Abstract
In many species, β3-adrenergic receptors (β3-ARs) have been reported to play a primary role in pharmacologically induced detrusor smooth muscle (DSM) relaxation. However, their role in guinea pig DSM remains controversial. The aim of this study was to investigate whether β3-ARs are expressed in guinea pig DSM and to evaluate how BRL37344 and L-755,507, two selective β3-AR agonists, modulate guinea pig DSM excitability and contractility. We used a combined experimental approach including RT-PCR, patch-clamp electrophysiology, and isometric DSM tension recordings. β3-AR mRNA message was detected in freshly isolated guinea pig DSM single cells. BRL37344 but not L-755,507 caused a slight decrease in DSM spontaneous phasic contraction amplitude and frequency in a concentration-dependent manner. In the presence of atropine (1 μM), only the spontaneous phasic contractions frequency was inhibited by BRL37344 at higher concentrations. Both BRL37344 and L-755,507 significantly decreased DSM carbachol-induced phasic and tonic contractions in a concentration-dependent manner. However, only BRL37344 inhibitory effect was partially antagonized by SR59230A (10 μM), a β3-AR antagonist. In the presence of atropine, BRL37344 and L-755,507 had no inhibitory effect on electrical field stimulation-induced contractions. Patch-clamp experiments showed that BRL37344 (100 μM) did not affect the DSM cell resting membrane potential and K+ conductance. Although β3-ARs are expressed at the mRNA level, they play a minor to no role in guinea pig DSM spontaneous contractility without affecting cell excitability. However, BRL37344 and L-755,507 have pronounced inhibitory effects on guinea pig DSM carbachol-induced contractions. The study outlines important DSM β3-ARs species differences.
Keywords: BRL37344; muscarinic receptor; L-755,507; large-conductance Ca2+-activated K+ channel
for many years, pharmacological treatment of overactive bladder (OAB) and associated urinary incontinence has relied exclusively on antimuscarinic drugs (1, 32, 43). However, β-adrenergic receptors (β-ARs) could represent alternative targets for OAB because pharmacological stimulation of β-ARs causes detrusor smooth muscle (DSM) relaxation (5, 23). Among all β-AR subtypes (β1, β2, and β3), the β3-ARs may be the most promising new pharmacological targets for OAB treatment because they represent 97% of all β-ARs expressed in human DSM (41). Such predominant expression in DSM suggests that drugs targeting β3-ARs will potentially have fewer side effects compared with antimuscarinic drugs, which can cause dry mouth, constipation, headaches, blurred vision, etc. (1, 3, 43). The activation of β-ARs stimulates adenylyl cyclase which converts ATP into cAMP. Increased intracellular cAMP activates protein kinase A (PKA), which then can phosphorylate various proteins and relax DSM (31, 35). In mouse and guinea pig, stimulation of β-ARs by isoproterenol, a nonselective β-AR agonist, relaxes DSM by activation of the large-conductance calcium-activated potassium (BK) channels (5, 35). Similarly in rat, stimulation of β3-ARs by the selective β3-AR agonist, BRL37344, relaxes DSM by activation of the ryanodine receptors (RyRs) and BK channels (23). The increase in RyRs activity in rat DSM by BRL37344 and in guinea pig DSM by isoproterenol leads to an increase in transient BK currents (TBKCs) frequency, which causes membrane hyperpolarization and thus relaxation (23, 35). Furthermore, in DSM of BK channel knockout mice, β-AR-mediated relaxation is significantly compromised (5). However, to our knowledge, β3-AR mRNA or protein expression has not yet been confirmed in guinea pig DSM, and the functional role of β3-ARs in guinea pig DSM remains controversial (28, 42). Clarification of this role is important because the guinea pig is a widely used animal model to study DSM function.
Our goal was to provide molecular evidence for β3-AR mRNA message expression in guinea pig DSM using RT-PCR and establish whether β3-ARs have a functional role in guinea pig DSM excitability and contractility. Our molecular studies indicate the presence of the β3-AR mRNA message in guinea pig DSM cells and that the selective β3-AR agonists BRL37344 and L-755,507 substantially inhibit carbachol-induced contractions. The compounds have a negligible inhibitory effect on spontaneous contractions and no inhibitory effect on the nerve-evoked contractions of guinea pig DSM in the presence of atropine. Finally, with the patch-clamp technique, we established that BRL37344 had no effect on guinea pig resting membrane potential, voltage step-induced steady-state K+ currents, and TBKCs.
METHODS
Animal studies and DSM tissue harvesting.
The animal studies were carried out in accordance with the Animal Use Protocol #1747 reviewed and approved by the University of South Carolina Institutional Animal Care and Use Committee. For this study, 76 adult (63 males, and 13 females) Hartley-Albino guinea pigs, weighing on average 366.6 ± 21.1 g, were euthanized and DSM tissue was collected for functional, molecular, and electrophysiological studies as previously described (5–7, 23, 25).
DSM single-cell isolation and individual collection.
DSM single cells for electrophysiological and single cells for RT-PCR experiments were collected as previously described (5–7, 22, 23, 25). Briefly, urothelium- and mucosa-free DSM was cut in strips (5–8 mm long, 2–4 mm wide), and 1–2 strips were placed in dissection solution (2 ml), supplemented with 1 mg/ml bovine serum albumin (BSA), 1 mg/ml papain, 1 mg/ml DL-dithiothreitol, and incubated for 25–30 min at 37°C. The DSM strips were transferred to dissection solution (2 ml) containing 1 mg/ml BSA, 1 mg/ml collagenase type II, and 100 μM CaCl2 and incubated for 5–6 min at 37°C. DSM strips were then washed with fresh BSA containing dissection solution. The individual DSM cells were dispersed when the enzyme-treated tissue was passed through the tip of a Pasteur pipette. The isolated DSM cells responded with contraction to mechanical stimulation. This indicates functional contractile proteins and that the cells are physiologically active and therefore ideal for both patch-clamp and single-cell RT-PCR experiments. Dispersed DSM cells were put in a glass chamber and allowed to settle at the bottom for ∼20 min. Next, DSM cells were washed out four to five times using a perfusion system that removes non-DSM cells that do not adhere to the glass bottom of the chamber. Individual DSM cell collection was performed based on cell morphology using an Axiovert 40CFL microscope (Carl Zeiss) with Nomarski interference contrast. DSM cells were collected individually by suction into a glass micropipette using an MP-285/ROE micromanipulator (Sutter Instruments, San Rafael, CA). Only spindle-shape, bright, and shiny DSM cells were collected for further RNA extraction. Approximately 100–200 single cells were collected for RNA extraction and further RT-PCR experiments.
RNA extraction/RT-PCR/sequencing.
Total RNA from whole guinea pig DSM tissue, mucosa-free ileal tissue, and enzymatically isolated DSM single cells was extracted using the RNeasy Mini Kit (Qiagen, Hilden, Germany) as previously described (6, 7, 22, 25). The extracted total RNA was reverse-transcribed into cDNA using M-MLV reverse transcriptase (Promega, Madison, WI) and oligo d(T) primers. Specific primer sequences for β3-AR were obtained from a previous publication (19) and synthesized by Integrated DNA Technologies (IDT, Coralville, IA). The sense and antisense primers were 5′-GTGGGAGGCAACCTGCTGGT-3′ and 5′-CGCCACCACTGGCTCAT-3′, respectively. The cDNA product was PCR amplified in the presence of GoTaq Green Master Mix (Promega) and the specific primers for β3-AR using the mastercycler gradient from Eppendorf (Hamburg, Germany). cDNA was heated for 15 min at 95°C and then amplified by 35 cycles (95°C for 30 s, 60°C for 30 s, 72°C for 30 s) followed by a 5-min extension at 72°C. PCR products were visualized by electrophoresis through 2% agarose ethidium bromide-stained gels. The expected length of the fragment was 387 bp, which was identified by using a 100-bp extended range DNA ladder from Lonza (Rockland, ME). All RT-PCR products from the intact DSM tissue, mucosa-free ileal tissue, and DSM isolated single cells were purified using the GenElute PCR Clean-Up Kit (Sigma, St. Louis, MO) and sequenced at the University of South Carolina Environmental Genomics Core Facility to confirm their identity.
Isometric DSM tension recordings.
Isometric tension recording experiments were conducted as previously described (5, 6, 23, 25). Guinea pig DSM strips were stretched to 1 g of tension to follow the protocol established in previous studies (10, 12, 18, 22, 29, 38). Strips were washed out with fresh physiological saline solution (PSS) every 15 min and allowed to equilibrate for a period of 45 min to 1 h. Next, we separated DSM strips into three experimental groups. The first group was characterized by DSM isolated strips that exhibited spontaneous contraction amplitude >0.1 g after the initial stretch to 1 g of tension following the equilibration period. The second and third groups were characterized by strips that exhibited phasic contraction amplitude <0.1 g. In the second and third group, contractions were amplified by carbachol (1 μM), a cholinergic agonist, or by electrical field stimulation (EFS), respectively. On DSM strips with spontaneous phasic contractions or carbachol-induced contractions, the neuronal Na+ channel blocker tetrodotoxin (1 μM TTX) was first added to the bath for at least 15 min after a 45-min equilibration period. TTX was added to restrain neuronal activity. Then, increasing BRL37344 or L-755,507 concentrations (1 nM-100 μM) were applied at 10-min intervals directly into the bath in the absence or presence of SR59230A (10 μM), a selective β3-AR antagonist (5, 8, 20, 36). On a separate set of strips, BRL37344 (100 μM) was added into the tissue baths containing DSM strips exhibiting spontaneous phasic contractions in the presence or absence of atropine (1 μM), a muscarinic receptor antagonist. For the EFS experimental series, TTX was not added to the bath, because TTX (1 μM) effectively suppresses EFS-induced contractions of DSM isolated strips (6, 17). Furthermore, we separated the cholinergic component of the EFS-induced contractions from the purinergic component by blocking purinergic receptors with α,β-methylene-ATP and suramin. DSM isolated strips were preincubated with suramin (10 μM) and α,β-methylene-ATP (10 μM) for 15 min before the EFS-control protocol. The purinergic component of the EFS-induced contractions was assessed by addition of 1 μM atropine to block the cholinergic component. This experimental series was also performed in the presence or absence of SR59230A (10 μM). The EFS pulses were generated by the PHM I stimulator (Med Associates) and had the following parameters: pulse amplitude (20 V), pulse width (0.75 ms), stimulus duration (3 s), polarity was reversed for alternating pulses, and frequencies (0.5, 2, 3.5, 5, 7.5, 10, 12.5, 15, 20, 30, 40, 50 Hz) applied at 3-min intervals.
Electrophysiological (patch-clamp) experiments.
To preserve the physiological environment of the DSM cells, amphotericin-perforated whole cell mode of the patch-clamp technique was applied in all electrophysiological experiments (13, 21). Whole cell currents and membrane potential were recorded as previously described (5, 22, 23, 25). Briefly, freshly isolated single DSM cells were placed onto an experimental chamber for 20 min to allow them to adhere to the glass bottom and were then washed with extracellular solution. The ionic currents and resting membrane potential were recorded using Axopatch 200B amplifier; pCLAMP version 10.2 software; Digidata 1322A and Digidata 1440A (Molecular Devices, Union City, CA). The signals were filtered using an eight-pole Bessel filter (model 900CT/9L8L; Frequency Devices, Ottawa, IL). Patch-clamp pipettes were pulled from borosilicate glass (Sutter Instruments, Novato, CA) using a Narishige PP-830 vertical puller, coated with sticky dental wax to reduce capacitance, and then polished with a Micro Forge MF-830 fire polisher (Narishige Group, East Meadow, NY) to give a final tip resistance of 4–6 MΩ. Leak currents were not subtracted. TBKCs were recorded at a holding potential of −40 or −20 mV (corrected for junction potential) for at least a 10-min period in the absence (control) and then in the presence of BRL37344 (100 μM). Voltage-step protocols were used to elicit steady-state K+ outward current. Resting membrane potentials of DSM cells were recorded using the current-clamp mode of the perforated patch-clamp technique. To determine the mean amplitude and frequency of TBKCs, analysis was performed off-line using Clampfit of the pCLAMP version 10.2 and MiniAnalysis software (Synaptosoft, Decatur, GA). All patch-clamp experiments were conducted at room temperature (22–23°C).
Solutions and drugs.
The Ca2+-free dissection solution, the Ca2+-containing PSS, the extracellular (bath) solution used for electrophysiological recordings, and the patch pipette solution were prepared daily and had the same composition as previously described (5, 23, 25). BSA and amphotericin-B were purchased from Fisher Scientific (Pittsburgh, PA). Papain was obtained from Worthington Biochemical (Lakewood, NJ). L-755,507 was purchased from Tocris Bioscience (Ellisville, MO). The rest of the drugs was purchased from Sigma. BRL37344 stock solution was prepared daily in double distilled H2O and was heated to 60°C to fully dissolve the drug according to the manufacturer's instructions. L-755,507 was also prepared daily and dissolved in DMSO at a final DMSO concentration not greater than 0.1%.
Data analysis and statistics.
Isometric DSM tension was recorded using MyoMed software (Med Associates). MiniAnalysis software (Synaptosoft) was used for data analysis of DSM phasic contraction amplitude, muscle force integral (determined by integrating the area under the curve of the phasic contractions), phasic contraction frequency, phasic contraction duration (determined by the sum of contraction rise and decay times), and muscle tone (determined by phasic contraction baseline curve). GraphPad Prism 4.03 software (GraphPad Software, La Jolla, CA) was used for statistical analysis and CorelDraw Graphic Suite software (Corel, Ottawa, Ontario, Canada) was used for data illustration. In the summary data, n represents the number of individual DSM isolated strips or single cells and N is the number of guinea pigs. Paired Student's t-test was used to analyze single-concentration drug effects and electrophysiology data. Two-way ANOVA followed by Bonferroni's posttest was used to calculate the statistical difference between concentration-response and frequency-response curves. All results are expressed as means ± SE; P < 0.05 was considered to be statistically significant.
RESULTS
Detection of mRNA message for β3-AR in guinea pig DSM.
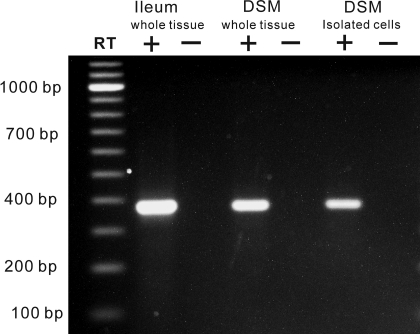
RT-PCR experiments detected a β3-AR mRNA message in whole DSM and isolated DSM single cells (Fig. 1). Using isolated DSM single cells is advantageous, eliminating possible contamination from other cell types such as neurons, fibroblasts, vascular myocytes, and other interstitial cells present within the DSM layers (6, 7, 25). These results demonstrate that guinea pig isolated DSM cells express β3-AR mRNA message. Negative controls performed in the absence of reverse transcriptase (−RT) demonstrated an absence of genomic DNA contamination. Next, we sought to evaluate the β3-ARs functional role in guinea pig DSM contractility.
Fig. 1.
RT-PCR detection of β3-adrenergic receptor (β3-AR) mRNA in detrusor smooth muscle (DSM) whole tissue and isolated DSM single cells. The expected size of the product was 387 bp. RT (+), reverse transcription; RT (−), no reverse transcription. Guinea pig ileal tissue was used as a positive control. Illustrated gel image is a selected representation of at least 5 independent RT-PCR experiments based on RNA extracted from 4 animals.
BRL37344 but not L-755,507 has a minor inhibitory effect on the spontaneous phasic and tonic contractions in guinea pig DSM isolated strips.
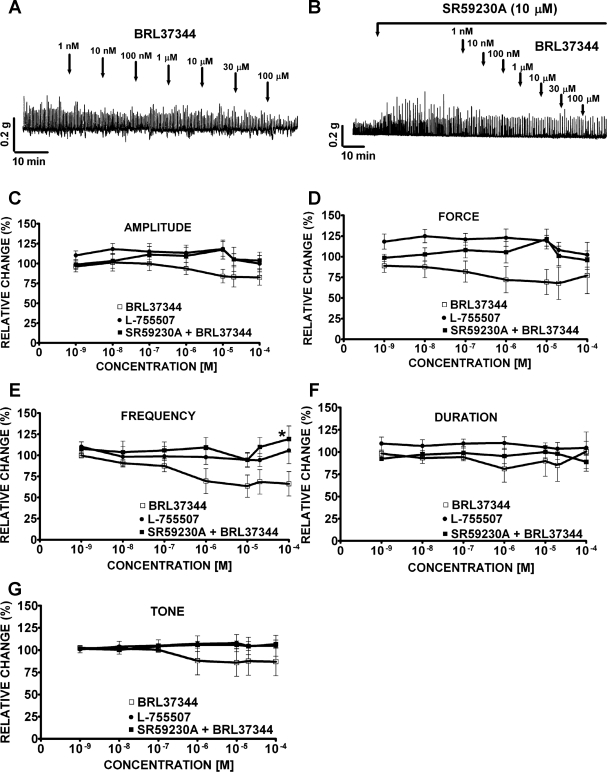
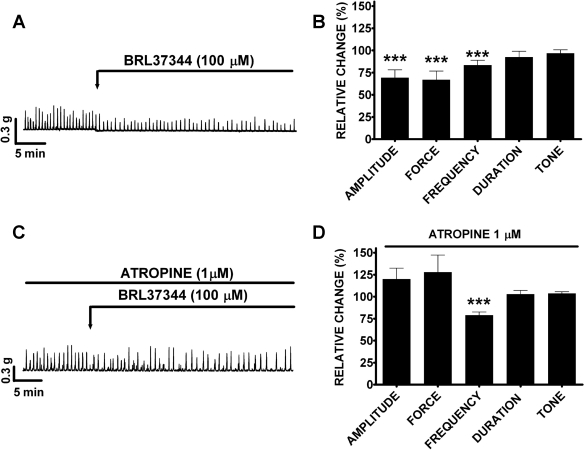
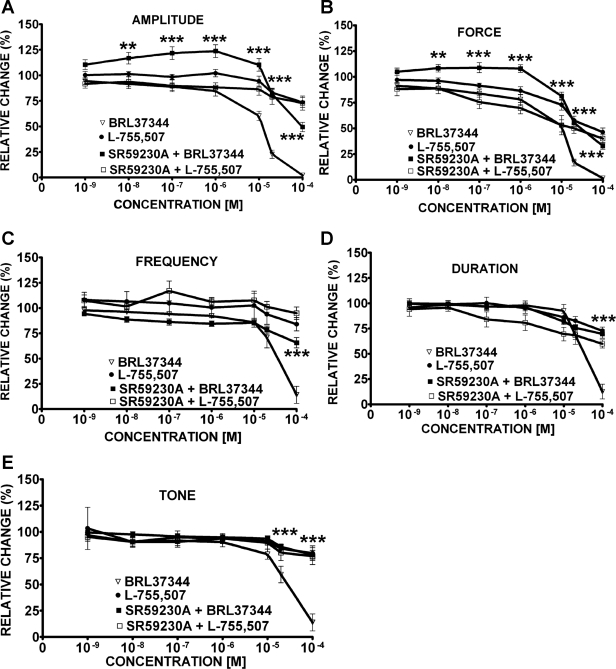
Under control conditions before compound (BRL37344, L-755,507, or SR59230A) application (taken to be 100%), guinea pig DSM spontaneous phasic contractions average amplitude was 0.4 ± 0.1 g, force was 1.4 ± 0.5 g/s, frequency was 3.0 ± 0.01 contractions/min, duration was 8.0 ± 0.9 s/contraction, and tone was 0.2 ± 0.03 g (n = 41, N = 23). In the absence of SR59230A, BRL37344 (1 nM-100 μM) slightly inhibited the spontaneous phasic contraction amplitude and frequency without statistically significant effects on muscle force integral, phasic contraction duration, and muscle tone (Fig. 2A). At the maximal concentration of 100 μM, BRL37344 significantly reduced the phasic contractions amplitude by 17.6 ± 9.7% and frequency by 20.7 ± 8.5% (n = 7, N = 7, P < 0.05; Fig. 2, C and E). In the presence of SR59230A (10 μM), the BRL37344 (100 μM) inhibitory effect on the spontaneous phasic contraction frequency was antagonized (n = 8, N = 4, P < 0.05; Fig. 2E) but no antagonistic effect on the spontaneous phasic contraction amplitude was observed (n = 8, N = 4, P > 0.05; Fig. 2C). SR59230A alone increased the amplitude and muscle force integral of the spontaneous phasic contractions. SR59230A increased the spontaneous phasic contraction amplitude by 196.0 ± 20.7% (n = 8, N = 4, P < 0.005) and muscle force integral by 166.0 ± 57.6% (n = 8, N = 4, P < 0.01) with no significant effect on the phasic contraction frequency, duration, or muscle tone (n = 8, N = 4; Fig. 2B). Unlike BRL37344, the other selective β3-AR agonist L-755,507 (1 nM-100 μM) had no significant effects on any of the five parameters of the spontaneous contractions (n = 12, N = 6, P > 0.05; Fig. 2, C–G). To prove that the BRL37344 inhibitory effect on spontaneous contractions in the concentration-response experiments was not due to a run-down of the preparation or a desensitization of the β3-AR, we further examined the effect of a single supramaximal BRL37344 concentration (100 μM) on spontaneous contractions. BRL37344 (100 μM) significantly decreased spontaneous phasic contraction amplitude, muscle force integral, phasic contraction frequency, but not phasic contraction duration or muscle tone. The amplitude of the spontaneous phasic contractions decreased by 31.0 ± 9.1%, muscle force integral by 33.2 ± 10.0%, and phasic contraction frequency by 16.6 ± 5.5% (n = 14, N = 5, P < 0.05; Fig. 3, A and B). BRL37344 has been reported to have some antimuscarinic effects in addition to its β3-AR agonist properties (23, 26). To separate these two effects, we used the cholinergic antagonist atropine. In the presence of 1 μM atropine, BRL37344 (100 μM) significantly inhibited only the DSM spontaneous contraction frequency by 21.2 ± 3.8% without any significant effect on phasic contraction amplitude, muscle force integral, phasic contraction duration, and muscle tone (n = 9, N = 5, P < 0.05; Fig. 3, C and D). These results suggest that BRL37344 but not L-755,507 has a minor inhibitory effect on the spontaneous contractions of guinea pig DSM isolated strips. This BRL37344 inhibitory effect is partially blocked by atropine (Fig. 3).
Fig. 2.
Effects of BRL37344 and L-755,507 on the spontaneous phasic and tonic contractions of guinea pig DSM isolated strips. A–B: original recordings of DSM spontaneous phasic contractions illustrating BRL37344 inhibitory effect (1 nM-100 μM) in the absence (A) or presence (B) of SR59230A (10 μM). C–G: concentration-response curves for BRL37344 (1 nM-100 μM) inhibitory effects on the spontaneous phasic contraction amplitude (C), muscle force integral (D), phasic contraction frequency (E), phasic contraction duration (F), and muscle tone (G) in the presence or absence of SR59230A (10 μM). Concentration-response curves for L-755,507 (1 nM-100 μM) showed no inhibitory effect on the spontaneous phasic and tonic contractions of DSM isolated strips (C–G). Spontaneous contractions were taken to be 100% (n = 7, N = 7 for BRL37344; n = 12, N = 6 for L-755,507; and n = 8, N = 4 for SR59230A + BRL37344; *P < 0.05 for the SR59230A effect). Tetrodotoxin (TTX; 1 μM) was present throughout the experiments.
Fig. 3.
Application of a single concentration of BRL37344 (100 μM) decreased the guinea pig DSM spontaneous phasic contraction amplitude, muscle force integral, and phasic contraction frequency in the absence of atropine. A, C: original recording of DSM contractions illustrating BRL37344 inhibitory effect in the absence (A) or presence of 1 μM atropine (C). B, D: summary data showing BRL37344 (100 μM) inhibitory effect in the absence (B; n = 14, N = 5, ***P < 0.005) or presence of 1 μM atropine (D; n = 9, N = 5, ***P < 0.005). Spontaneous contractions were taken to be 100%. TTX (1 μM) was present throughout the experiments.
Both BRL37344 and L-755,507 inhibit carbachol-induced contractions in guinea pig DSM isolated strips.
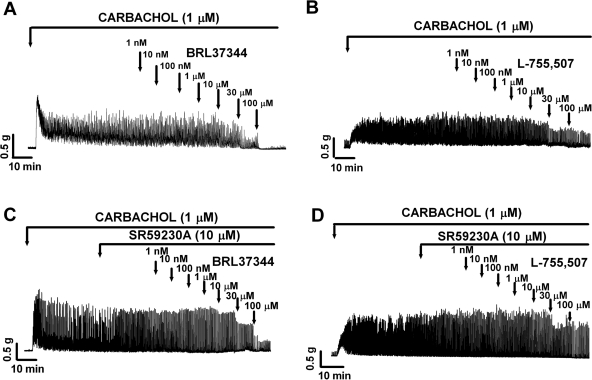
Under control conditions (taken to be 100%), guinea pig DSM carbachol-induced phasic contraction average amplitude was 1.4 ± 0.3 g, force was 6.8 ± 1.3 g/s, frequency was 3.0 ± 0.01 contractions/min, contraction duration was 11.0 ± 0.6 s, and tone was 0.4 ± 0.06 g (n = 59, N = 21). BRL37344 (1 nM-100 μM) almost completely inhibited all five parameters of the carbachol-induced contractions (Figs. 4A and 5). L-755,507 (1 nM-100 μM) caused a less dramatic but still statistically significant decrease in all five parameters of the carbachol-induced contractions (Fig. 4B). However, only at concentrations higher than 10 μM L-755,507 did significantly inhibit DSM carbachol-induced contractions. At a concentration of 100 μM, L-755,507 decreased the phasic contraction amplitude by 26.4 ± 4.8%, muscle force integral by 53.6 ± 4.0%, phasic contraction frequency by 16.1 ± 6.6%, phasic contraction duration by 27.4 ± 3.8%, and muscle tone by 20.3 ± 7.0% (n = 12, N = 3, P < 0.05; Fig. 5).
Fig. 4.
Original DSM contraction recordings illustrating BRL37344 (1 nM-100 μM) and L-755,507 (1 nM-100 μM) concentration-dependent inhibitory effects on carbachol-induced phasic and tonic contractions of DSM isolated strips in the absence (A, B) or presence (C, D) of SR59230A (10 μM). TTX (1 μM) was present throughout the experiments.
Fig. 5.
Concentration-response curves for BRL37344 (1 nM-100 μM) and L-755,507 (1 nM-100 μM) inhibitory effects on carbachol-induced phasic contraction amplitude (A), muscle force integral (B), phasic contraction frequency (C), phasic contraction duration (D), and muscle tone (E) in the presence or absence of SR59230A (10 μM). Carbachol-induced contractions were taken to be 100% (n = 14, N = 6 for BRL37344; n = 25, N = 6 for BRL37344 + SR59230A; **P < 0.01, ***P < 0.005 for the SR59230A effect) and (n = 12, N = 3 for L-755,507; n = 7, N = 3 for L-755,507 + SR59230A; P > 0.05 for the SR59230A effect). TTX (1 μM) was present throughout the experiments.
In the presence of SR59230A (10 μM), BRL37344 still inhibited DSM carbachol-induced contractions; however, its inhibitory effect was partially antagonized (Figs. 4C and 5). BRL37344 (100 μM) inhibitory effect was partially reduced as follows: phasic contraction amplitude was reduced by only 47.1 ± 3.1% (Fig. 5A); muscle force integral was reduced by 30.4 ± 2.2% (Fig. 5B); phasic contraction frequency was reduced by 51.6 ± 6.7% (Fig. 5C); phasic contraction duration was reduced by 57.1 ± 5.7% (Fig. 5D); muscle tone was reduced by 64.2 ± 6.2% (Fig. 5E; n = 14, N = 6 for control and n = 25, N = 6, in presence of 10 μM SR59230A; P < 0.01). There was no significant change in the inhibitory effect of L-755,507 (1 nM-100 μM) on the carbachol-induced contractions in the presence of 10 μM SR59230A (n = 7, N = 3, P > 0.05; Fig. 5).
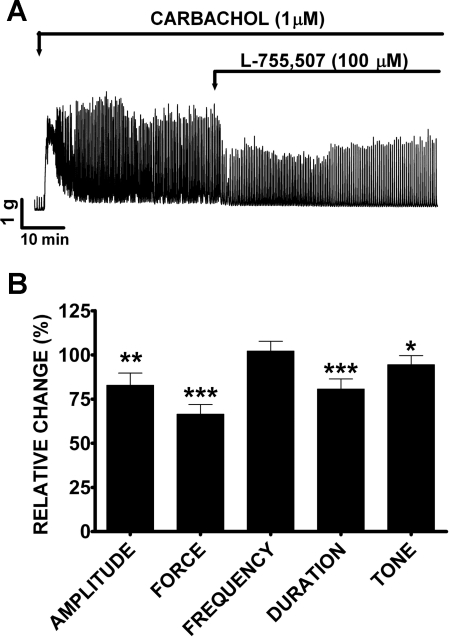
We further examined the effect of a single supramaximal concentration of L-755,507 (100 μM) on carbachol-induced contractions. L-755,507 (100 μM) significantly decreased the amplitude, muscle force integral, duration, and tone but not the frequency of carbachol-induced contractions (Fig. 6A). The amplitude of carbachol-induced phasic contractions decreased by 18.1 ± 6.2%, muscle force integral by 34.4 ± 5.2%, phasic contraction duration by 18.0 ± 5.1%, and muscle tone by 12.2 ± 5.0% (n = 12, N = 3, P < 0.05; Fig. 6B). These data suggest that BRL37344 and L-755,507 can substantially reduce carbachol-induced contractions. Next, we evaluated whether pharmacological activation of β3-ARs can modulate nerve-evoked contractions of guinea pig DSM isolated strips.
Fig. 6.
L-755,507 (100 μM) significantly reduced carbachol-induced phasic and tonic contractions of DSM isolated strips. A: original DSM contraction recording illustrating L-755,507 (100 μM) inhibitory effect in the absence of SR59230A (10 μM). B: summary data showing that L-755,507 (100 μM) significantly reduced the amplitude, muscle force integral, phasic contraction duration, and muscle tone (n = 12, N = 3, *P < 0.05, **P < 0.01, ***P < 0.005). TTX (1 μM) was present throughout the experiments.
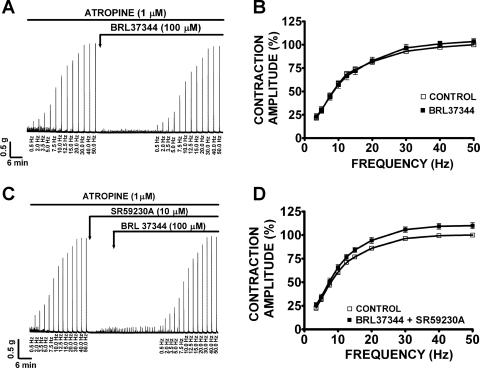
BRL37344 decreases the amplitude of the EFS-induced contractions in guinea pig DSM isolated strips.
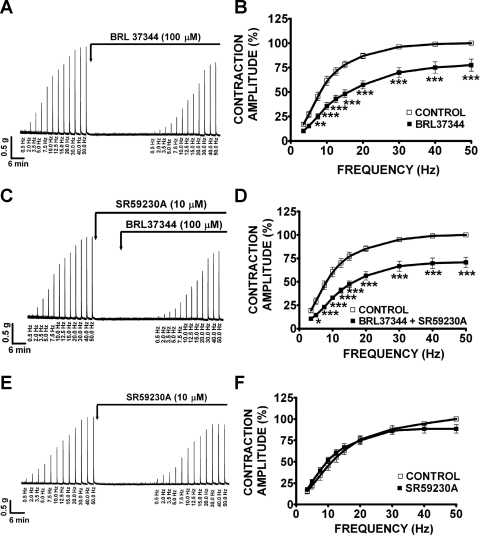
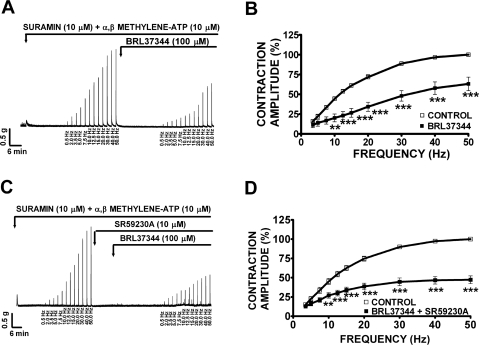
Under control conditions at 50 Hz (taken to be 100%), guinea pig DSM EFS-induced contractions average amplitude was 4.0 ± 0.3 g (n = 36, N = 12). In the absence of atropine, BRL37344 (100 μM) significantly reduced the amplitude of the EFS-induced contractions at all stimulation frequencies between 7.5 and 50 Hz (Fig. 7A). At 50 Hz, BRL37344 (100 μM) significantly reduced the amplitude of the EFS-induced contraction by 22.5 ± 6.2% (n = 10, N = 4, P < 0.005; Fig. 7B). In the presence of SR59230A (10 μM), BRL37344 (100 μM) still decreased the amplitude of the EFS-induced contractions (Fig. 7C). At 50 Hz, in the presence of SR59230A (10 μM), BRL37344 inhibited EFS-induced contractions by 29.3 ± 5.4% (n = 11, N = 5, P < 0.005; Fig. 7D). SR59230A (10 μM) alone had no effect on the EFS-induced contractions as illustrated in Fig. 7, E and F (n = 9, N = 6, P > 0.05). These results suggest that BRL37344 inhibitory effect on EFS-induced contractions is not mediated by β3-ARs but it is most likely due to BRL37344 antimuscarinic effects. In the presence of suramin (10 μM) and α,β-methylene-ATP (10 μM), which were used to block the purinergic component of EFS-induced contraction, BRL37344 (100 μM) still decreased the amplitude of the EFS-induced contractions as shown in Fig. 8A. In the presence of suramin and α,β-methylene-ATP, guinea pig DSM EFS-induced contraction average amplitude at 50 Hz was 2.4 ± 0.4 g and was taken to be 100% (n = 20, N = 10). At the maximal stimulation frequency of 50 Hz, BRL37344 (100 μM) significantly reduced the amplitude of the EFS-induced contractions by 36.9 ± 8.5% (n = 10, N = 5, P < 0.005; Fig. 8B). SR59230A (10 μM) again did not block the BRL37344 inhibitory effect on contraction amplitude at EFS stimulation frequencies between 10 and 50 Hz (Fig. 8C). At the maximal stimulation frequency of 50 Hz, in the presence of SR59230A (10 μM), BRL37344 (100 μM) decreased the EFS-induced contraction amplitude by 52.7 ± 5.0% (n = 10, N = 5, P < 0.005; Fig. 8D). In the presence of atropine (1 μM), which was used to block the cholinergic component of the EFS-induced contraction, BRL37344 (100 μM) had no effect on the EFS-induced contractions in the absence (n = 13, N = 6, P > 0.05) or presence of 10 μM SR59230A (n = 12, N = 5, P > 0.05; Fig. 9). In the presence of atropine, guinea pig DSM EFS-induced contraction average amplitude at 50 Hz was 2.3 ± 0.2 g and was taken to be 100% (n = 25, N = 11). These data also show that BRL37344's inhibitory effect on EFS-induced contractions is not mediated by β3-AR but is rather due to BRL37344 antimuscarinic properties. Next, we sought to evaluate L-755,507 effect on EFS-induced contractions.
Fig. 7.
BRL37344 (100 μM) significantly decreases the amplitude of the electrical field stimulation (EFS)-induced contractions of guinea pig DSM isolated strips in the absence or presence of SR59230A (10 μM). Original recordings illustrating EFS-induced (0.5–50 Hz) contractions of DSM isolated strips in response to 100 μM BRL37344 (A), 10 μM SR59230A and 100 μM BRL37344 combined (C), and 10 μM SR59230A (E). Frequency-response curves for the EFS-induced (0.5–50 Hz) contractions in response to 100 μM BRL37344 (B), 10 μM SR59230A and 100 μM BRL37344 combined (D), 10 μM SR59230A (F; n = 10, N = 4 for B; n = 11, N = 5 for D; n = 9, N = 6, P > 0.05 for F; *P < 0.05, **P < 0.01, ***P < 0.005). EFS-induced contraction amplitude at 50 Hz under control conditions was taken to be 100%.
Fig. 8.
In the presence of suramin (10 μM) and α,β-methylene-ATP (10 μM), BRL37344 (100 μM) decreased the amplitude of the EFS-induced (0.5–50 Hz) contractions in DSM isolated strips. A, C: original EFS-induced contraction recordings showing BRL37344 (100 μM) inhibitory effect in the absence (A) or presence (C) of SR59230A (10 μM). B, D: frequency-response curves showing BRL37344 inhibitory effect on EFS-induced contraction amplitude in the absence (B; n = 10, N = 5, **P < 0.01, ***P < 0.005) or presence (D) of 10 μM SR59230A (n = 10, N = 5, **P < 0.01, ***P < 0.005). EFS-induced contraction amplitude at 50 Hz under control conditions was taken to be 100%.
Fig. 9.
In the presence of atropine (1 μM), BRL37344 (100 μM) does not affect the amplitude of the EFS-induced (0.5–50 Hz) contractions of DSM isolated strips. A, C: original recordings illustrating the lack of BRL37344 (100 μM) effect on EFS-induced contractions in the absence (A) or presence (C) of SR59230A (10 μM). B, D: frequency-response curves showing the lack of BRL37344 effect on the EFS-induced contraction amplitude in the absence (B; n = 13, N = 6, P > 0.05) or presence (D) of 10 μM SR59230A (n = 12, N = 5, P > 0.05).
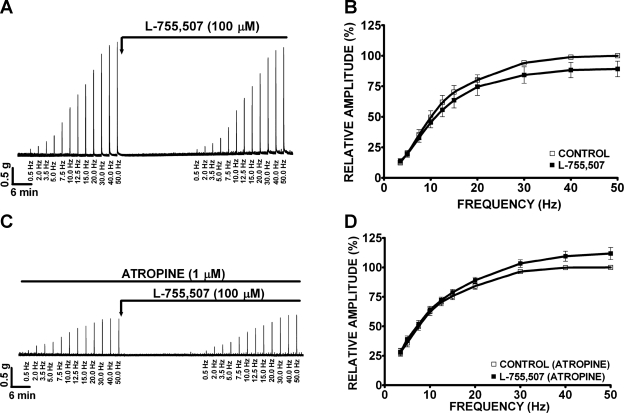
L-755,507 has no effect on the EFS-induced contractions of guinea pig DSM isolated strips.
In this experimental series, we investigated the effect of L-755,507 on EFS-induced contractions. We observed that L-755,507 (100 μM) did not significantly inhibit EFS-induced contractions at all stimulation frequencies (0.5–50 Hz) in the absence (n = 9, N = 4, P > 0.05) or presence of 1 μM atropine (n = 6, N = 4, P > 0.05; Fig. 10).
Fig. 10.
L-755,507 (100 μM) does not affect the amplitude of the EFS-induced (0.5–50 Hz) contractions of DSM isolated strips. A, C: original recordings illustrating a lack of L-755,507 (100 μM) effect on EFS-induced contractions in the absence (A) or presence (C) of atropine (1 μM). B, D: frequency-response curves showing the lack of inhibitory effect on EFS-induced contractions by L-755,507 in the absence (B; n = 9, N = 4, P > 0.05) or presence (D) of 1 μM atropine (n = 6, N = 4, P > 0.05).
Pharmacological activation of β3-ARs with BRL37344 does not modulate cell excitability in guinea pig DSM freshly isolated cells.
BRL37344 was preferred over L-755,507 due to its more pronounced effects on guinea pig DSM contractility. We previously reported that in rat DSM, BRL37344 (100 μM) induces relaxation by activation of TBKCs and membrane hyperpolarization (23).
The average capacitance of guinea pig DSM cells was 34.7 ± 0.8 pF (n = 123 cells, N = 30). In the first patch-clamp experimental series, we investigated BRL37344 (100 μM) effect on guinea pig DSM TBKCs amplitude and frequency. Our results showed no statistically significant change in the mean amplitude and frequency of TBKCs after application of 100 μM BRL37344 (n = 6–9, N = 5–7, P > 0.05; Table 1). Next, we examined whether the application of BRL37344 (100 μM) activates the voltage step-induced steady-state K+ current in single DSM cells. From a holding potential −70 mV, we applied a voltage-step protocol from 0 to +80 mV in 20-mV increments and 200-ms duration. BRL37344 (100 μM) did not activate the depolarization-induced steady-state outward K+ currents (n = 4, N = 2, P > 0.05; Table 1). In current-clamp mode, we tested the effect of BRL37344 (100 μM) on the resting membrane potential of freshly isolated guinea pig DSM cells, and BRL37344 did not affect the resting membrane potential (n = 8, N = 4, P > 0.05; Table 1). These data suggest that activation of β3-ARs with BRL37344 in guinea pig DSM does not modulate basal cell excitability.
Table 1.
Summarized data illustrating a lack of effect of BRL37344 (100 μM) on cell membrane excitability in guinea pig DSM freshly isolated cells
| Control | BRL37344 (100 μM) | Statistics | ||
|---|---|---|---|---|
| TBKCs | Amplitude −40 mV | 24.0 ± 4.6 pA | 26.3 ± 6.5 pA | n = 9; N = 7; P > 0.05 |
| Amplitude −20 mV | 19.8 ± 2.4 pA | 18.16 ± 1.1 pA | n = 6; N = 5; P > 0.05 | |
| Frequency −40 mV | 50.2 ± 22.8 TBKCs/min | 65.76 ± 30.4 TBKCs/min | n = 9; N = 7; P > 0.05 | |
| Frequency −20 mV | 45.3 ± 18.2 TBKCs/min | 74.23 ± 31.2 TBKCs/min | n = 6; N = 5; P > 0.05 | |
| Voltage step-induced whole cell current | 0 mV | 2.3 ± 1.0 pA/pF | 1.1 ± 0.3 pA/pF | n = 4; N = 2; P > 0.05 |
| 20 mV | 3.5 ± 1.1 pA/pF | 1.8 ± 0.2 pA/pF | n = 4; N = 2; P > 0.05 | |
| 40 mV | 5.9 ± 1.7 pA/pF | 3.6 ± 0.6 pA/pF | n = 4; N = 2; P > 0.05 | |
| 60 mV | 11.4 ± 3.3 pA/pF | 10.8 ± 1.9 pA/pF | n = 4; N = 2; P > 0.05 | |
| 80 mV | 22.8 ± 7.5 pA/pF | 22.7 ± 5.1 pA/pF | n = 8; N = 4; P > 0.05 | |
| Resting membrane potential recorded in current-clamp mode | −22.8 ± 3.5 mV | −21.6 ± 4.0 mV |
Values are means ± SE. Perforated whole cell patch-clamp experiments revealed no effect of 100 μM BRL37344 on transient BK currents (TBKCs) amplitude and frequency recorded at −40 and −20 mV. Whole cell outward K+ current amplitude was also unchanged after application of 100 μM BRL37344. Current-clamp experiments revealed that 100 μM BRL37344 did not significantly change the level of the resting membrane potential in guinea pig detrusor smooth muscle (DSM) freshly isolated cells.
DISCUSSION
In this study, we applied a multidisciplinary approach including molecular, cellular, and tissue studies to evaluate the expression and potential functional role of β3-ARs in guinea pig DSM. Because β3-ARs are considered viable therapeutic targets for OAB, it is crucial to understand β3-ARs expression and function in the bladder of guinea pig, a commonly used animal research model. Our data suggest that β3-ARs are expressed at the mRNA level but are minor contributors (if at all) in guinea pig DSM function. Unlike earlier studies (42), the novelty of our study is that we systematically spanned for all five parameters of the phasic contractions including amplitude, muscle force integral, frequency, duration, and muscle tone in guinea pig DSM isolated strips.
The physiological significance of our study derives from the fact that β3-ARs have been shown to be important facilitators of bladder relaxation by controlling spontaneous phasic and nerve-evoked contractions as well as TBKCs that contribute to DSM excitability (5, 23, 35). Previous studies established a functional link between the β3-AR and the BK channel, which plays a key role in DSM function (5, 22, 23, 35). These findings have led researchers to suggest that β3-ARs could be suitable targets for the pharmacological treatment of OAB. As a consequence, there are now a number of β3-AR agonists, which are currently under investigation for the treatment of OAB. In anesthetized mice, CL316,243, a selective β3-AR agonist, given intravenously at doses of 0.03 and 0.1 mg/kg, significantly increases bladder capacity and threshold pressure without a significant change in bladder compliance (8). CL316,243 also induces a significant decrease in the amplitude of both micturition and nonvoiding contractions (8). In anesthetized rats, intravenous injections of selective β3-AR agonists such as BRL37344, TAK-677, and FK175 (0.1–500 μg/kg) have been proven to significantly decrease voiding frequency, bladder pressure, and the amplitude of bladder contractions (27).
Whether β3-ARs are expressed in guinea pig DSM has been debated: no reports identify their presence at the single DSM cell level. Pharmacological studies on guinea pig DSM using various β3-AR agonists and antagonists yielded controversial results (11, 28, 42). Clarification of β3-AR functional role has a strong physiological significance because the guinea pig is an established animal model to study DSM function added to the fact that species differences could also be a determining factor. Some authors suggest that the β1-AR is the most predominant β-AR in guinea pig DSM, and thus is mainly responsible for guinea pig DSM relaxation (28, 31, 42). Other studies show that the selective β3-AR agonist BRL37344 (300 μM) reduced the amplitude of phasic rises in intravesical pressure of guinea pig isolated whole bladder, suggesting a role of β3-ARs in guinea pig DSM relaxation (11). Our molecular data support the latter publication that the β3-AR mRNA message is expressed in isolated single cells of guinea pig DSM (Fig. 1). The advantage of using isolated single cells for RT-PCR is that this approach eliminates any possible contamination by other cell types present within the DSM (6, 7, 22, 23). Due to the lack of specific antibodies for guinea pig β3-ARs (31), we performed no Western blot or immunohistochemistry studies at this time to further prove β3-AR protein expression. To our knowledge, this is the first report in which molecular evidence for the presence of β3-ARs in guinea pig DSM has been provided.
The functional role of β-ARs activation in DSM is to induce relaxation (5, 23, 25, 35, 42), which is suggested to be mediated via activation of the β-AR/PKA pathway (23, 31, 35). Our results indicate that at higher concentrations BRL37344 induced a slight concentration-dependent reduction of the amplitude and frequency of the spontaneous phasic contractions of guinea pig DSM isolated strips (Fig. 2). Furthermore, a single supramaximal concentration of BRL37344 (100 μM) also decreased the amplitude, muscle force integral, and frequency of the spontaneous phasic contractions of guinea pig DSM isolated strips (Fig. 3). The use of this supramaximal concentration of 100 μM is justified by the fact that BRL37344 concentrations ≤30 μM seem insufficient to induce inhibition of spontaneous DSM contractions (5, 11, 42). However, at these high concentrations BRL37344 may also nonselectively activate β1-ARs and/or β2-ARs (39).
Studies on rat DSM have shown that BRL37744 also exhibit some antimuscarinic properties (5, 26, 30). In our study, we addressed this issue by using the muscarinic receptor antagonist atropine. In the presence of atropine, BRL37344 (100 μM) lost its inhibitory effect on the DSM phasic contraction amplitude and muscle force integral (Fig. 3), further supporting its antimuscarinic properties. Our data (consistent with early findings) (42) indicate that in the guinea pig, β3-ARs have limited effects on DSM relaxation because their pharmacological activation with BRL37344 significantly modulates only one (frequency) spontaneous phasic contraction parameter and only at high concentrations (10–100 μM). At these high concentrations, BRL37344 may also nonselectively activate β1-ARs and/or β2-ARs (39).
In DSM, the β-adrenergic relaxation is primarily mediated by activation of the BK channels (5, 23, 35). Our electrophysiological data supported the idea that β3-ARs, although expressed in guinea pig DSM, do not regulate the DSM excitability. Perforated patch-clamp experiments revealed no effect of BRL37344 on whole cell steady-state K+ current, TBKCs amplitude and frequency, and the resting membrane potential (Table 1). These findings clearly suggest species differences. Our previous studies on rat DSM showed that the activation of β3-AR by BRL37344 increased TBKCs frequency and hyperpolarized the cell membrane (23). In rat DSM, a functional link between β3-ARs and BK channel is mediated by the RyRs of the sarcoplasmic reticulum (23). Our study shows that in guinea pig DSM, unlike rat and human DSM, β3-ARs are not the primary regulator of DSM function.
Since the relationship between length and force can be converted to volume and pressure using the law of Laplace (2), the evaluation of muscle force integral provides information on the increase in bladder volume and intravesical pressure during bladder filling and voiding phases. It is known that the BK channels shape guinea pig DSM spontaneous action potentials that determine the spontaneous phasic contractions including contraction amplitude, duration, frequency, muscle force integral, and tone (5, 14, 18, 22, 23, 34). Pharmacological inhibition of DSM BK channels increases the action potential duration and frequency, causes membrane potential depolarization (16, 22), and increases the amplitude of phasic contractions (5, 18, 22, 34). The increase in the amplitude of a phasic contraction is provoked by the increase in intracellular Ca2+ during the action potential depolarization phase and activation of L-type voltage-gated Ca2+ channels, whereas the duration of a phasic contraction depended on the duration of the single action potential or a whole burst of action potentials (14, 16). Thus, there is a clear association between the action potential parameters, BK channel activity, and the related phasic contraction parameters. However, our data on guinea pig DSM show that BRL37344 does not affect BK channel activity (Table 1).
We also evaluated the effects of β3-AR agonists during cholinergic stimulation using carbachol, a stable pharmacological analog of acetylcholine, as previously done by others (11, 25). Under such conditions, BRL37344 and L-755,507 significantly decreased all phasic and tonic contraction parameters in a concentration-dependent manner (Figs. 4–5). BRL37344 inhibitory effect could be explained by its pronounced antimuscarinic properties since this inhibitory effect on spontaneous contractions was partially blocked by atropine (Fig. 3). Consistent with our results, previous studies by Klausner et al. (25) showed that isoproterenol, a nonselective β3-AR agonist, decreased the contractions induced by low concentrations of carbachol in rabbit DSM strips suggesting the involvement of β-ARs. Using arecaidine, a muscarinic receptor agonist, to induce contractions in guinea pig isolated whole bladder, Gillespie (11) also showed that BRL37344 (300 μM) could significantly reduce the amplitude of phasic rises in intravesical pressure of guinea pig isolated whole bladder. Furthermore, SR59230A did not antagonize L-755,507 inhibitory effect on the carbachol-induced contractions (Figs. 4–5), suggesting that L-755,507-induced relaxation of the carbachol-induced contractions is not mediated through β3-ARs. Indeed, at higher concentrations (10–100 μM), L-755,507 may also act on β1-/β2-AR directly. L-755, 507 EC50 values for β3-, β1-, and β2-AR are 0.43 nM, 530 nM, and >10 μM, respectively (9, 33). Therefore, the inhibitory effect of L-755,507 observed at such high concentrations (10–100 μM) on carbachol-induced contractions could be due to its direct effects on β1- and/or β2-ARs (33), which tend to corroborate previous findings by others (28, 42). One explanation for BRL37344 inhibitory effects on carbachol-induced contractions is that during cholinergic stimulation with carbachol, muscarinic M2 receptors, which are linked to the cAMP/PKA inhibitory pathway, are activated. M2 receptors and β3-ARs have opposing effects at the cAMP/PKA level that may account for the inhibitory effects of BRL37344 and L-755,507 on carbachol-induced contractions. We find that the relaxation of guinea pig DSM isolated strips by BRL37344 and L-755,507 is predominant during cholinergic stimulation with carbachol and minor during spontaneous contractions, consistent with previous studies (42).
Nerve stimulation also plays an important role in the micturition process during which acetylcholine and ATP are released from parasympathetic nerve terminals and act on DSM muscarinic (M2/M3) and purinergic (P2X) receptors to generate DSM contractions (15, 17). The observed decrease of the EFS-induced contraction amplitude by BRL37344 can be misleading (Fig. 7). This BRL37344 inhibitory effect was suppressed by atropine, suggesting that BRL37344 acted directly on muscarinic receptors rather than β3-ARs during nerve stimulation (Fig. 9). In addition to DSM cells, muscarinic receptors are also present in bladder nerves (24). It has been reported that presynaptic muscarinic receptors on parasympathetic nerve terminals in DSM strips are involved in an autofacilitatory mechanism that enhances acetylcholine release during continuous EFS (4). BRL37344 may therefore block muscarinic receptors in both DSM cells and bladder nerves. Furthermore, the β3-AR agonist L-755,507 at concentration as high as 100 μM has no effect on the EFS-induced contractions in the absence or presence of atropine, suggesting that β3-ARs do not play any role in the nerve-evoked contraction of guinea pig DSM (Fig. 10). This latter observation confirms the concept that the decrease in EFS-induced contraction caused by BRL37344 was a direct antimuscarinic effect. An alternative explanation is that the β3-ARs in guinea pig DSM have a distinct pharmacological profile compared with β3-ARs in other species or that β3-ARs might not be functionally expressed in guinea pig DSM.
In summary, four key points can be drawn from our study: 1) β3-ARs are expressed at the mRNA level but play a negligible functional role, if any, in guinea pig DSM relaxation; 2) BRL37344 inhibitory effect on guinea pig DSM spontaneous and nerve-evoked contractions is likely mediated through its antimuscarinic properties; 3) BRL37344 and L-755,507 have significant inhibitory effects on guinea pig carbachol-induced contractions; and 4) BRL37344 does not modulate guinea pig basal cell membrane excitability. Collectively, our data suggest that unlike rat and human DSM (23, 37, 40, 41), in guinea pig DSM β3-ARs have a minor to no functional role in DSM excitability and spontaneous contractility.
We recommend particular prudence when interpreting data generated with BRL37344 because of its pronounced antimuscarinic effects. We also suggest that, although the guinea pig DSM is widely used for experimental studies, future investigations on β3-ARs should not use this animal model. Our study does not rule out β3-ARs functional role in guinea pig urothelium and other non-DSM bladder cells (11, 41).
GRANTS
This study was supported by National Institutes of Health Grants DK084284 and DK083687 to G. V. Petkov.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.V.P., S.A.Y.A. and K.L.H. performed experiments; G.V.P., S.A.Y.A. and K.L.H. analyzed data; G.V.P., S.A.Y.A. and K.L.H. interpreted results of experiments; G.V.P., S.A.Y.A. prepared figures; G.V.P., S.A.Y.A. and K.L.H. drafted manuscript; S.A.Y.A., K.L.H., and G.V.P. edited and revised manuscript; S.A.Y.A., K.L.H., and G.V.P. approved final version of manuscript; G.V.P. conception and design of research.
ACKNOWLEDGMENTS
We thank Sean Brown and Dr. Xiangli Cui for help with the initial contraction and patch-clamp experiments, respectively. We also thank Drs. Jennifer G. Schnellmann, John Malysz, Shankar Parajuli, Wenkuan Xin, Rupal P. Soder, Amy Smith, and Qiuping Cheng for the critical evaluation of the manuscript.
REFERENCES
- 1. Andersson KE. Pharmacotherapy of the overactive bladder. Discov Med 8: 118–124, 2009 [PubMed] [Google Scholar]
- 2. Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84: 935–986, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Biers SM, Reynard JM, Brading AF. The effects of a new selective beta3-adrenoceptor agonist (GW427353) on spontaneous activity and detrusor relaxation in human bladder. BJU Int 98: 1310–1314, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Biers SM, Reynard JM, Doore T, Brading AF. The functional effects of a c-kit tyrosine inhibitor on guinea-pig and human detrusor. BJU Int 97: 612–616, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Brown SM, Bentcheva-Petkova LM, Liu L, Hristov KL, Chen M, Kellett WF, Meredith AL, Aldrich RW, Nelson MT, Petkov GV. β-Adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am J Physiol Renal Physiol 295: F1149–F1157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen M, Kellett WF, Petkov GV. Voltage-gated K+ channels sensitive to stromatoxin-1 regulate myogenic and neurogenic contractions of rat urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 299: R177–R184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen M, Petkov GV. Identification of large conductance calcium activated potassium channel accessory beta4 subunit in rat and mouse bladder smooth muscle. J Urol 182: 374–381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deba A, Palea S, Rouget C, Westfall TD, Lluel P. Involvement of beta(3)-adrenoceptors in mouse urinary bladder function: role in detrusor muscle relaxation and micturition reflex. Eur J Pharmacol 618: 76–83, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Fisher MH, Amend AM, Bach TJ, Barker JM, Brady EJ, Candelore MR, Carroll D, Cascieri MA, Chiu SH, Deng L, Forrest MJ, Hegarty-Friscino B, Guan XM, Hom GJ, Hutchins JE, Kelly LJ, Mathvink RJ, Metzger JM, Miller RR, Ok HO, Parmee ER, Saperstein R, Strader CD, Stearns RA, Thompson GM, Tota L, Vicario PP, Weber AE, Woods JW, Wyvratt MJ, Zafian PT, MacIntyre DE. A selective human beta3 adrenergic receptor agonist increases metabolic rate in rhesus monkeys. J Clin Invest 101: 2387–2393, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Foster CD, Fujii K, Kingdon J, Brading AF. The effect of cromakalim on the smooth muscle of the guinea-pig urinary bladder. Br J Pharmacol 97: 281–291, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gillespie JI. Noradrenaline inhibits autonomous activity in the isolated guinea pig bladder. BJU Int 93: 401–409, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Gopalakrishnan M, Whiteaker KL, Molinari EJ, Davis-Taber R, Scott VE, Shieh CC, Buckner SA, Milicic I, Cain JC, Postl S, Sullivan JP, Brioni JD. Characterization of the ATP-sensitive potassium channels (KATP) expressed in guinea pig bladder smooth muscle cells. J Pharmacol Exp Ther 289: 551–558, 1999 [PubMed] [Google Scholar]
- 13. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- 14. Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol 141: 183–193, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hashitani H, Bramich NJ, Hirst GD. Mechanisms of excitatory neuromuscular transmission in the guinea-pig urinary bladder. J Physiol 524: 565–579, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol 273: C110–C117, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Herrera GM, Etherton B, Nausch B, Nelson MT. Negative feedback regulation of nerve-mediated contractions by KCa channels in mouse urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 289: R402–R409, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol 279: R60–R68, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Horinouchi T, Tanaka Y, Koike K. Function of beta1-adrenoceptors and mRNA expression of beta1- and beta2-adrenoceptors in guinea-pig esophagus. Eur J Pharmacol 473: 79–82, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Horinouchi T, Yamamoto Y, Koike K. Effect of SR59230A on atypical beta-adrenoceptor mediating relaxation in the guinea Pig gastric fundus. Pharmacology 62: 98–102, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole cell recording method. J Gen Physiol 92: 145–159, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hristov KL, Chen M, Kellett WF, Rovner ES, Petkov GV. Large conductance voltage- and Ca2+-activated K+ channels regulate human detrusor smooth muscle function. Am J Physiol Cell Physiol 301: C903–C912, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hristov KL, Cui X, Brown SM, Liu L, Kellett WF, Petkov GV. Stimulation of beta3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 295: C1344–C1353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kennedy C, Tasker PN, Gallacher G, Westfall TD. Identification of atropine- and P2X1 receptor antagonist-resistant, neurogenic contractions of the urinary bladder. J Neurosci 27: 845–851, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klausner AP, Rourke KF, Miner AS, Ratz PH. Potentiation of carbachol-induced detrusor smooth muscle contractions by beta-adrenoceptor activation. Eur J Pharmacol 606: 191–198, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kubota Y, Nakahara T, Yunoki M, Mitani A, Maruko T, Sakamoto K, Ishii K. Inhibitory mechanism of BRL37344 on muscarinic receptor-mediated contractions of the rat urinary bladder smooth muscle. Naunyn Schmiedebergs Arch Pharmacol 366: 198–203, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Kullmann FA, Limberg BJ, Artim DE, Shah M, Downs TR, Contract D, Wos J, Rosenbaum JS, de Groat WC. Effects of beta3-adrenergic receptor activation on rat urinary bladder hyperactivity induced by ovariectomy. J Pharmacol Exp Ther 330: 704–717, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li JH, Yasay GD, Kau ST. Beta-adrenoceptor subtypes in the detrusor of guinea-pig urinary bladder. Pharmacology 44: 13–18, 1992 [DOI] [PubMed] [Google Scholar]
- 29. Malysz J, Buckner SA, Daza AV, Milicic I, Perez-Medrano A, Gopalakrishnan M. Functional characterization of large conductance calcium-activated K+ channel openers in bladder and vascular smooth muscle. Naunyn Schmiedebergs Arch Pharmacol 369: 481–489, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Michel MC, Ochodnicky P, Summers RJ. Tissue functions mediated by beta(3)-adrenoceptors–findings and challenges. Naunyn Schmiedebergs Arch Pharmacol 382: 103–108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Michel MC, Vrydag W. Alpha1-, alpha2- and beta-adrenoceptors in the urinary bladder, urethra and prostate. Br J Pharmacol 147, Suppl 2: S88–S119, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Muhlstein J, Deval B. Anticholinergic drugs in overactive bladder. Gynecol Obstet Fertil 36: 90–96, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Parmee ER, Ok HO, Candelore MR, Tota L, Deng L, Strader CD, Wyvratt MJ, Fisher MH, Weber AE. Discovery of L-755,507: a subnanomolar human beta3 adrenergic receptor agonist. Bioorg Med Chem Lett 8: 1107–1112, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Petkov GV, Heppner TJ, Bonev AD, Herrera GM, Nelson MT. Low levels of K(ATP) channel activation decrease excitability and contractility of urinary bladder. Am J Physiol Regul Integr Comp Physiol 280: R1427–R1433, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Petkov GV, Nelson MT. Differential regulation of Ca2+-activated K+ channels by β-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol 288: C1255–C1263, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Sarma DN, Banwait K, Basak A, DiMarino AJ, Rattan S. Inhibitory effect of beta3-adrenoceptor agonist in lower esophageal sphincter smooth muscle: in vitro studies. J Pharmacol Exp Ther 304: 48–55, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Sato M, Hutchinson DS, Evans BA, Summers RJ. The beta3-adrenoceptor agonist 4-[[(Hexylamino)carbonyl]amino]-N-[4-[2-[[(2S)-2-hydroxy-3-(4-hydroxypheno xy)propyl]amino]ethyl]-phenyl]-benzenesulfonamide (L755507) and antagonist (S)-N-[4-[2-[[3-[3-(acetamidomethyl)phenoxy]-2-hydroxypropyl]amino]-ethyl] phenyl]benzenesulfonamide (L748337) activate different signaling pathways in Chinese hamster ovary-K1 cells stably expressing the human beta3-adrenoceptor. Mol Pharmacol 74: 1417–1428, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Shieh CC, Feng J, Buckner SA, Brioni JD, Coghlan MJ, Sullivan JP, Gopalakrishnan M. Functional implication of spare ATP-sensitive K+ channels in bladder smooth muscle cells. J Pharmacol Exp Ther 296: 669–675, 2001 [PubMed] [Google Scholar]
- 39. Takasu T, Ukai M, Sato S, Matsui T, Nagase I, Maruyama T, Sasamata M, Miyata K, Uchida H, Yamaguchi O. Effect of (R)-2-(2-aminothiazol-4-yl)-4′-{2-[(2-hydroxy-2-phenylethyl)amino]ethyl} acetanilide (YM178), a novel selective beta3-adrenoceptor agonist, on bladder function. J Pharmacol Exp Ther 321: 642–647, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Takeda M, Obara K, Mizusawa T, Tomita Y, Arai K, Tsutsui T, Hatano A, Takahashi K, Nomura S. Evidence for beta3-adrenoceptor subtypes in relaxation of the human urinary bladder detrusor: analysis by molecular biological and pharmacological methods. J Pharmacol Exp Ther 288: 1367–1373, 1999 [PubMed] [Google Scholar]
- 41. Yamaguchi O, Chapple CR. Beta3-adrenoceptors in urinary bladder. Neurourol Urodyn 26: 752–756, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Yamamoto Y, Mori A, Koike K. Beta-adrenoceptors in the detrusor of guinea pig bladder. J Smooth Muscle Res 34: 233–242, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Yarker YE, Goa KL, Fitton A. Oxybutynin. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic use in detrusor instability. Drugs Aging 6: 243–262, 1995 [DOI] [PubMed] [Google Scholar]