Bulk 6.2.2 (www.gestation.net). This adjusts infant birth weight for infant gender, gestational age, and maternal constitutional factors, i.e., height, weight, ethnicity, and parity, therefore providing a more accurate assessment of infant birth weight (15). SGA infants were defined as below the 10th centile, AGA between the 10th and 90th centile, and LGA above the 90th centile. There were no cases of preeclampsia reported in this population of teenagers.
Placentas were also obtained from a cohort of adults (20–39 yr, n = 19), attending the same hospital during the same period, to serve as a control group. The same exclusion criteria were applied as for teenage pregnancies. Placentas from adults delivering SGA infants were positively selected to ensure adequate comparison with the teenage cohort.
Placental sampling and processing.
Placentas were collected within 30–60 min of delivery, and all experiments on fresh tissue were completed within 4 h of delivery. From each placenta, four samples of villous tissue (2–3 cm3) were dissected by selective random sampling. Each sample was rinsed in phosphate-buffered saline (PBS; GIBCO, Invitrogen, Paisley, UK) and then subdivided for immunohistochemical (fixed and paraffin embedded), molecular (treated with RNA Later; Ambion, Applied Biosciences, Huntingdon, UK), and nutrient transport analyses [collected in DMEM, containing 1 g/l glucose (GIBCO, Invitrogen)/Tyrode's buffer solution (ratio 1:1)] (24).
Analysis of placental system A activity.
The activity of the system A transporter was used as a marker of placental function and was measured in placentas collected from 33 teenagers and 19 adults. Maternal growth data were collected from 15 of these teenagers, who were subdivided into growing (n = 6) and nongrowing (n = 9) cohorts; the remainder failed to attend a second visit. Teenagers were also grouped into those with AGA (n = 26), SGA (n = 6), and LGA (n = 1) births. Of the 19 adults, there were 12 AGA, 6 SGA, and 1 LGA births. Power calculations based on previously reported differences in system A activity between placentas of AGA and SGA/FGR pregnancies (34, 47) determined that samples sizes of four to six were required to show a similar difference (50–60%) in system A activity at 90% power at the 5% significance level. For this reason, placentas from LGA pregnancies were excluded from the analyses. The demographic and biophysical profile of study participants assessed for placental system A activity is shown in Table 1.
Table 1.
Demographic and biophysical data for teenage and adult cohorts assessed for placental system A activity
| Category | Growing | Nongrowing | Adults |
|---|---|---|---|
| n | 6 | 9 | 19 |
| Age, yr | 17.4*** | 17.3*** | 29.0 |
| (15.9–18.6) | (16.1–18.8) | (20.0–43.0) | |
| Gynecological age, yr | 5.1 | 5.4 | |
| (2.9–6.3) | (3.1–7.5) | ||
| Primiparous, no. (%) | 6 (100.0)** | 9 (100.0)** | 13 (68.4) |
| Smokers, no. (%) | 4 (66.7)†††*** | 2 (22.2) | 3 (15.8) |
| BMI at booking, kg/m2 | 22.3 | 21.7 | 23.4 |
| (19.8–36.9) | (18.2–35.2) | (15.9–34.7) | |
| Underweight, no. (%) | 0 (0.0)†††*** | 3 (33.3)*** | 3 (15.8) |
| Normal, no. (%) | 4 (66.7) | 4 (44.4) | 11 (57.9) |
| Overweight, no. (%) | 1 (16.7) | 1 (11.1)* | 4 (21.0) |
| Obese, no. (%) | 1 (16.7)*** | 1 (11.1)* | 1 (5.3) |
| Knee height change, mm/90 days | 3.7††† | −0.02 | |
| (2.6–6.1) | (−1.2–1.0) | ||
| SGA <10th centile, no. (%) | 1 (16.7)** | 1 (11.1)** | 6 (31.5) |
| AGA 10th–90th centile, no. (%) | 5 (83.3)* | 8 (88.9)** | 12 (63.2) |
| LGA >90th centile, no. (%) | 0 (0.0)* | 0 (0.0)* | 1 (5.3) |
Values are median (and range) unless otherwise stated. Of the 33 teenagers from whom placentas were collected, maternal growth data were collected from 15 teenagers, subdivided into 6 growing and 9 nongrowing. Gynecological age defined as no. of years since menarche. Parous teenagers and adults had only one previous birth. BMI, body mass index; IBC, individual birthweight centile; SGA, small-for-gestational-age; AGA, appropriate-for-gestational-age; LGA, large-for-gestational-age.
P < 0.05,
P < 0.01,
P < 0.0001 teenagers vs. adults;
P < 0.0001 growing vs. nongrowing teenagers.
System A activity was quantified using the placental villous fragment method (19, 24). In brief, individual villous fragments were secured with cotton thread to each of three hooks supported on a Perspex comb (Stopford Workshop, Manchester, UK), enabling measurements to be made in triplicate. Fragments were immersed in DMEM-Tyrode's buffer solution (1:1) to preequilibrate for 20 min at 37°C. One set of triplicates was prewashed for 2 min in control Tyrode's (containing Na+) and the other set in Na+-free Tyrode's (containing choline chloride in place of NaCl), immediately prior to incubation with tracer concentrations of the system A-specific substrate [14C]MeAIB (0.5 μCi/ml, 8.5–9.9 μM; PerkinElmer Life and Analytical Sciences, Boston, MA) for 10, 20, and 30 min in either control or Na+-free Tyrode's. Fragment uptake was stopped by vigorously washing the tissue in ice-cold Tyrode's buffer (control or Na+-free as appropriate). The tissue was lysed in dH2O for 18 h at room temperature to release the [14C]MeAIB accumulated during the experiment. Scintillation fluid was added to each water lysate and counted for 5 min using a Tri-Carb 2100 TR Liquid Scintillation Analyzer (Packard Bioscience, Berkshire, UK). Villous fragments were transferred to 0.3 M NaOH and incubated at 37°C for 18 h until fully lysed. The protein content of each fragment was determined using a Bio-Rad protein assay (Bio-Rad Laboratories, Hertfordshire, UK). The uptake of [14C]MeAIB was calculated as picomoles per milligram of fragment protein. The average was taken of triplicates for [14C]MeAIB uptake in control and Na+-free Tyrode's buffer for each time point. Na+-dependent accumulation of [14C]MeAIB was measured to represent system A activity in the microvillous membrane of the syncytiotrophoblast, by subtraction of uptake in Na+-free conditions from that in control Tyrode's buffer. The mean system A activity was plotted against time.
Placental gene expression of system A transporters.
Power calculations based on previous studies (48) indicated that a greater number of samples was required for molecular analyses (7 per group required to show a difference of 20% at 80% power at the 5% significance level); thus, samples in which placental system A activity was measured were supplemented with 18 samples collected as part of the ATE study. Placental mRNA expression of system A isoforms SLC38A1, -2 and -4 was assessed in 28 teenagers participating in the ATE study and 10 adults, using real-time reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR). Maternal growth data were collected from 26 of the 28 teenagers, who were subdivided into 13 growing and 13 nongrowing. Of the 28 teenagers, 9 delivered SGA, 14 AGA, and 5 LGA infants. The adult cohort included 5 AGA and 5 SGA pregnancies. There were no differences in the demographic and biophysical profiles of study participants assessed for placental mRNA expression and those used for the transport studies.
Reverse transcription (RT) was performed on all RNA samples in duplicate using the AffintyScript Multiple Temperature cDNA Synthesis Kit (Agilent Technologies, Wokingham, UK) according to the manufacturer's instructions. Human reference RNAs (total human reference, human placenta and human liver; Agilent) were reverse transcribed to produce standards. Duplicate cDNA samples were diluted 1:10 and assessed by qPCR for TATA box binding protein (TBP) to verify the reproducibility of the RT reaction and were pooled if percent coefficient of variance was <15%. The pooled samples were used for subsequent qPCR reactions to amplify SLC38A1, -2, and -4 and TBP genes.
cDNA samples were diluted 1:10 using a mastermix containing Brilliant SYBR Green Master Mix (Agilent), reference dye ROX, and specific primer pairs. The SLC38A1 (5′-GTGTATGCTTTACCCACCATTGC-3′ and 3′-GCACGTTGTCATAGAATGTCAAGT-5′), SLC38A2 (5′ACGAAACAATAAACACCACCTTAA-3′ and 3′-AGATCAGAATTGGCACAGCATA-5′), SLC38A4 (5′-TTGCCGCCCTCTTTGGTTAC-3′ and 3′-GAGGACAATGGGCACAGTTAGT-5′), and TBP (5′-CACGAACCACGGCACTGATT-3′ and 3′-TTTTCTTGCTGCCAGTCTGGAC-5′) primers were purchased from Invitrogen and have been used previously (8, 31). Standards were serially diluted and included in every run together with human placental reference RNA (1:10) as a quality control (QC). All standards, samples, and QCs were pipetted in duplicate, and qPCR runs were repeated using an MX3005P thermal cycler for initial denaturation for 10 min at 95°C, 40 cycles of 30 s denaturation at 95°C, annealing at a primer-specific temperature (58°C for SLC38A1, -2 and -4 and 60°C for TBP) for 1 min, and 1 min amplification/extension at 72°C. Endpoint fluorescence values were measured at the end of each annealing and amplification stage of each cycle. Values for unknown samples were extrapolated from the standard curve (human reference, human placental reference, or human liver reference). Melting curve analysis data were recorded and analyzed using MxPro-Mx3005P QPCR Software (Agilent). Average values were calculated for sample duplicates on the same plate, and mean expression values were calculated from the average of both qPCR runs. Expression levels were presented as ratios of the QC to minimize the variability between each RT-qPCR reaction and were normalized to the housekeeping gene TBP (37).
Placental protein expression of system A transporters.
Immunohistochemistry for SNAT1 and SNAT2 was carried out to assess the expression and distribution of system A isoforms in the placenta by using antibodies that had been optimized previously (9). In brief, placental tissue sections (5 μm) were dewaxed, rehydrated, and microwaved for antigen retrieval in 0.01 M sodium citrate buffer (pH 6.0). Sections were incubated with 3% H2O2 to quench endogenous peroxidase activity and then with nonimmune block: 10% swine serum (Sigma-Aldrich) and 2% human serum (“in house”) in hi-salt TBS containing 0.1% Tween 20 (Bio-Rad Laboratories, Hertfordshire, UK) for 30 min at room temperature. Primary rabbit polyclonal antibodies (SNAT1 or SNAT2, 2 μg/ml) were diluted in nonimmune block and applied overnight at 4°C. Negative controls were performed by preabsorption of the primary antibody with a fivefold excess of the appropriate SNAT peptide. Antibody binding was detected by applying biotinylated swine anti-rabbit immunoglobulins (Dako, Ely, UK) followed by avidin-peroxidase (5 μg/ml, Sigma). Diaminobenzidine (DAB; SigmaFAST, Sigma-Aldrich) was the chromogen. Tissue sections were counterstained with Harris's hematoxylin (Sigma-Aldrich), dehydrated, and mounted.
SNAT1 and SNAT2 staining was assessed on two placental villous tissue sections from 10 teenagers and 5 adults. The teenage cohort comprised 5 growing and 5 nongrowing teenagers, all of whom delivered AGA infants. All adults delivered AGA infants and were included as a control group. A scoring system between 0 and 4 was utilized to allow semiquantitative analysis of SNAT immunostaining intensity in the placental syncytiotrophoblast, vessels, and stroma over the whole tissue section, and the mean value for each tissue compartment was calculated: 0, no staining; 1, minimal/patchy; 2, moderate staining; 3, strong staining; and 4, very strong staining. Two independent scorers were blinded to sample identity.
Statistical analysis.
Data were processed for statistical analysis and graphic presentation using GraphPad Prism 5 for Windows. Differences between subject demographic data were detected using χ2, Mann-Whitney U, and Kruskal-Wallis tests. The time course of Na+-dependent [14C]MeAIB accumulation was analyzed by least squares linear regression. Differences in the fragment protein content were assessed using a Mann-Whitney U-test. Placental gene and protein expression were analyzed using Mann-Whitney U and Kruskal-Wallis tests. P < 0.05 was considered significant.
RESULTS
Impact of maternal age on placental system A activity.
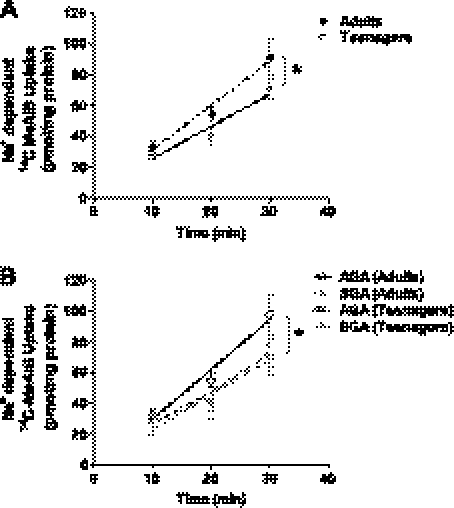
The Na+-dependent uptake of [14C]MeAIB, taken as a measure of placental system A activity, was linear over 10–30 min in placental fragments from all participants. To examine the effect of maternal age on placental system A activity, primiparous teenagers and adults were compared. Teenagers had significantly lower placental system A activity than adults (P = 0.016; Fig. 1A). This difference could not be attributed to differences in the placental fragment protein content or differences in the uptake of [14C]MeAIB under Na+-free conditions between the two cohorts (data not shown).
Fig. 1.
Impact of maternal age on placental system A activity. A: placental system A activity was significantly lower in placentas of primiparous teenagers (n = 31) vs. primiparous adults (n = 13). B: system A activity was significantly reduced in placentas from adults delivering small-for-gestational-age (SGA) infants (n = 6) vs. appropriate-for-gestational-age (AGA) infants (n = 12). There was no significant difference in system A activity in placentas of AGA (n = 26) and SGA (n = 6) infants delivered to teenagers. Teenagers delivering both AGA and SGA infants had comparable placental system A activity to adults delivering SGA infants and significantly lower activity than AGA infants delivered to adults. Values are means ± SE. Least squares linear regression: *P < 0.05 AGA vs. SGA in adults; and AGA (Teenagers) and SGA (Teenagers) vs. AGA (Adults).
To determine whether lower placental system A activity in teenagers than adults was related to differences in infant birth weight between the two cohorts, activity was examined according to birth weight category. As expected, lower system A activity was measured in placentas from adults delivering SGA compared with AGA infants (P = 0.026; Fig. 1B). However, no difference was detected in placental system A activity between teenagers delivering AGA and SGA infants (Fig. 1B). In fact, in teenagers, levels of placental system A activity for both AGA and SGA births were equivalent to those detected for SGA births to adults, and were significantly lower than that of AGA births to adults (P = 0.038; Fig. 1B). Placental system A activity was not compared in LGA infants delivered to teenagers and adults due to insufficient numbers.
Impact of maternal growth on placental system A activity.
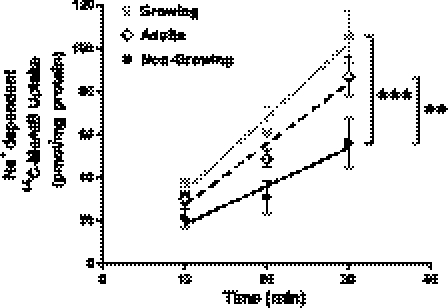
The teenage cohort was subdivided into growing and nongrowing cohorts. System A activity was significantly elevated in placentas from growing compared with nongrowing teenagers (P = 0.0002; Fig. 2). Placentas from the adult cohort were included for comparison. Placentas from growing teenagers exhibited system A activity equivalent to that of placentas from adults. However, system A activity was significantly lower in placentas from nongrowing teenagers compared with adults (P = 0.002). These differences could not be attributed to differences in the placental fragment protein content or differences in the uptake of [14C]MeAIB under Na+-free conditions (data not shown).
Fig. 2.
Impact of maternal growth on placental system A activity. Placental system A activity was significantly elevated in placentas of growing teenagers (n = 6) vs. nongrowing teenagers (n = 9). Nongrowing teenagers had significantly lower placental system A activity vs. adults (n = 19). Placental system A activity was comparable in the growing teenage and adult cohorts. Values are means ± SE. Least squares linear regression: ***P < 0.001 growing vs. nongrowing; **P < 0.01 nongrowing vs. adults.
Impact of confounding factors on placental variables.
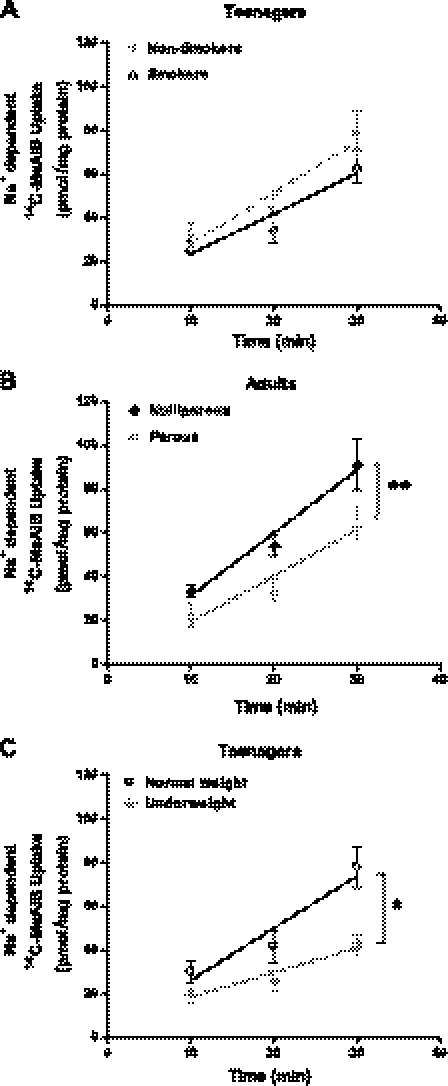
Despite our best efforts, there were some differences between the adult and teenage populations studied; hence, further comparisons were made to rule out any effect of potential confounding variables on the reduced placental system A activity in teenagers: 45.2% of teenagers smoked during pregnancy compared with 15.8% of adults; however, placental system A activity was unaffected by maternal smoking status in both teenagers (Fig. 3A) and adults (data not shown). Placental system A activity was also unaffected by infant gender in both cohorts (teenagers: 24 males and 9 females; adults: 7 males and 12 females; data not shown). However, an effect of parity on placental system A was observed in the adult cohort, with significantly lower system A activity in placentas from parous adults (one previous birth) compared with primiparous adults (P = 0.009; Fig. 3B).
Fig. 3.
Impact of maternal smoking status, parity, and BMI on placental system A activity. A: placental system A activity was unaffected by maternal smoking in teenagers (15 smokers and 17 nonsmokers). B: primiparous adults (n = 13) had significantly higher placental system A activity than parous adults (n = 6). C: impact of maternal BMI at booking on placental system A activity in teenagers (6 underweight and 20 normal weight). Na+-dependent [14C]MeAIB uptake was significantly lower in placentas of underweight teenagers vs. overweight teenagers. Values are means ± SE. Least squares linear regression: *P < 0.05.
Our previous studies demonstrated that nongrowing teenagers have a lower BMI at booking than growing teenagers (29); hence, we assessed placental system A activity in relation to low maternal BMI. Teenagers who were underweight had lower placental system A activity than normal-weight teenagers (P = 0.017; Fig. 3C). There were too few overweight and obese teenagers to include in these assessments. In addition, placental system A activity in relation to low maternal BMI in the adult cohort was not examined due to insufficient numbers of underweight adults.
Placental gene expression of placental system A transporters.
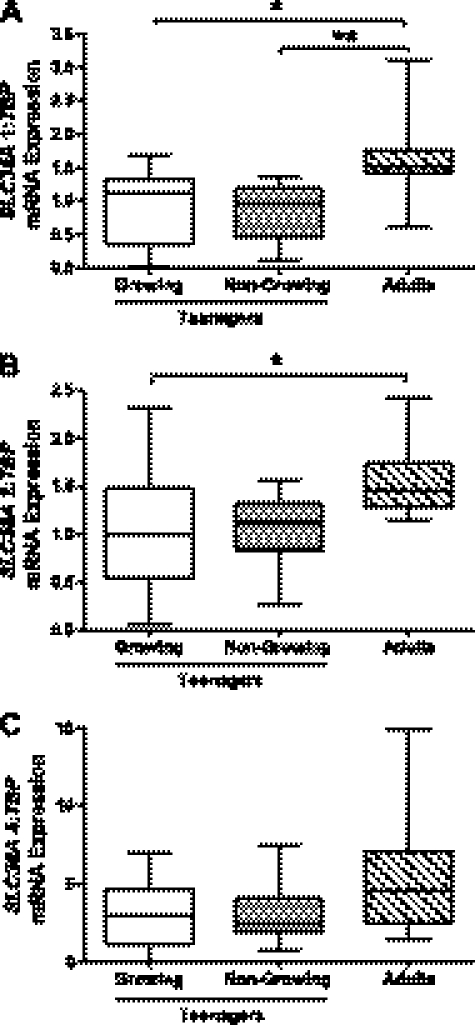
To explore the potential mechanisms underlying reduced placental transport in teenagers, mRNA expression of the system A transporter isoforms was measured. There was a tendency for lower SLC38A placental gene expression in teenage cohorts (growing and nongrowing) compared with adults. This reached significance for SLC38A1 for both growing (P < 0.05) and nongrowing teenagers (P < 0.01; Fig. 4A) and for SLC38A2 for growing teenagers (P < 0.05; Fig. 4B). Placental SLC38A4 mRNA expression was not significantly different between groups (Fig. 4C). There were no differences in placental gene expression between growing and nongrowing teenagers.
Fig. 4.
Impact of maternal growth on placental system A transporter mRNA expression. A: SLC38A1. B: SLC38A2. C: SLC38A4. Growing (n = 13) and nongrowing (n = 13) teenagers had significantly lower placental SLC38A1 mRNA expression than adults (n = 10). Growing teenagers had significantly lower placental SLC38A2 mRNA expression than adults. TBP, TATA box binding protein. Box and whisker plots. Kruskal-Wallis test with Dunn's post hoc test: *P < 0.05, **P < 0.01.
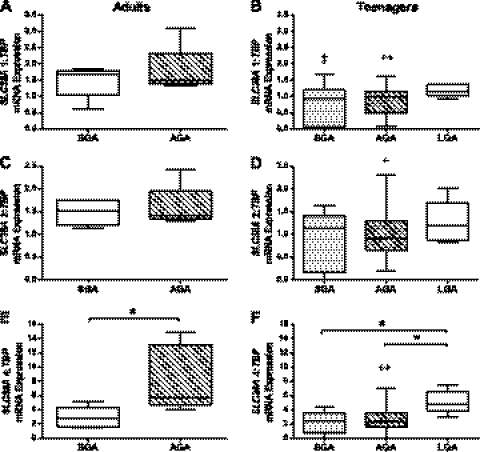
In both adult and teenage cohorts, SLC38A1 and -2 mRNA expression was comparable in placentas from AGA and SGA infants (Fig. 5, A–D). However, SLC38A4 mRNA expression was significantly lower in adults delivering SGA compared with AGA infants (P < 0.05; Fig. 5E). SLC38A4 expression was significantly higher in LGA infants compared with SGA and AGA infants in the teenage cohort (P < 0.05; Fig. 5F). Placental mRNA expression of SLC38A1 (P < 0.01), -2 (P < 0.05), and -4 (P < 0.01) was significantly lower in AGA infants delivered to teenagers compared with adults. SGA infants delivered to teenagers had significantly lower placental SLC38A1 expression than adults (P < 0.05). There was no significant difference between SLC38A2 and -4 mRNA expression between placentas of SGA infants delivered to teenagers and adults. There was no effect of smoking, maternal BMI or infant gender on SLC38A gene expression (data not shown).
Fig. 5.
Impact of infant birth weight on placental system A transporter mRNA expression in adults and teenagers: A and B: SLC38A1. C and D: SLC38A2. E and F: SLC38A4. Placentas of SGA infants (n = 5) had significantly lower SLC38A4 mRNA expression than AGA infants (n = 5) delivered to adults. In teenagers, large-for-gestational-age (LGA) infants (n = 5) had significantly higher placental SLC38A4 mRNA expression than those of AGA (n = 14) and SGA (n = 9) infants. AGA infants delivered to teenagers had significantly lower placental mRNA expression of SLC38A1, -2, and -4 than AGA infants delivered to adults. Placentas of SGA infants delivered to teenagers had significantly lower SLC38A1 mRNA expression than SGA infants delivered to adults. Box and whisker plots. Kruskal-Wallis with Dunn's post hoc test: *P < 0.05 SGA vs. AGA in adults; LGA vs. SGA and AGA in teenagers; +P < 0.05, ++P < 0.01 AGA (teenagers) vs. AGA (adults); ‡P < 0.05 SGA (teenagers) vs. SGA (adults).
Placental protein expression of system A transporters SNAT1 and SNAT2.
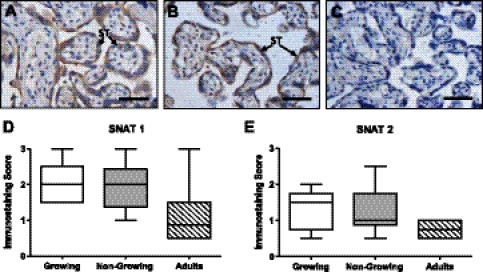
SNAT1 and -2 immunostaining was extremely variable between placentas. SNAT1 (Fig. 6A) and -2 (Fig. 6B) were predominantly localized to the syncytiotrophoblast but were also present in the stroma and in vascular endothelial cells. There was no difference in the distribution or intensity of immunostaining in the syncytiotrophoblast for SNAT1 (Fig. 6D) and -2 (Fig. 6E) or in the other tissue compartments (data not shown) between teenagers and adults or between growing and nongrowing teenagers.
Fig. 6.
Localization of SNAT1 and SNAT2 proteins in teenage and adult placentas. Representative images of SNAT1 (A) and SNAT2 (B) immunostaining and negative control (C). Scale bars, 50 μm. Semiquantitative analysis was used to assess SNAT1 (D) and SNAT2 (E) expression in the syncytiotrophoblast of placentas of AGA infants delivered to growing (n = 5) and nongrowing (n = 5) teenagers vs. adults (n = 5). Box and whisker plots.
DISCUSSION
Teenagers are more susceptible to delivering SGA infants than adults are, but the predisposing factors and underlying mechanisms remain poorly understood. Studies that eliminated confounding variables (such as ethnicity, marital status, adequate antenatal care, smoking, multiparity, and educational level) by examining cohorts of married, white, nonsmoking teenagers with adequate antenatal care and age-appropriate education confirmed the detrimental effect of young maternal age on outcome (4, 13). These findings suggest that biological susceptibility to SGA is related to young maternal age. These data reported in the present study support this, showing lower system A amino acid transporter activity (a well-characterized biomarker of placental function) in teenagers compared with adults. Furthermore, the growth status of teenagers appears to be a key factor in this association, as growing teenagers have higher system A activity than nongrowing teenagers. These data, and our finding of lower placental system A in underweight teenagers, are consistent with those of Lewis et al. (33), demonstrating that maternal body composition influences placental system A activity.
To exclude any complicating effects of maternal parity on system A, only placentas from primiparous teenagers and adults were compared. System A activity and SLC38A1 and -2 mRNA expression were significantly lower in placentas from teenagers than those from adults. This relationship for lower placental system A activity in teenage pregnancies was also observed when placentas from AGA infants delivered to teenagers and adults were compared. Together, these data indicate that placental amino acid transport is inherently lower in teenagers, suggesting a potential mechanism for their susceptibility to delivering SGA infants. However, in our study, teenagers being able to produce AGA infants indicate that there must be other mechanisms that compensate for this inherently lower placental system A activity; this may include changes in the activity of other amino acid transporters; however, further research would be required to confirm this hypothesis.
In contrast to previous reports that implicate maternal growth as an underlying causal factor of reduced infant birth weight, our recent findings demonstrate that teenagers who continue growing deliver higher birth weight infants than teenagers who do not grow during pregnancy (29). These data suggest that growing teenagers have comparable pregnancy outcomes to those of adults, whereas nongrowing teenagers are the more susceptible group for SGA birth. In the current study, we demonstrated that system A amino acid transporter activity is significantly higher in placentas from growing teenagers compared with nongrowing teenagers and is equivalent to that seen in adult pregnancies. This is consistent with our recent finding that birth weight:placental weight ratios are higher in growing teenagers, suggesting increased placental efficiency (22). These data therefore strengthen and extend our findings that, in the UK contemporary population studied, continued maternal growth in teenage pregnancies is not detrimental to fetal growth.
The significantly higher placental system A activity observed in growing teenagers compared with nongrowing teenagers was independent of alterations in the mRNA or protein expression of SNAT isoforms. Thus, increased system A activity in growing teenagers does not appear to be due to increased transcription or translation of the system A isoforms. Moreover, the higher system A activity in growing teenagers occurs despite lower placental gene expression than in adults and indicates upregulation at the level of activity. This is consistent with previous studies showing disconnection between system A activity and mRNA expression levels (1, 36). The ATE study demonstrated that growing teenagers had significantly higher plasma concentrations of leptin and IGF-I at 28–32 wk compared with their nongrowing counterparts (29). Leptin and IGF-I have been shown previously to stimulate system A activity (24, 30) and thus may play an important role in promoting placental amino acid transfer in growing teenagers. Future studies are required to investigate whether these findings of higher system A transporter activity reflect global changes in the activity of other placental nutrient transporters.
The novel observation of impaired placental system A activity in nongrowing teenagers is in accordance with their susceptibility for delivering SGA infants, as observed in the ATE study (29). In the ATE study, this was attributed, in part, to nongrowing teenagers having lower nutritional status, as indicated by lower booking BMI, gestational weight gain, red cell folate concentrations, and lower dietary intake of a number of key micronutrients for fetal growth, e.g., iron and calcium. This suggested that the nongrowing cohort encompassed teenagers who were skeletally mature, together with those who could not sustain their own growth due to poor nourishment (29). The reduced placental amino acid transport in nongrowing teenagers would further impact on fetal nutrient supply. We also found that teenagers who were underweight at booking had lower placental system A activity than those teenagers with normal BMI. These data are in line with observations of lower placental system A activity in undernourished rats (25, 35, 44) and in women with lower upper arm muscle mass (33), and they further support the hypothesis that low maternal nutritional status contributes to reduced placental nutrient transport.
Interestingly, when underweight teenagers and adults were excluded from the comparisons of primiparous teenagers and adults, and AGA births in teenagers and adults, the difference in placental system A activity between these groups becomes smaller (and the P value increases to 0.06 in both cases; data not shown). This supports the theory that poor nutritional status may be a contributory factor to reduced placental amino acid transport in teenage pregnancies. Nevertheless the influence of maternal age in these comparisons cannot be completely excluded, particularly as pregnant teenagers are susceptible to lower nutritional status and poor dietary quality (2, 32, 39). This is consistent with pregnant adolescent sheep being more susceptible to nutritional insult that adults (54).
In the current study, system A activity in placental fragments was significantly lower from SGA infants delivered to adults. This is consistent with studies in which system A activity was assessed using microvillous membrane vesicles isolated from the syncytiotrophoblast (17, 21, 34). However, system A activity from AGA and SGA births in teenagers was comparable to that measured in placentas from adults delivering SGA infants. This may be a consequence of the relatively small number of SGA births in the teenage cohort examined or the severity of the growth restriction. Within the SGA cohorts, five of the six infants delivered to adults had IBCs (individualized birth weight centiles) <5th centile compared with two of the six infants delivered to teenagers, indicating that the infants were less severely growth restricted in the teenage cohort. Our data are consistent with previous studies that showed that placental system A activity is associated with the severity of the FGR (17).
There was no difference in placental mRNA expression of SLC38A1 or -2 in women who delivered SGA infants compared with those who had normal pregnancies. This is consistent with observations by Malina et al. (36), who found no difference in placental mRNA expression of SLC38A1 and -2 in women delivering SGA infants compared with those with normal pregnancies, indicating that the reduced system A activity is not caused by reduced transcription of transporter isoforms (36). However, in adults, placentas of SGA infants had significantly lower SLC38A4 mRNA expression than AGA infants, consistent with reduced placental system A activity in SGA infants. SNAT4 may therefore have an important role to play in placental system A activity. Studies of the P0 knockout mouse, which resulted in lower placental weight and FGR, provided evidence that SLC38A4 is important in fetal growth, as changes in expression closely match the changes in placental system A activity (7). Additionally, gene ablation of SLC38A4 in mice has been associated with FGR (9). In human pregnancies, the relative contribution of each SNAT isoform to system A activity is unknown. However, recent studies have suggested that SNAT4 may contribute more to system A activity in early pregnancy than in late pregnancy, when SNAT1 and -2 appear more important (9). Interestingly, teenagers delivering LGA infants had significantly higher placental mRNA expression of SLC38A4 than those delivering AGA and SGA infants. Previous studies have shown that fetal overgrowth in mice, induced by a high-fat diet, is associated with an increased transplacental transfer of amino acids (28). However, system A activity is unaffected in microvillous membrane vesicles isolated from placentas of human LGA pregnancies compared with those isolated from normal pregnancies (26).
There were significant differences in the incidence of maternal smoking between the adults and teenagers studied. To ensure that this potential influencing factor was not the cause of reduced placental system A activity in teenagers, we subdivided the participants for comparison. In contrast to previous in vitro studies showing placental amino acid transport to be reduced by nicotine (42, 43), we found that system A activity and gene expression was unaffected by smoking in both teenagers and adults. The previous studies had used the placental perfusion model and placental tissue slices to observe the isolated effects of nicotine on system A activity at concentrations higher (potentially up to 10-fold) than those expected in vivo (6, 43). The majority of our teenagers reported smoking fewer than 10 cigarettes a day, which was supported by cotinine concentrations measured by the ATE study (2). In addition, cigarette smoke contains over 4,000 components other than nicotine, which may differentially affect placental development and function (11).
Parity is a potential confounding factor on pregnancy outcome, and, unexpectedly, in the current study, parous adults had significantly lower placental system A activity than primiparous adults. This is contrary to published observations in a larger cohort of pregnant women showing that parity does not affect placental system A activity (33) and is likely to be a reflection of two of the six parous adults delivering SGA infants. Alternatively, lower system A activity in parous women may be related to more successful transformation of uterine spiral arteries in second pregnancies, as these vessels fail to return to their prepregnant state (18, 20). The resultant increase in maternal perfusion of the placenta and nutrient supply may thus mean that a lower rate of system A activity is sufficient to achieve adequate nutrient transfer and optimal fetal growth. This highlights the importance of collecting demographic data and information of obstetric history when recruiting patients for studies of placental development and function.
In summary, this study supports the hypothesis that placental nutrient transporter activity is affected by young maternal age and growth status and confirms and extends our observations that maternal growth in teenage pregnancies is not detrimental to fetal growth. The opposite seems to be true; lack of growth in teenagers, potentially due to poor nutritional status, can impact placental function and increase the risk of SGA birth.
GRANTS
This work was funded by Action Medical Research, Tommy's the Baby Charity, and the Big Lottery Fund. Infrastructure support was provided by the NIHR Biomedical Research Centre.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.E.H., S.L.G., and R.L.J. conception and design of research; C.E.H., S.L.G., and R.L.J. performed experiments; C.E.H. and R.L.J. analyzed data; C.E.H., S.L.G., and R.L.J. interpreted results of experiments; C.E.H. and R.L.J. prepared figures; C.E.H. and R.L.J. drafted manuscript; C.E.H., S.L.G., C.P.S., P.N.B., J.R.G.C., and R.L.J. edited and revised manuscript; C.E.H., S.L.G., C.P.S., P.N.B., J.R.G.C., and R.L.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We offer our sincere thanks to our research midwives Georgina Bennett, Gemma Wild, Lorna Carruthers, Jane Guarino, and Cara Taylor, who recruited and collected data from the participants, as well as Dr. Henna Cederberg, Louise Walkin, and Fiona Mackie, who helped collect placentas and carry out the uptake experiments. Many thanks are due to Prof. Lucilla Poston and the ATE study steering committee. Our thanks also go to the women and staff of St. Mary's and Wythenshawe Hospitals, Manchester, UK, for participating in the current study.
REFERENCES
- 1. Audette MC, Greenwood SL, Sibley CP, Jones CJP, Challis JRG, Matthews SG, Jones RL. Dexamethasone stimulates placental system A transport and trophoblast differentiation in term villous explants. Placenta 31: 97–105, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Baker PN, Wheeler SJ, Sanders TA, Thomas JE, Hutchinson CJ, Clarke K, Berry JL, Jones RL, Seed PT, Poston L. A prospective study of micronutrient status in adolescent pregnancy. Am J Clin Nutr 89: 1114–1124, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Bukulmez O, Deren O. Perinatal outcome in adolescent pregnancies: a case-control study from a Turkish university hospital. Eur J Obstet Gynecol Reprod Biol 88: 207–212, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Chen XK, Wen SW, Fleming N, Demissie K, Rhoads GG, Walker M. Teenage pregnancy and adverse birth outcomes: a large population based retrospective cohort study. Int J Epidemiol 36: 368–373, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Christensen HN, Oxender DL, Liang M, Vatz KA. The use of N-methylation to direct the route of mediated transport of amino acids. J Biol Chem 240: 3609–3616, 1965 [PubMed] [Google Scholar]
- 6. Christianson RE. Gross differences observed in the placentas of smokers and nonsmokers. Am J Epidemiol 110: 178–187, 1979 [DOI] [PubMed] [Google Scholar]
- 7. Constância M, Angiolini E, Sandovici I, Smith P, Smith R, Kelsey G, Dean W, Ferguson-Smith A, Sibley CP, Reik W, Fowden A. Adaptation of nutrient supply to fetal demand in the mouse involves interaction between the Igf2 gene and placental transporter systems. Proc Natl Acad Sci USA 102: 19219–19224, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Desforges M, Lacey HA, Glazier JD, Greenwood SL, Mynett KJ, Speake PF, Sibley CP. SNAT4 isoform of system A amino acid transporter is expressed in human placenta. Am J Physiol Cell Physiol 290: C305–C312, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Desforges M, Mynett KJ, Jones RL, Greenwood SL, Westwood M, Sibley CP, Glazier JD. The SNAT4 isoform of the system A amino acid transporter is functional in human placental microvillous plasma membrane. J Physiol 587: 61–72, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dicke JM, Henderson GI. Placental amino acid uptake in normal and complicated pregnancies. Am J Med Sci 295: 223–227, 1988 [DOI] [PubMed] [Google Scholar]
- 11. EPAUS Respiratory Health Effects of Passive Smoking. Washington, DC: p. 2–5, 1992 [Google Scholar]
- 12. Eure CR, Lindsay MK, Graves WL. Risk of adverse pregnancy outcomes in young adolescent parturients in an inner-city hospital. Am J Obstet Gynecol 186: 918–920, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Fraser AM, Brockert JE, Ward RH. Association of young maternal age with adverse reproductive outcomes. N Engl J Med 332: 1113–1117, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Frisancho AR, Matos J, Bollettino LA. Influence of growth status and placental function on birth weight of infants born to young still-growing teenagers. Am J Clin Nutr 40: 801–807, 1984 [DOI] [PubMed] [Google Scholar]
- 15. Gardosi J, Chang A, Kalyan B, Sahota D, Symonds EM. Customised antenatal growth charts. Lancet 339: 283–287, 1992 [DOI] [PubMed] [Google Scholar]
- 16. Gilbert W, Jandial D, Field N, Bigelow P, Danielsen B. Birth outcomes in teenage pregnancies. J Matern Fetal Neonatal Med 16: 265–270, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Glazier JD, Cetin I, Perugino G, Ronzoni S, Grey AM, Mahendran D, Marconi AM, Pardi G, Sibley CP. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res 42: 514–519, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Gluckman PD, Hanson MA. Maternal constraint of fetal growth and its consequences. Semin Fetal Neonatal Med 9: 419–425, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Greenwood SL, Sibley CP. In vitro methods for studying human placental amino acid transport placental villous fragments. Methods Mol Med 122: 253–264, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Hafner E, Schuchter K, Metzenbauer M, Philipp K. Uterine artery Doppler perfusion in the first and second pregnancies. Ultrasound Obstet Gynecol 16: 625–629, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Harrington B, Glazier J, D'Souza S, Sibley C. System A amino acid transporter activity in human placental microvillous membrane vesicles in relation to various anthropometric measurements in appropriate and small for gestational age babies. Pediatr Res 45: 810–814, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Hayward CE, Greenwood SL, Sibley CP, Baker PN, Jones RL. Effect of young maternal age and skeletal growth on placental growth and development. Placenta 32: 990–998, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Hyde R, Christie GR, Litherland GJ, Hajduch E, Taylor PM, Hundal HS. Subcellular localization and adaptive up-regulation of the System A (SAT2) amino acid transporter in skeletal-muscle cells and adipocytes. Biochem J 355: 563–568, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jansson N, Greenwood SL, Johansson BR, Powell TL, Jansson T. Leptin stimulates the activity of the system A amino acid transporter in human placental villous fragments. J Clin Endocrinol Metab 88: 1205–1211, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. Placenta 576: 935–946, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jansson T, Ekstrand Y, Bjőrn C, Wennergren M, Powell TL. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes 51: 2214–2219, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Jolly MC, Sebire N, Harris J, Robinson S, Regan L. Obstetric risks of pregnancy in women less than 18 years old. Obstet Gynecol 96: 962–966, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J 23: 271–278, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones RL, Cederberg HMS, Wheeler SJ, Poston L, Hutchinson CJ, Seed PT, Oliver RL, Baker PN. Relationship between maternal growth, infant birthweight and nutrient partitioning in teenage pregnancies. Br J Obstet Gynaecol 117: 200–211, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Karl PI. Insulin-like growth factor-1 stimulates amino acid uptake by the cultured human placental trophoblast. J Cell Physiol 165: 83–88, 1995 [DOI] [PubMed] [Google Scholar]
- 31. Latil A, Morant P, Fournier G, Mangin P, Berthon P, Cussenot O. CHC1-L, a candidate gene for prostate carcinogenesis at 13q14.2, is frequently affected by loss of heterozygosity and underexpressed in human prostate cancer. Int J Cancer 99: 689–696, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Lenders CM, McElrath TF, Scholl TO. Nutrition in adolescent pregnancy. Curr Opin Pediatr 12: 291–296, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Lewis RM, Greenwood SL, Cleal JK, Crozier SR, Verrall L, Inskip HM, Cameron IT, Cooper C, Sibley CP, Hanson MA, Godfrey KM. Maternal muscle mass may influence system A activity in human placenta. Placenta 31: 418–422, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Mahendran D, Donnai P, Glazier JD, D'Souza SW, Boyd RD, Sibley CP. Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr Res 34: 661–665, 1993 [DOI] [PubMed] [Google Scholar]
- 35. Malandro MS, Beveridge MJ, Kilberg MS, Novak DA. Effect of low-protein diet-induced intrauterine growth retardation on rat placental amino acid transport. Am J Physiol Cell Physiol 271: C295–C303, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Malina A, Daftary A, Crombleholme W, Markovic N, Roberts JM. Placental system A transporter mRNA is not different in preeclampsia, normal pregnancy, or pregnancies with small-for-gestational-age infants. Hypertens Preg 24: 65–74, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Meller M, Vadachkoria S, Luthy DA, Williams MA. Evaluation of housekeeping genes in placental comparative expression studies. Placenta 26: 601–607, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Miller HS, Lesser KB, Reed KL. Adolescence and very low birth weight infants: a disproportionate association. Obstet Gynecol 87: 83–88, 1996 [DOI] [PubMed] [Google Scholar]
- 39. Moran VH. Nutritional status in pregnant adolescents: a systematic review of biochemical markers. Matern Child Nutr 3: 74–93, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Olausson PO, Cnattingius S, Haglund B. Teenage pregnancies and risk of late fetal death and infant mortality. Br J Obstet Gynaecol 106: 116–121, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Office of National Statistics Birth Statistics—Review of the National Statistician on Births and Patterns of Family Building in England and Wales. Series FM1, no. 36, Tables 3.2 and 7.4, 2007 [Google Scholar]
- 42. Pastrakuljic A, Derewlany LO, Knie B, Koren G. The effects of cocaine and nicotine on amino acid transport across the human placental cotyledon perfused in vitro. J Pharmacol Exp Ther 294: 141–146, 2000 [PubMed] [Google Scholar]
- 43. Pastrakuljic A, Derewlany LO, Koren G. Maternal cocaine use and cigarette smoking in pregnancy in relation to amino acid transport and fetal growth. Placenta 20: 499–512, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Rosso P. Maternal-fetal exchange during protein malnutrition in the rat. Placental transfer of alpha-amino isobutyric acid. J Nutr 107: 2002–2005, 1977 [DOI] [PubMed] [Google Scholar]
- 45. Scholl TO, Hediger ML, Ances IG. Maternal growth during pregnancy and decreased infant birth weight. Am J Clin Nutr 51: 790–793, 1990 [DOI] [PubMed] [Google Scholar]
- 46. Scholl TO, Hediger ML, Schall JI, Khoo CS, Fischer RL. Maternal growth during pregnancy and the competition for nutrients. Am J Clin Nutr 60: 183–188, 1994 [DOI] [PubMed] [Google Scholar]
- 47. Shibata E, Hubel CA, Powers RW, von Versen-Hoeynck F, Gammill H, Rajakumar A, Roberts JM. Placental system A amino acid transport is reduced in pregnancies with small for gestational age (SGA) infants but not in preeclampsia with SGA infants. Placenta 29: 879–882, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sifakis S, Soufla G, Koukoura O, Soulitzis N, Koutroulakis D, Maiz N, Konstantinidou A, Melissari E, Spandidos DA. Decreased Annexin A5 mRNA placental expression in pregnancies complicated by fetal growth restriction. Thromb Res 125: 326–331, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Smith GC, Pell JP. Teenage pregnancy and risk of adverse perinatal outcomes associated with first and second births: population based retrospective cohort study. BMJ 323: 476, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. UNICEF A League Table of Teenage Births in Rich Nations. Innocenti Report Card Issue no. 3, July 2011, p. 4–7 [Google Scholar]
- 51. Wallace J, Bourke D, Da Silva P, Aitken R. Nutrient partitioning during adolescent pregnancy. Reproduction 122: 347–357, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Wallace JM, Bourke DA, Aitken RP, Leitch N, Hay WW., Jr Blood flows and nutrient uptakes in growth-restricted pregnancies induced by overnourishing adolescent sheep. Am J Physiol Regul Integr Comp Physiol 282: R1027–R1036, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Wallace JM, Bourke DA, Aitken RP, Palmer RM, Da Silva P, Cruickshank MA. Relationship between nutritionally-mediated placental growth restriction and fetal growth, body composition and endocrine status during late gestation in adolescent sheep. Placenta 21: 100–108, 2000 [DOI] [PubMed] [Google Scholar]
- 54. Wallace JM, Luther JS, Milne JS, Aitken RP, Redmer DA, Reynolds LP, Hay WW., Jr Nutritional modulation of adolescent pregnancy outcome—a review. Placenta 27, Suppl A: S61–68, 2006 [DOI] [PubMed] [Google Scholar]