Abstract
Colony stimulating factor 1 (CSF-1), required for macrophage (Mø) survival, proliferation and activation, is upregulated in the tubular epithelial cells (TEC) during kidney inflammation. CSF-1 mediates Mø-dependent destruction in lupus-susceptible mice with nephritis and paradoxically, Mø-dependent renal repair in lupus-resistant mice after transient ischemia/reperfusion injury (I/R). We now report that I/R leads to defective renal repair, non-resolving inflammation and in turn, early-onset lupus nephritis in pre-clinical MRL-Faslpr mice. Moreover, defective renal repair is not unique to MRL-Faslpr mice as flawed healing is a feature of other lupus-susceptible mice (Sle 123) and MRL mice without the Faslpr mutation. Increasing CSF-1 hastens renal healing after I/R in lupus-resistant mice, but hinders healing, exacerbates non-resolving inflammation, and triggers more severe early-onset lupus nephritis in MRL-Faslpr mice. Probing further, the time-related balance of M1 “destroyer” Mø shifts towards the M2 “healer” phenotype in lupus-resistant mice after I/R, but M1 Mø continue to dominate in MRL-Faslpr mice. Moreover, hypoxic TEC release mediators, including CSF-1, that are responsible for stimulating the expansion of M1 Mø inherently poised to destroy the kidney in MRL-Faslpr mice. In conclusion, I/R induces CSF-1 in injured TEC that expands aberrant Mø (M1 phenotype) mediating defective renal repair and non-resolving inflammation, and thereby hasten the onset of lupus nephritis.
Introduction
Identifying molecular mechanisms that mediate experimental lupus nephritis offer the promise of uncovering novel therapeutic targets for human lupus. MRL-Faslpr mice are powerful tools for dissecting mechanisms central to lupus nephritis and are a multi-organ (kidney, skin, lung, salivary/lacrimal glands, etc) disease animal model similar to human lupus (1–3). As in human lupus, kidney disease in MRL-Faslpr mice is the major cause of mortality. Macrophages (Mø) are prominent within the inflamed kidneys (4, 5) and are key mediators of lupus nephritis in MRL-Faslpr mice (6–10). Thus, Mø are prime candidates as key regulators of lupus nephritis.
Colony stimulating factor 1 (CSF-1), required for Mø survival, differentiation, proliferation, incites inflammation that leads to Mø-mediated destruction. We established that CSF-1 and Mø are pivotal in the pathogenesis of lupus nephritis in MRL-Faslpr mice based on the following evidence. CSF-1 is detected in tubular epithelial cells (TEC) that are surrounded by Mø at the onset of lupus nephritis (4, 10). Mice deficient in CSF-1 (Csf1op/op;MRL-Faslpr), nearly depleted of Mø, are protected from lupus nephritis (11). Local, discrete intra-renal CSF-1 over-expression incites Mø-rich inflammation in the adjacent tissue (12), and systemic CSF-1 over-expression hastens the onset and progression of Mø-rich lupus nephritis (10). Probing further, CSF-1 is upregulated largely by TEC at the onset of renal inflammation, and spills over into the circulation, thereby increasing the number of circulating monocytes and shifting their phenotype towards an “inflammatory”, activated population (10). These “inflammatory” Mø are more readily recruited to the kidney (10) and release mediators that induce apoptosis of renal parenchymal cells, thereby damaging the kidney (10, 13). Moreover, CSF-1 and Mø are highly relevant to human lupus nephritis. Enhanced intra-renal CSF-1 and Mø correlate with a higher histopathology activity index, and heightened serum and urine CSF-1 levels correlate with increased disease activity (10). Taken together, CSF-1 and Mø are closely intertwined in mediating lupus nephritis.
Paradoxically, CSF-1 and Mø mediate renal repair in lupus-resistant mice. As inflammation during a transient insult is meant to set the stage for repair, and Mø are implicated in tissue repair (14, 15), we selected a model of transient renal injury leading to repair, ischemia/reperfusion injury (I/R), and determined that CSF-1 promotes Mø-dependent renal repair. Tubular damage is a cardinal feature of ischemic renal injury and is reversible as uninjured TEC proliferate to replenish faulty TEC (16). Injecting CSF-1 after I/R hastened healing (decreased tubular pathology, and fibrosis, improved renal function) in lupus-resistant mice (17). Notably, CSF-1 increased TEC proliferation, and reduced further damage by suppressing TEC apoptosis. We determined that Mø contributed to renal repair as eliminating Mø increased tubular pathology and renal fibrosis, suppressed TEC proliferation and increased TEC apoptosis (17). Taken together, Mø and CSF-1 are intertwined and central to tubular repair in lupus-resistant mice.
How do CSF-1 and Mø mediate renal repair in lupus-resistant mice, and conversely, injury in lupus-susceptible mice? Mø regulate a dynamic balance between destructive and repair signals in the kidney. These mononuclear phagocytes are broadly, and simplistically divided into a operationally useful conceptual framework that describes Mø along a continuum of functional states subsets referred to as classically activated, “destroyers” (M1) and alternatively activated “healers” (M2) (14, 18–20). The M1 Mø release molecules that initiate tissue injury (21) evident in the kidney after I/R (20), while the M2 Mø generate molecules, such as TGF-β, that suppress further Mø activation, and promote repair (22). Moreover, evidence suggests that phagocytosis of tissue debris may switch Mø from pro-inflammatory to anti-inflammatory (23, 24). However, phagocytosis is impaired in Mø derived from lupus-susceptible mice, and these Mø are not able to efficiently clear apoptotic TEC (25) and other debris (unpublished data). Thus, the dominance of M1 Mø may fail to shift to M2 Mø in lupus-susceptible mice. Moreover, inefficient engulfment of apoptotic cells may activate the immune system and trigger lupus (26).
The goal of this study was to uncover mechanisms responsible for CSF-1 leading to Mø-mediated kidney destruction in lupus-susceptible mice and Mø-mediated kidney repair in lupus-resistant mice. We used a transient insult (I/R) to unmask the molecular mechanisms that mediate defective innate immunity bridging adaptive immunity in lupus-susceptible mice. We now report that transient injury leads to defective repair, non-resolving inflammation and early-onset lupus nephritis as aberrant Mø fail to shift from phenotypes that “destroy” (M1) to phenotypes that “heal” (M2) in lupus-susceptible MRL-Faslpr mice.
Materials and Methods
Mice
We purchased C57BL/6 (B6), BALB/c, MRL/MpJ-Faslpr/Faslpr (MRL-Faslpr), MRL/MpJ (MRL-++), and B6.Sle1Sle2Sle3Sle5 (Sle 123) mice from The Jackson Laboratory (Bar Harbor, ME). Mice were housed and bred at Harvard Medical School. The CSF-1op/op;MRL-Faslpr and TgC/+;MRL-Faslpr mice were constructed as previously detailed (10). Use of mice in this study was reviewed and approved by the Standing Committee on Animals in the Harvard Medical School in adherence to standards set in the Guide for the care and use of laboratory animals (NIH publication no. 86-23 Revised 1996).
I/R
We anesthetized mice and exposed the left kidney through a flank incision. We induced ischemia by clamping the renal pedicle with non-traumatic micro-aneurysm clamps (Roboz). We removed the clamps after 30 min in male, 45 min in female. The body temperature was controlled at 36.6–37.5°C throughout the procedure. We removed and prepared the kidneys as previously described (17, 27). We initiated I/R at: 6 wks of age in MRL-Faslpr, B6, BALB/c, TgC/+;MRL-Faslpr, CSF-1op/op;MRL-Faslpr mice; 6 wks and 8–10 mo of age in MRL-++ mice; and 10 wks of age in Sle 123 mice.
Renal histopathology
We fixed kidneys in 10% neutral buffered formalin, embedded them in paraffin, and stained paraffin sections with periodic acid-Schiff reagent. Kidney pathology was assessed as previously detailed (17).
Collagen detection
We stained paraffin sections after rehydration in picrosirius red solution for 1 hour and rinsed (×2) with acidified water. We dehydrated and mounted the sections and analyzed the amount of stain using a Nikon Eclipse E1000 upright fluorescence microscope and Adobe Photoshop CS4 extended.
Renal function
We measured albuminuria as previously described (17).
Immunohistochemistry
We stained frozen kidney sections, fixed in 25% ethanol/75% acetone for 10 min at room temperature, for the presence of Mø, neutrophils and T cell populations, using anti-mouse F4/80 antibody (BM-8; Invitrogen, Carlsbad, CA), anti-mouse GR-1 antibody (RB6-8C5; BD pharmingen, San Diego, CA), anti-mouse CD4 antibody (RM4-5; eBioscience, San Diego, CA), and anti-mouse CD8 antibody (53-6.7; eBioscience) (10). Optimal concentrations of primary antibodies were diluted in antibody dilution buffer, and incubated with the tissue sections overnight in a humidified chamber at 4 °C. We incubated tissue sections with biotinylated anti-rat IgG antibody (BA-4001; Vector lab, Burlingame, CA) for 1h at room temperature, followed by the incubation with ABC complex (PK-6100; Vector lab) for 1h at room temperature. Then, the stain was developed using DAB peroxidase substrate (SK-4100; Vector lab) followed by counterstain with Mayer’s Hematoxylin (Sigma-Aldrich).
To determine the number of M1 and M2 Mø, we fixed frozen kidney sections in 4% paraformaldehyde (for iNOS staining) and washed non-fixed sections for 10 min in PBS (for CD206), stained them with anti-mouse F4/80 antibody (Invitrogen) followed by Alexa Fluor 594 goat anti-rat IgG antibody (Invitrogen). Then, we stained them with FITC-conjugated rat anti-mouse CD206 (MR5D3; Serotec, Raleigh, NC). For the staining of iNOS, the kidney sections were permeabilized in saponin buffer then stained with FITC-conjugated anti-mouse iNOS antibody (BD pharmingen). We enumerated the number of F4/80+ iNOS+ cells and F4/80+ CD206+ cells in 10 high power fields (HPF) and divided the number of F4/80+ iNOS+ cells by the number of F4/80+ CD206+ cells for the M1/M2 ratio.
Kim-1 expression
Cryosections were washed in PBS for 10 min, and stained with polyclonal anti–Kim-1 (anti-sera R9) to identify tubular injury as previously detailed (17). Real-time PCR was performed as described before (17). Kim-1 primers (Invitrogen, sense 5’-GGA AGT AAA GGG GGT AGT GGG-3’, anti-sense, 5’-AAG CAG AAG ATG GGC ATT GC-3’) were used and mRNA levels were normalized to GAPDH (GAPDH: sense, 5-CAT GGC CTC CAA GGA GTA AG-3; antisense, 5-CCT AGG CCC CTC CTG TTA TT-3).
IgG and C3 deposits within renal glomeruli
Cryosections were fixed in 20% acetone/80% methanol for 15 min at −20°C, and stained with FITC-conjugated goat anti-mouse IgG and FITC-conjugated goat IgG fraction of mouse C3 (Cappel, Malvern, PA) (10). The fluorescence intensity within the peripheral glomerular capillary walls and the mesangium was scored on a scale of 0–3 (0 = none; 1 = weak; 2 = moderate; 3 = strong). At least 10 glomeruli/section were analyzed.
Flow Cytometry
We prepared and stained single-cell suspensions from kidneys or primary cultured TEC/BMMø as described previously (17). To identify intra-cellular cytokine expression, kidneys or cultured cells were incubated in RPMI containing Monensin (Sigma-Aldrich) for 4h. We washed the cells in PBS, suspended them in FACS buffer (PBS, 5% FBS, and 0.09% NaN3), and incubated them with several cell surface markers for 30 min on ice. Then we fixed and permeabilized the cells using Fix/Perm buffer (Biolegend, San Diego, CA). After staining of intracellular cytokines, cells were washed twice in Fix/Perm buffer, and suspended in FACS buffer to allow the resealing of permeabilized membrane. We collected 1.0 to 5.0×105 total kidney cells and 1.0 to 3.0×104 cultured cells using the FACSCalibur (Becton Dickinson, San Jose, CA) and analyzed data using FlowJo software 9.3 (Tree Star, Palo Alto, CA).
Antibodies
We used the following antibodies for FACS analysis: FITC-conjugated anti-CD4 (L3T4, eBioscience), anti-CD8 (53-6.7, eBioscience), anti-CD3 (145-2C11), anti-B220 (RA3-6B2, eBioscience), anti-CD206 (MCA2235FA, Serotech), anti-TNF-α (MP6-XT22, BD pharmingen), anti-CD80 (16-10A1, BD pharmingen), anti-CD11b (M1/70, BD pharmingen), anti-CD45.1 (A20, BD pharmingen), and anti-CD45.2 (104, eBioscience); PE-conjugated anti-IL-10 (JES5-16E3, eBioscience), anti-IL-4R (mIL4R-M1, BD pharmingen), anti-GR-1 (RB6-8C5, BD pharmingen), anti-Ly6G (1A8, BD pharmingen), anti-CD86 (GL1, eBioscience), anti-CD45.1 (A20) and anti-CD45.2 (104) PerCP-Cy5.5 conjugated anti CD11b (M1/70, BD pharmingen) and allophycocyanin-conjugated anti-F4/80 (BM8, Biolegend), anti-CD45.1 (A20) and anti-CD45.2 (104). We used biotin conjugated anti-Ly6C (AL-21, BD pharmingen), followed by Streptavidine-PerCP-Cy5.5 (BD pharmingen).
Serum Ig profile
We measured total IgG and total anti-dsDNA antibodies as previously reported (3).
Mø cell culture
We isolated and cultured BMMø as previously described from mice 6–8 wks of age (5). BM cells were cultured in L929 cell-conditioned medium to separate adherent differentiated cells. Non-adherent, immature cells were removed and cultured for 3–4 days as previously detailed (10).
C1 cell culture
The immortalized mouse proximal TEC cell line (C1) (28) were grown to be sub-confluent on collagen-coated plates in modified K1 media as previously detailed (17).
Antibody/cytokine administration
We injected mice (i.p.) every 12h with purified recombinant human CSF-1 (gift from Chiron Corporation Emeryville, CA) using a dose of 50µg/kg/body wt beginning at 1.5d and ending at 5d (17) after I/R. Mice injected with PBS were used as controls.
Mø stimulation using supernatant from hypoxic TEC (C1)
C1 cells were exposed to 5% CO2, balance N2 (3% O2) for 24h in hypoxic chamber with 1% FCS media. Supernatant was collected after 24h (d1). Re-oxygenation was performed by placing the cells in an incubator gassed with 95% air/5% CO2 with C1 cultured media. Then supernatant was changed to 1% FCS media at 4d, and collected at 5d. Mø were incubated with C1 supernatant for 24h before Mø profiling.
Cell Survival/Proliferation
We analyzed proliferation using the MTT colorimetric assay (Roche, Palo Alto, CA) according to the manufacturer's instructions.
Real-Time PCR
Real-time PCR was performed as described before (17). We detected TNF-α, iNOS, arginase-1, IL-10 and GAPDH expression using QuantiTect Primer Assays (Qiagen, Valencia, CA). The data were analyzed by delta-delta-Ct method.
Statistics
Data represent the mean ± SEM prepared using GraphPad Prism software, version 4.0. We used the nonparametric Mann-Whitney U test to evaluate P values. P < 0.05 was considered significant.
Results
Renal repair is defect in lupus-susceptible MRL-Faslpr mice after transient kidney injury (I/R)
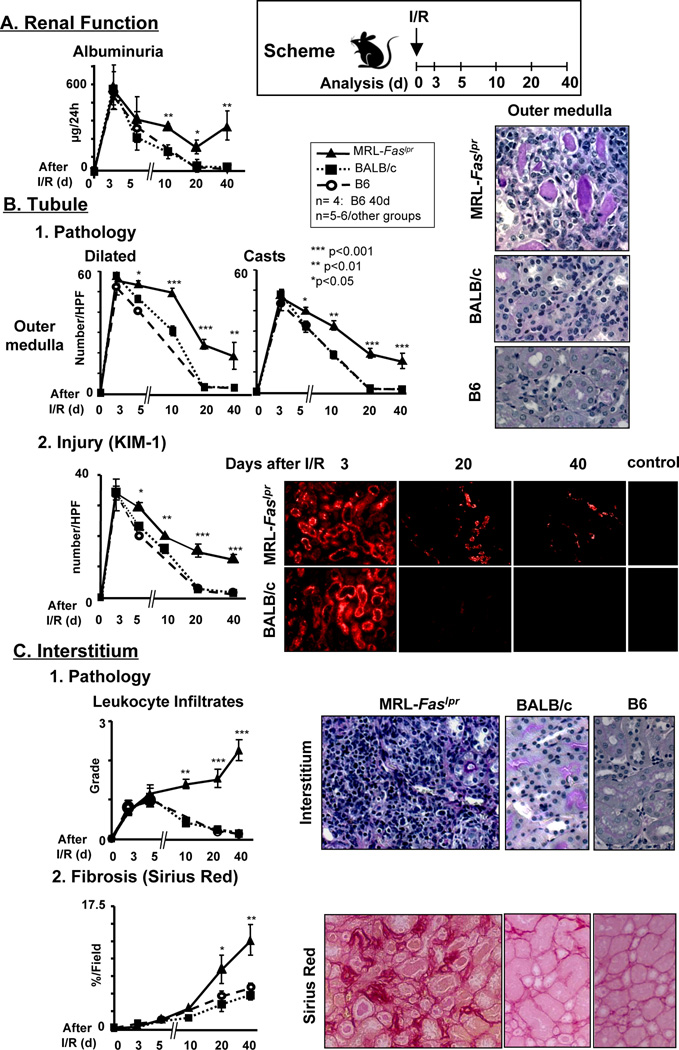
Our prior studies show that CSF-1 hastens renal repair after transient renal injury (I/R) and is expressed by TEC in normal (lupus-resistant) mice (17). In contrast, CSF-1 hastens the tempo of lupus nephritis in MRL-Faslpr mice (10). Thus, we tested the hypothesis that transient renal injury (unilateral I/R) leads to un-resolving inflammation and defective repair in MRL-Faslpr mice. To test this hypothesis we compared the time-related magnitude of renal injury and repair in pre-clinical MRL-Faslpr mice and lupus-resistant, C57Bl/6 (B6) and BALB/c, mice. We detected a similar initial loss of renal function (albuminuria), an index of renal injury and repair, that peaks at 3d after I/R in lupus-resistant and MRL-Faslpr mice, and returns to normal (baseline) levels in lupus-resistant (20d), but not in MRL-Faslpr mice (Figure 1A). This suggests that renal repair is defective in MRL-Faslpr mice. As tubular injury in the outer medulla of the kidney is exquisitely sensitive to hypoxia injury after I/R we evaluated tubular pathology and kidney injury molecule 1 (Kim-1), an index of tubule injury (29), in MRL-Faslpr and lupus-resistant mice. The magnitude of tubular pathology (casts, dilated tubules) in the outer medulla reached an apex at 3d after I/R in lupus-resistant and MRL-Faslpr mice, declined and returned to baseline at 20d after I/R in lupus-resistant mice. In contrast, tubular pathology only partially resolved in MRL-Faslpr mice even by 40d after I/R (Figure 1B1). Defective renal repair in the less severely impacted renal cortex was similar to the outer medulla in MRL-Faslpr (data not shown). Consistent with renal tubule pathology, we found maximal Kim-1 expression in lupus-resistant and MRL-Faslpr mice at 3d after I/R that declined and returned to baseline by 20d in lupus-resistant mice, but remained elevated in MRL-Faslpr mice (Figure 1B2). Strikingly, leukocyte infiltrates, an index of inflammation that accompanies tubular injury, descended rapidly in lupus-resistant mice, but continued to ascend in MRL-Faslpr mice. This suggests that infiltrating leukocytes may be central to non-resolving inflammation, and may mediate further renal destruction (Figure 1C1). In keeping with increasing renal injury, rather than repair, in MRL-Faslpr mice, the time-related magnitude of fibrosis (Sirius red) more dramatically increased in MRL-Faslpr compared with lupus-resistant mice (Figure 1C2). As expected the contralateral kidneys in MRL-Faslpr and lupus-resistant mice resemble age-matched sham controls (data not shown). Of note, male mice are more sensitive than female mice to ischemic injury, thus, we initially used MRL-Faslpr male mice (30). By extending the ischemic injury (from 30 min to 45 min) in female mice, we detect renal injury and repair comparable to males in lupus-resistant mice. Using this protocol the magnitude of defective repair after I/R in age-matched MRL-Faslpr female mice mimicked males (data not shown). Moreover, over-aggressive inflammation at the onset of injury is not responsible for defective renal repair in MRL-Faslpr mice after I/R. We detected a similar magnitude of time-related tubular pathology (0, 2, 12 24, 72h) in the outer medulla and cortex, Kim-1 expression (0, 24, 48, 72h), loss of renal function (72h) and infiltrating leukocytes (Mø [F4/80+], T cells [CD4, CD8 and CD3+B220+CD4−CD8−], B cells (B220+) and granulocytes (GR1+) (72h) in MRL-Faslpr and lupus-resistant mice (data not shown). Taken together, renal repair is defective in lupus-susceptible MRL-Faslpr mice after transient renal injury (I/R).
Figure 1.
Defective renal repair in MRL-Faslpr mice compared to lupus-resistant mice after I/R injury. Male MRL-Faslpr and lupus-resistant (BALB/c and B6) mice were analyzed at increasing times (d) after I/R. We initiated I/R at 6 wks of age. (A) Renal function (albuminuria, µg/24h). (B) Tubular histopathology (dilated tubules, casts) in the outer medulla and Kim-1 expression (index of tubule injury). (C) Interstitial leukocyte infiltrates and fibrosis (collagen staining, Sirius red). Representative photomicrographs 40d after I/R (magnification 40×, except Kim-1 20×). Data represent means ± SEM. Data is representative of three separate experiments.
Renal I/R triggers early-onset lupus nephritis in MRL-Faslpr mice
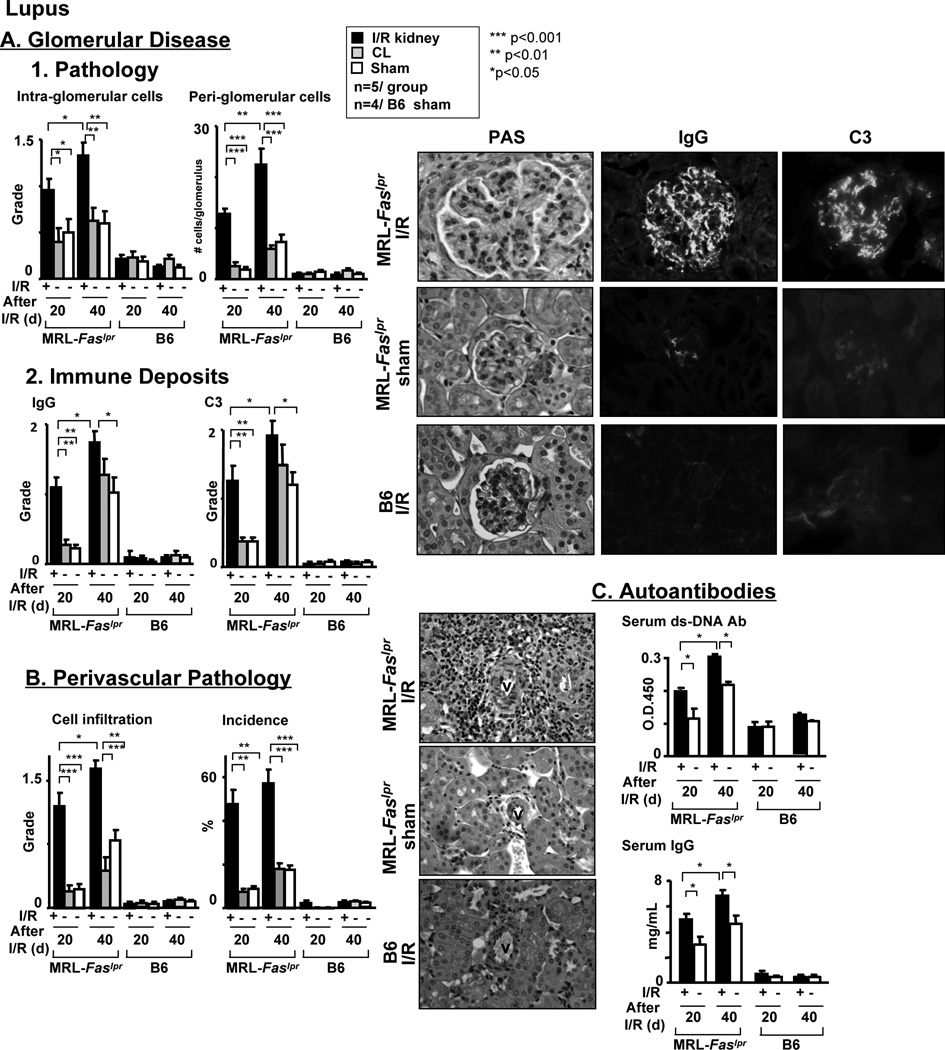
As renal repair after transient injury (I/R) is defective in MRL-Faslpr mice, we tested the hypothesis that transient renal injury leads to non-resolving inflammation that triggers early-onset lupus nephritis. Lupus nephritis and renal I/R share several pathologic features including tubular pathology, an influx of leukocytes into the interstitium and the loss of renal function. However, glomerular disease, perivascular leukocyte infiltrates and autoantibodies are features of lupus nephritis in MRL-Faslpr mice, and not renal I/R. Therefore, to test our hypothesis we compared the time-related magnitude of features specific to lupus nephritis (glomerular disease, perivascular pathology and serum autoantibodies) in MRL-Faslpr mice after I/R with controls (MRL-Faslpr age-matched shams). We detected a greater time-related increase in glomerular pathology in MRL-Faslpr kidneys after I/R compared to the contralateral kidney and control kidneys (Figure 2A1). Consistent with this finding the time-related magnitude of IgG and C3 deposits in glomeruli was greater in MRL-Faslpr kidneys after I/R compared with the contralateral kidney and control kidneys (Figure 2A2). Even more dramatic was the prominent time-related rise in the number of leukocyte infiltrates accumulating around a large proportion (incidence) of renal vasculature after I/R in MRL-Faslpr mice that was barely elevated in age-matched MRL-Faslpr control mice compared with lupus-resistant controls (Figure 2B). In addition, the time-related rise in serum autoantibodies (anti-dsDNA), IgG (IgG1, IgG2a, IgG2b, IgG3, not shown) and C3 was higher in MRL-Faslpr mice after I/R compared with controls (Figure 2C). Lupus-resistant, B6 (Figure 2) and BALB/c (data not shown) kidneys with and without I/R were similar and served as negative controls. As transient injury to the kidney (I/R) is responsible for increasing the circulating autoantibodies, this suggests that neo-antigens released from the injured kidney may be responsible for triggering the production of autoantibodies. Importantly, the contralateral kidneys remained similar to control kidneys, thus elevating serum autoantibodies alone, without direct kidney injury, is not sufficient to trigger lupus nephritis (Figure 2). Taken together, transient ischemic kidney injury triggers non-resolving inflammation, thereby leading to a rise in circulating autoantibodies and early-onset lupus nephritis in the injured kidney.
Figure 2.
Renal I/R triggers the onset of lupus nephritis in MRL-Faslpr mice. Features of lupus nephritis in MRL-Faslpr male mice assessed at increasing times (20d, 40d) after I/R. Lupus-resistant B6 male mice after I/R serve as controls. We initiated I/R at 6 wks of age. (A) Glomerular histopathology and immune deposits (IgG and C3) in MRL-Faslpr mice after I/R compared to sham-treated mice and contra-lateral (CL) kidney. (B) Peri-vascular histopathology in MRL-Faslpr mice after I/R compared to sham treated mice and contra-lateral (CL) kidney. (C) Total serum ds-DNA and IgG antibodies in MRL-Faslpr mice after I/R compared to sham and WT mice. Representative photomicrographs (magnification, 60× glomerulus and 40× perivascular leukocytes). Data represent means ± SEM.
Defective repair after I/R is not unique to MRL-Faslpr mice, but linked to lupus-susceptibility and the MRL background
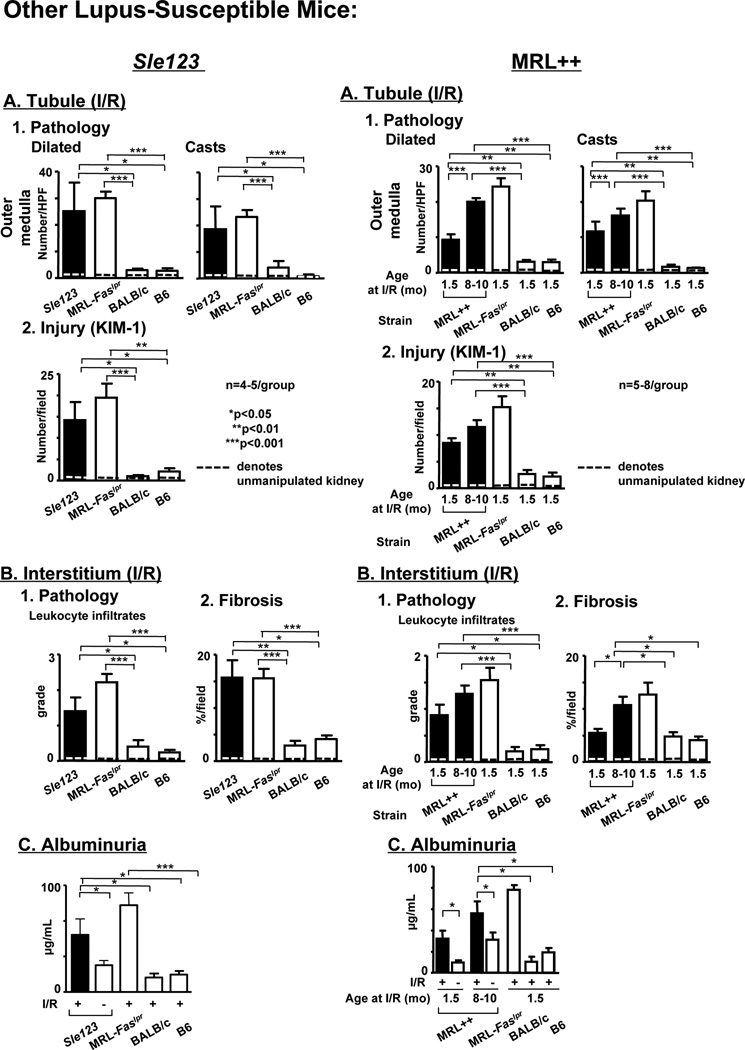
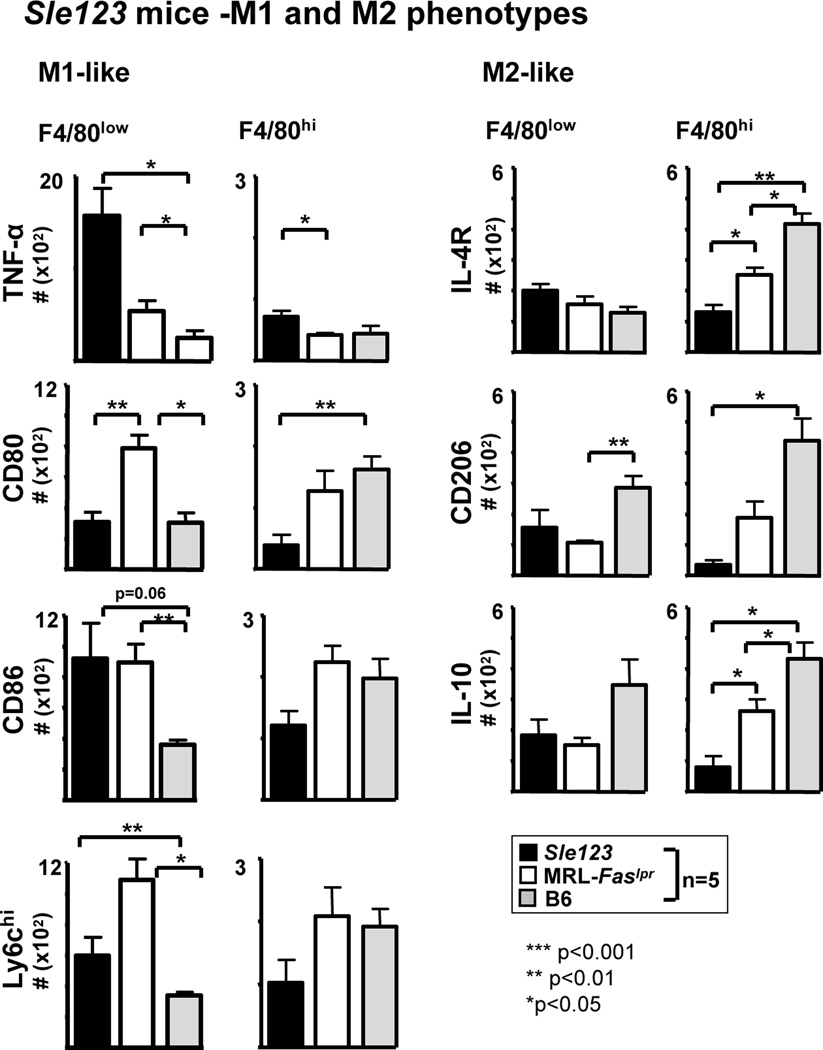
To determine if defective repair is a more global feature of lupus-susceptible mice, and not unique to MRL-Faslpr mice, we evaluated renal repair after I/R in another lupus-susceptible strain, Sle 123, that develops lupus nephritis (31, 32). We compared renal injury and repair in pre-clinical Sle 123, MRL-Faslpr mice (positive control) and lupus-resistant, B6 and BALB/c mice (negative controls) after I/R. Recall, renal injury is repaired by 20d after I/R in lupus-resistant mice (Figure 1). In contrast, renal repair is defective in Sle 123 mice after I/R. The magnitude of tubule pathology (outer medulla) and tubule injury (Kim-1) is substantial in Sle 123 mice compared to lupus-resistant mice after I/R (d20) (Figure 3A). Defective tubular repair in Sle 123 mice in the cortex is similar to the outer medulla (data not shown). Moreover, the magnitude of interstitial pathology, leukocyte infiltrates and fibrosis, and loss of renal function (albuminuria) are elevated in Sle 123 mice compared to lupus-resistant mice after I/R (20d) (Figure 3B). Of note, the extent of defective repair in Sle 123 and MRL-Faslpr mice are comparable (Figure 3). Taken together, as in lupus-susceptible MRL-Faslpr mice, renal repair is defective in lupus-susceptible Sle 123 mice after I/R.
Figure 3.
Defective renal repair after I/R is not unique to MRL-Faslpr mice as it is a feature in other lupus-susceptible mice without the Faslpr mutation. Renal repair in Sle 123 female mice (left panel) and MRL-++ male mice (right panel) after I/R (20d) are displayed. We initiated I/R in Sle123 mice at 10 wks of age and MRL-++ mice at 1.5 and 8–10 mo of age. Age matched lupus-resistant (BALB/c [female] and B6 [male]) mice after I/R served as a negative control and MRL-Faslpr male mice after I/R served as a positive control. Note: BALB/c and B6 males and females respond similarly. (A) Renal tubular histopathology (dilated tubules, casts) in the outer medulla and Kim-1 expression. (B) Interstitial leukocyte infiltrates and fibrosis (Sirius red), (C) renal function (albuminuria, µg/24h). Dotted line denotes baseline values for age-matched mice that are not manipulated. Data represent means ± SEM.
To test the hypothesis that the MRL background, not the Faslpr mutation alone, is responsible for defective repair, we compared renal repair in MRL-Faslpr mice with the Faslpr mutation (deficient in Fas) with MRL-++ mice without the Faslpr mutation (Fas intact) after I/R. It is important to appreciate that lupus nephritis in MRL-++ mice is latent (clinically evident in 2nd yr of life), progresses at a slower pace (50% mortality 17 mo of age) and is less severe compared with the fulminant (clinically evident at 3–4 mo of age), lethal (50% mortality 5 mo of age) renal disease in MRL-Faslpr mice. We evaluated pre-clinical MRL-++ mice at a point (20d) after I/R when the kidneys in lupus-resistant mice are healed. Renal tubular injury does not fully resolve in MRL-++ (1.5 mo of age) after I/R as the tubular pathology, Kim-1 expression and leukocyte infiltrates in the interstitium are substantially greater than in B6 and BALB/c mice (Figure 3A, right panel). Since lupus nephritis is latent in mice without the Fas, we tested the hypothesis that defective repair after I/R is more pronounced in pre-clinical MRL-++ mice that are aged closer to the emergence of spontaneous kidney disease (33), a situation more comparable to MRL-Faslpr mice. The magnitude of renal tubular (Figure 3A) and interstitial (Figure 3B) disease after I/R is greater in the pre-clinical MRL-++ (8–10 mo of age) compared to MRL-++ (1.5 mo of age) mice, and is similar to MRL-Faslpr mice (1.5 mo of age) (Figure 3, right panel). This finding is in keeping with CSF-1 producing cells implanted under the kidney capsule initiating non-resolving renal inflammation in pre-clinical similarly aged MRL-++ mice, but not in younger (1.5 mo of age) MRL-++ mice (unpublished data). In addition, defective renal repair is not a general feature of other autoimmune diseases as the kidneys of preclinical non-obese diabetic (NOD) mice healed as rapidly as BALB/c and B6 mice after I/R (data not shown). Of note, serum CSF-1 is elevated in MRL-Faslpr mice after I/R, but does not rise in NOD mice after I/R (data not shown) (17). Taken together, defective renal repair after I/R is not unique to MRL-Faslpr mice, but is characteristic of other lupus-susceptible mice expressing Fas.
Does defective repair lead to early-onset lupus nephritis in these other lupus-susceptible strains? We detected more glomerular pathology in the kidney of Sle 123 mice after I/R compared to the contralateral and age-matched control kidneys and serum IgG levels were higher in Sle 123 mice after I/R compared to age-matched controls (Supplement Figure 1, Left panel). Lupus resistant (B6 and BALB/c) mice served as negative controls. Moreover, glomerular and perivascular pathology in the preclinical MRL-++ mice at 1.5 mo of age was greater than the contralateral control kidney, and this is even more pronounced in MRL-++ at 8–10 mo of age after I/R (Supplement Figure 1, Right panel). In addition, the trend in serum IgG was higher in MRL-++ mice after I/R compared with controls. As the spontaneous time course of disease is somewhat slower in Sle 123 (50% mortality 8 mo of age) (31) and dramatically slower in MRL-++ mice (50% mortality 17 mo of age) (33, 34) and lupus nephritis in both strains is substantially more heterogeneous than in MRL-Faslpr mice, it is not surprising that early-onset lupus-nephritis is somewhat less striking in these strains compared with MRL-Faslpr mice.
CSF-1 mediates defective repair, not healing, in MRL-Faslpr mice
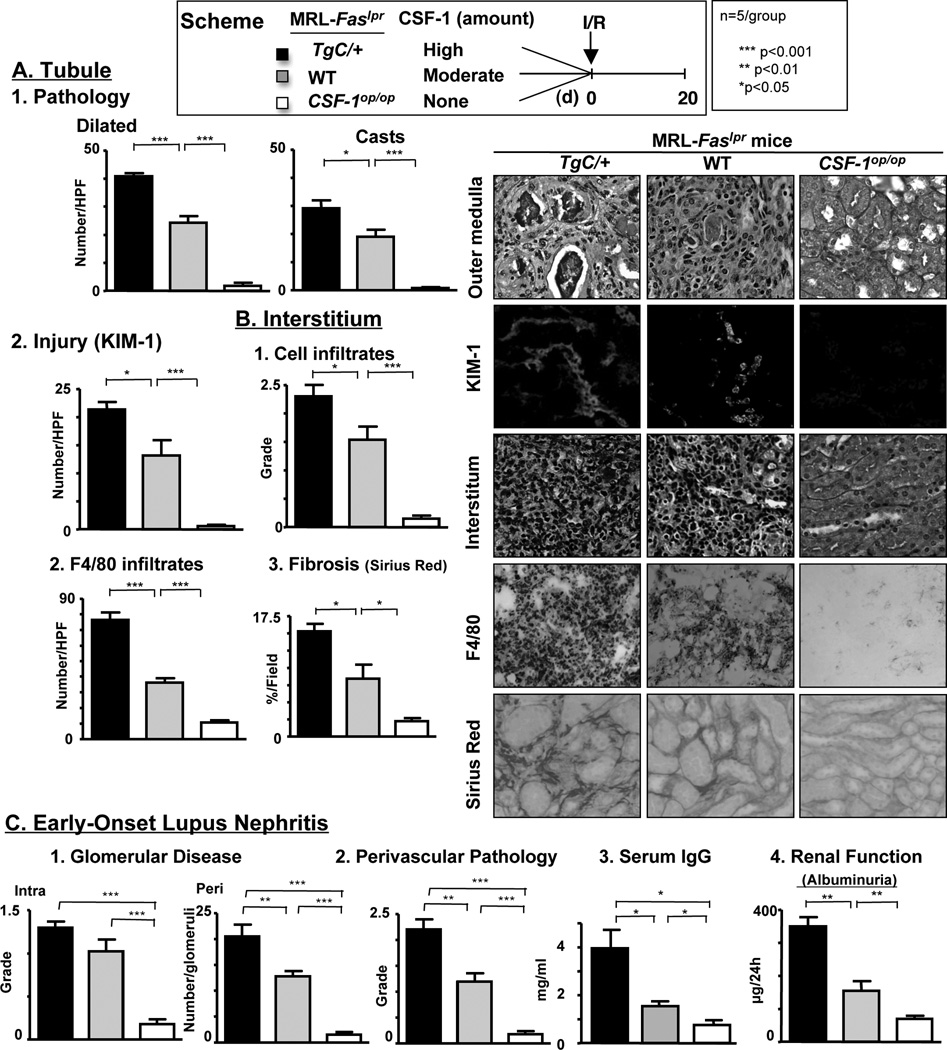
CSF-1 mediates healing in lupus-resistant mice (17). Contrastingly, CSF-1 mediates destructive inflammation and lupus nephritis in MRL-Faslpr mice (10). Therefore, we tested the hypothesis that increasing CSF-1 in MRL-Faslpr mice heightens the severity of defective repair and non-resolved inflammation leading to early-onset lupus nephritis after I/R. To test this hypothesis we used mutant MRL-Faslpr mice that we constructed to express differing levels of CSF-1 in the kidney and circulation: TgC/+;MRL-Faslpr (high), MRL-Faslpr (wildtype [WT], moderate) and CSF-1op/op;MRL-Faslpr (none) as previously detailed (10). We compared renal injury and repair in these MRL-Faslpr mice with differing levels of CSF-1 at a time point when lupus-resistant kidneys are repaired (20d) after I/R. We detected the greatest magnitude of tubular pathology and injury (Kim-1), interstitial infiltrating leukocytes and fibrosis in MRL-Faslpr mice expressing the highest level of CSF-1 (TgC/+;MRL-Faslpr > WT > CSF-1op/op;MRL-Faslpr) (Figure 4). Of note, MRL-Faslpr mice lacking CSF-1 are spared from renal pathology after I/R (20d). Consistent with this finding, injecting CSF-1 after I/R, using the same protocol that promotes healing in lupus-resistant mice (17), thwarts healing in MRL-Faslpr mice (data not shown). Moreover, specific characteristics of lupus nephritis that are not features of I/R, including glomerular pathology (Figure 4C) and immune deposits (IgG, C3) lodged in glomerular capillary walls (data not shown), perivascular infiltrates, serum autoantibodies (IgG, dsDNA) and the loss of renal function (Figure 4C) are most pronounced in MRL-Faslpr mice expressing the highest level of CSF-1 (TgC/+;MRL-Faslpr > WT > CSF-1op/op;MRL-Faslpr). Note, the contralateral kidneys for TgC/+;MRL-Faslpr, WT and CSF-1op/op;MRL-Faslpr mice did not have features of I/R or lupus nephritis. Taken together, increasing CSF-1 in the kidney and circulation diminishes renal repair, and in turn, promotes more severe early-onset lupus nephritis in MRL-Faslpr mice after I/R.
Figure 4.
CSF-1 mediates defective repair, not healing, in MRL-Faslpr mice. Analysis of renal repair in TgC/+;MRL-Faslpr mice, CSF-1op/op;MRL-Faslpr mice and WT MRL-Faslpr male mice 20d after I/R. We initiated I/R at 6 wks of age. (A) Tubular histopathology (dilated tubules and casts) in outer medulla and Kim-1 expression, an index of injured tubules (B) Interstitial leukocyte infiltrates, Mø (F4/80+) and fibrosis (Sirius red). (C) Early-onset lupus nephritis evaluated by glomerular disease, perivascular pathology, serum IgG and renal function (albuminuria, µg/24h). Representative photomicrographs at 20d after I/R (magnification, 40× except Kim-1 20×). Data represent means ± SEM.
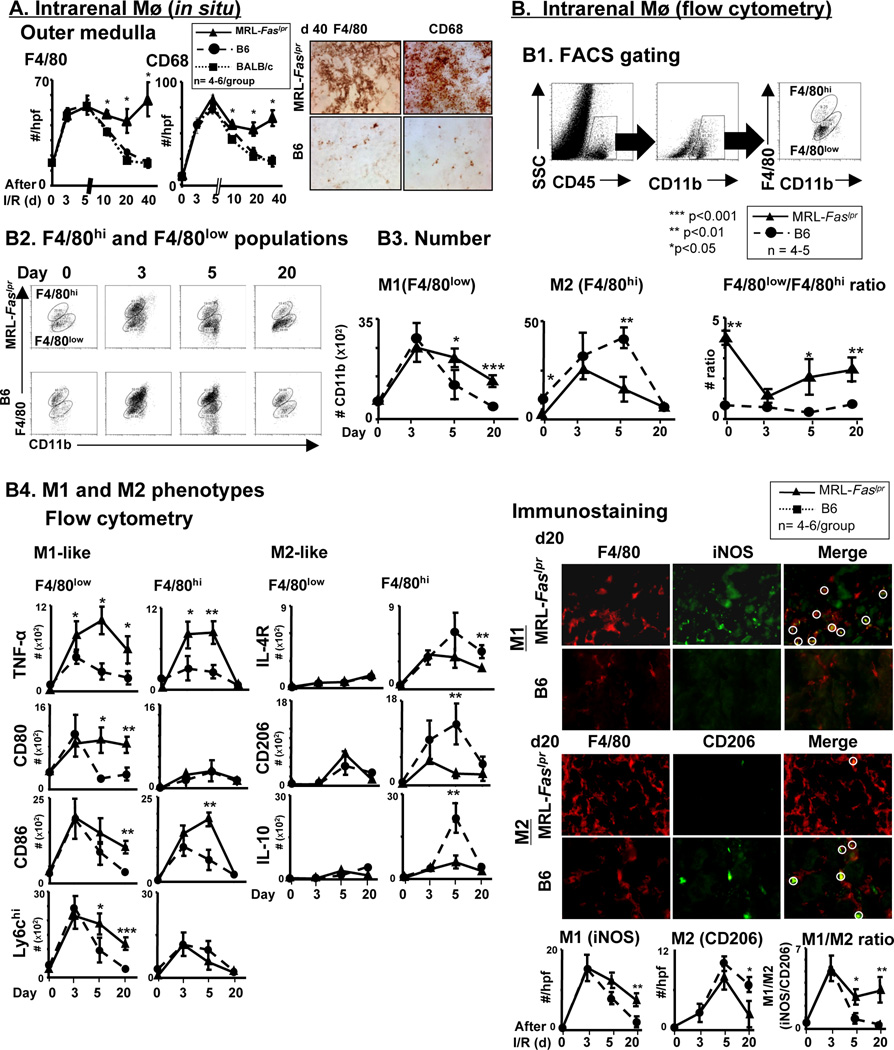
MRL-Faslpr Mø in the kidney are skewed towards an M1 (destroyer), rather than M2 (healer) phenotype, after I/R and during spontaneous lupus nephritis
Mø are integral in inflammation leading to repair and contrastingly, inflammation leading to injury. The initial (3,5d) increase in intra-renal Mø (F4/80+, CD68+) is similar in lupus-resistant and MRL-Faslpr mice after I/R. However, Mø dramatically and continuously decline (10, 20, 40d) in lupus-resistant mice, while Mø remain elevated (10, 20d) and then begin to re-ascend (40d) in MRL-Faslpr mice (Figure 5A). Of note, we did not detect a differential expression of neutrophils in MRL-Faslpr and B6 mice after I/R, as neutrophils (Ly6G+, F4/80−) peaked rapidly (d1) and similarly declined promptly, returning to baseline levels (d20) in MRL-Faslpr and B6 mice (Supplement Figure 2B). As inflammation is non-resolving and leads to lupus nephritis in MRL-Faslpr mice and contrastingly, leads to healing in lupus-resistant mice, this suggests that Mø, not neutrophils, in MRL-Faslpr are central to defective repair and escalating destructive inflammation.
Figure 5.
Mø are skewed towards M1 “destroyers”, rather than M2 “healers” in MRL-Faslpr mice after I/R. (A) Renal Mø (F4/80+ and CD68+) in MRL-Faslpr and lupus-resistant (BALB/c and B6) male mice was detected by immunostaining at increasing times after I/R. We initiated I/R at 6 wks of age. (B1-3) Intrarenal M1 and M2 Mø evaluated in MRL-Faslpr and B6 at increasing times after I/R using flow cytometry and immunostaining. (B1) Diagram analysis using flow cytometry. (B2) Representative FACS plot by gating on the CD45+ CD11+ F4/80hi/low cells are shown. (B3) Numbers of F4/80hi and F4/80low cells. (B4) Left: M1-like (TNF-α+/F4/80low/hi, CD80+/F4/80low/hi, CD86+/F4/80low/hi, Ly6Chi+/F4/80low/hi) and M2-like (CD206+/F4/80low/hi, IL-10+/F4/80low/hi, IL4R+/F4/80+low/hi) Mø were evaluated at increasing times after I/R using flow cytometry. Right: M1-like (iNOS+F4/80+) and M2-like (CD206+F4/80+) Mø were evaluated at increasing times after I/R using immunostaining. Representative photomicrographs at d20 after I/R (magnification 40×). Data represent means ± SEM.
Simplistically, Mø phenotypes are divided into classically activated Mø that are central to mediating tissue destruction, termed M1, and alternatively activated Mø that are central to repair, termed M2. We tested the hypothesis that Mø shift from M1-like ”destroyers” to M2-like “healers” in lupus-resistant mice, while M1 Mø continue to rise and fail to shift toward the M2 phenotype in MRL-Faslpr mice, after I/R. To phenotype Mø populations by flow cytometry, we identified the CD45+ hematopoietic cells, and within this population the CD11b+ cells. As F4/80low reflect the M1 phenotype and F4/80hi largely reflect the M2 phenotype (35), we further analyzed CD11b+ leukocytes into F4/80low and F4/80hi Mø populations (Figure 5B1). Moreover, we compared the time-related magnitude of CD11b+ F4/80low and F4/80hi Mø in the kidney using specific panels of M1 (iNOS, TNF-α, CD86, IL-12) and M2 (CD206, IL-10, Ym-1) markers in lupus-resistant and MRL-Faslpr mice after I/R. We arbitrarily divided the phases after I/R into initial phase (0–3d), initiation of injury in lupus-resistant mice, and later phase (5–20d), progression toward repair in lupus-resistant mice. We detected a dramatic and similar rise in M1 (F4/80low) Mø in lupus-resistant and MRL-Faslpr mice during the initial phase after I/R (Figures 5B2, 5B3), a finding that is similar using M1 specific markers (Figure 5B4). However, M1 Mø (number, Figures 5B3, 5B4; frequency and representative plots, Supplement Figure 3) markedly declined and returned to normal levels in lupus-resistant mice, but remain elevated in MRL-Faslpr mice. Moreover, we identified CD11b+ F4/80low GR1hi, that peaked at 5d after I/R in MRL-Faslpr and B6 kidneys, that decline in B6 mice, but remained elevated in MRL-Faslpr mice, at 20d after I/R (Supplement Figure 2C). By comparison during the initial phase after I/R, the magnitude of M2 Mø barely increases in lupus-resistant and MRL-Faslpr mice (Figures 5B3, 5B4). Moreover, there is a far more robust rise in M2 Mø in lupus-resistant mice compared with MRL-Faslpr mice at the beginning of the later phase after I/R (d5) that declines to near baseline levels (d20, d40) (Figures 5B3, 5B4). In addition, the failure to shift from M1 to M2 and non-resolving inflammation is a feature of Sle 123 after I/R, and thus is not unique to MRL-Faslpr mice (Figure 6). However, as the accumulation of Mø in the MRL-Faslpr kidneys is nearly 2 fold greater than in the Sle 123 kidneys after I/R (data not shown), lupus nephritis in MRL-Faslpr mice may be more dependent on Mø-mediated mechanisms than Sle 123 mice (Figure 6). Taken together, the balance of M1 and M2 Mø shifts from the M1 phenotype, at the onset of injury, to the M2 phenotype, during healing after I/R in lupus-resistant mice, while M1 Mø continue to dominant in MRL-Faslpr mice after I/R during defective repair and non-resolving inflammation that triggers early-onset lupus nephritis. We wish to point out that most, but not all, Mø express M1 or M2 markers (immunostaining, flow cytometry, Supplement Figure 4), suggesting that there are other Mø populations yet to be clearly defined in the inflamed kidney.
Figure 6.
Mø are skewed towards M1 “destroyers”, rather than M2 “healers” in other lupus-susceptible mice that express Fas. Intrarenal Mø phenotype (M1/M2) analysis in Sle 123 female mice 20d after I/R. We initiated I/R at 10 wks of age. Lupus-resistant (B6) female mice after I/R serve as a negative control and MRL-Faslpr female mice after I/R served as a positive control. Control mice were 6 wks of age at initiation of I/R. M1 (TNF-α+/F4/80low/hi, CD80+/F4/80low/hi, CD86+/F4/80low/hi, Ly6Chi+/F4/80low/hi) and M2 (CD206+/F4/80low/hi, IL-10+/F4/80low/hi, IL4R+/F4/80low/hi) Mø were evaluated by flow cytometry after I/R (d20). Data represent means ± SEM.
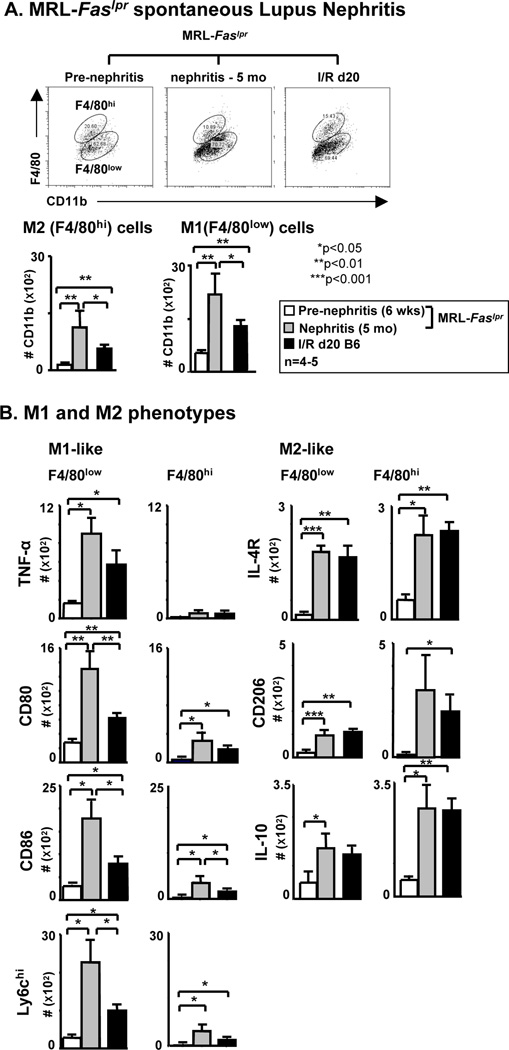
Is there a shift towards M1 Mø rather than M2 Mø during spontaneous lupus nephritis in MRL-Faslpr mice? We compared the Mø M1 and M2 phenotypes in the kidney prior to (6 wks of age) and during advanced (5 mo of age) lupus nephritis in MRL-Faslpr mice. Moreover, these Mø M1 and M2 phenotypes were compared with MRL-Faslpr mice after I/R (d20). The number of M1 (F4/80low and M1 markers) and M2 (F4/80hi and M2 markers) Mø increase in spontaneous lupus nephritis, however the rise in the number of M1 Mø is far more robust than M2 Mø, and even exceeds the number of M1 Mø in the kidney after I/R (Figure 7). Thus, there is a greater abundance of M1 compared with M2 Mø in the kidney during spontaneous lupus nephritis. We wish to re-emphasize that Mø are not crisply divided into M1 and M2 categories, but rather that we use this conceptual framework to describe a continuum of Mø along a spectrum of leukocytes with diverse functions.
Figure 7.
During spontaneous lupus nephritis (MRL-Faslpr) M1 increase more robustly than M2 Mø in MRL-Faslpr kidneys. B6 mice at 20d after I/R served as control. Pre-nephritic and nephritic MRL-Faslpr mice were 6 wks and 5 mo of age, respectively. B6 mice were 6 wks of age at intitiation of I/R. (A) Intrarenal Mø phenotype M1 (F4/80low) cells and M2 (F4/80hi) cells evaluated by flow cytometry. Representative FACS plots gating on the CD45+ CD11+ F4/80+ are shown. (B) Mø were further evaluated using the following markers: M1 (TNF-α+/F4/80low/hi, CD80+/F4/80low/hi, CD86+/F4/80low/hi, Ly6Chi/F4/80+low/hi) and M2 (CD206+/F4/80low/hi, IL-10+/F4/80low/hi, IL4R+/F4/80low/hi) (flow cytometry). Data represent means ± SEM and is representative of three separate experiments.
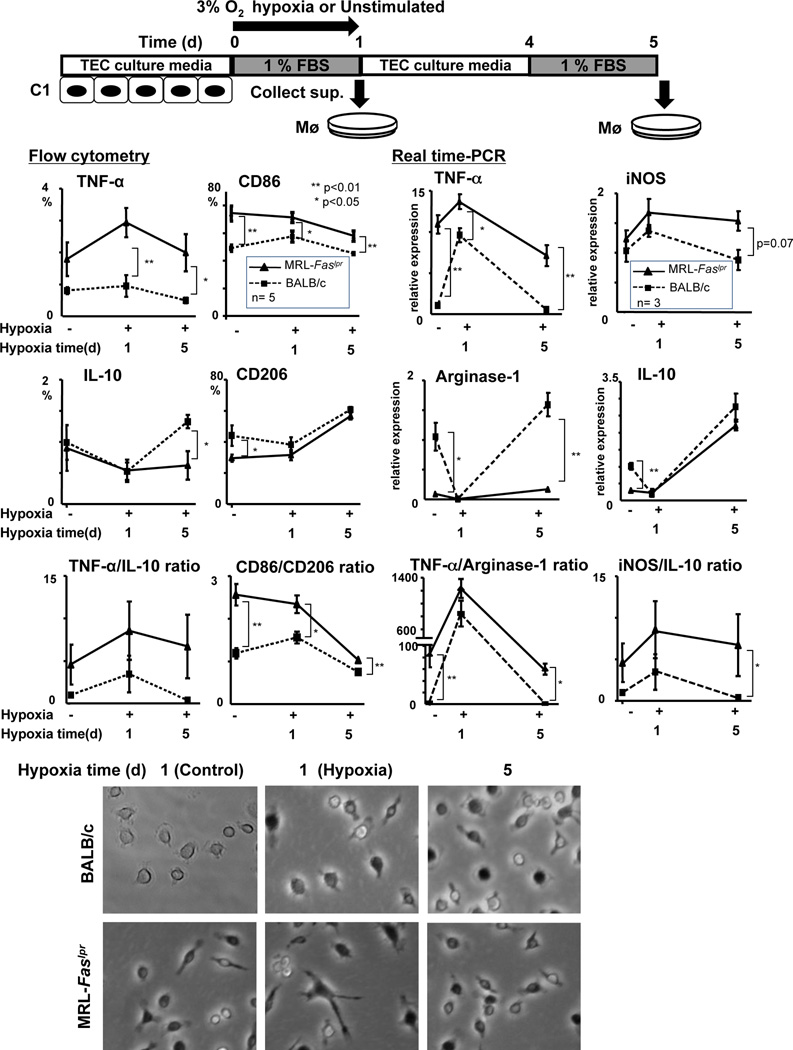
Hypoxic cultured TEC release mediators that skew Mø towards the M1 phenotype in MRL-Faslpr and lupus-resistant mice during injury, and shifts Mø towards the M2 phenotype in lupus-resistant, but not MRL-Faslpr mice, during repair
During injury TEC release mediators, including CSF-1, responsible for mediating repair in lupus-resistant mice (17) and lupus nephritis in MRL-Faslpr mice (10). As shown, in the later phase after I/R, intra-renal Mø are skewed toward the M1 phenotype in MRL-Faslpr mice when destructive inflammation is escalating, and toward the M2 phenotype in lupus-resistant mice when the kidney is healing (Figure 5). As hypoxia induces TEC injury, we tested the hypothesis that hypoxic tubules release mediators that skew Mø toward the M1 phenotype in MRL-Faslpr mice, and toward the M2 phenotype in lupus-resistant mice. To test this hypothesis, we induced hypoxia in a proximal TEC line (C1) that we previously constructed (28). We then stimulated bone marrow (BM) Mø from pre-clinical MRL-Faslpr and lupus-resistant mice with supernatant taken from hypoxic tubules just after injury (1d) and during proliferative repair (5d) as verified by Kim-1 expression and survival/proliferation (MTT assay) (data not shown). Mediators, including CSF-1, released from hypoxic cells during injury (1d) stimulated a greater magnitude of M1 BMMø (TNF-α, CD86 and iNOS, flow cytometry and/or Real time PCR) than M2 BMMø (IL-10, CD206, Arginase-1, flow cytometry and/or Real time PCR) in MRL-Faslpr mice compared to lupus-resistant mice. However, the magnitude of M1 Mø was greater in MRL-Faslpr than lupus-resistant mice (Figure 8). In contrast, hypoxic TEC released mediators during repair that stimulated more M2 BMMø (IL-10, CD206, Arginase-1, flow cytometry and/or Real time PCR) in lupus-resistant than in MRL-Faslpr mice (Figure 8). Of note, even prior to stimulation with hypoxic supernatant (0d), BMMø were more skewed toward the M1 phenotype in MRL-Faslpr mice and more skewed toward the M2 phenotype in lupus-resistant mice (Figure 8). In fact, prior to stimulation, MRL-Faslpr BMMø appear activated (large and elongated) compared to similarly prepared BMMø from lupus-resistant mice (Figure 8). As the same mediators released from hypoxic TEC skew Mø toward a M2 phenotype in lupus-resistant mice, but toward a M1 phenotype in MRL-Faslpr mice, this suggests that Mø in MRL-Faslpr mice are inherently poised to destroy, not repair, after transient injury.
Figure 8.
Hypoxia injured TEC release mediators that skew Mø towards M1 in MRL-Faslpr and lupus-resistant (BALB/c) mice, while repairing TEC release mediators that skew Mø towards M2 in lupus-resistant, but not MRL-Faslpr, mice. Scheme depicting experimental design using hypoxic C1 TEC supernatant (effectors) and BMMø (responders). Supernatant of C1 TEC collected at 1d and 5d after hypoxia. BMMø incubated with C1 supernatant for 24h. M1 (TNF-α and CD86) and M2 (IL-10 and CD206) phenotypes evaluated by FACS analysis (left panels). M1 (TNF-α and iNOS) and M2 (arginase-1 and IL-10) evaluated by Real time PCR. Representative photomicrographs of MRL-Faslpr and BALB/c BMMø stimulated with supernatant from C1 TEC after hypoxia (magnification 40×). BMMø were isolated from male mice at 6 wks of age. Data represent means ± SEM.
Discussion
We now report that transient ischemic kidney injury (I/R) elicits defective renal repair, and non-resolving inflammation leading to early-onset lupus nephritis in pre-clinical MRL-Faslpr mice. However, defective renal repair is not unique to MRL-Faslpr mice as flawed I/R elicited healing is a feature of other lupus-susceptible mice (Sle 123) and MRL mice without the Faslpr mutation (MRL-++). While CSF-1 hastens healing after I/R in lupus-resistant mice, CSF-1 impairs renal repair, exacerbates non-resolving inflammation, and triggers early-onset lupus nephritis in MRL-Faslpr mice. Probing further, Mø in MRL-Faslpr mice fail to shift from a M1 “destroyer” to M2 “healer” phenotype. Indeed, Mø in MRL-Faslpr mice are inherently skewed toward the M1 “destroyer” phenotype. The time-related balance of M1 to M2 Mø shifts towards the M2 “healer” phenotype in lupus-resistant mice after I/R, but M1 continue to dominate in lupus-susceptible (MRL-Faslpr and Sle 123) mice. Moreover, hypoxic TEC release mediators that stimulate the expansion of BMMø in MRL-Faslpr mice inherently poised to destroy the kidney. Taken together, ischemic injury induces CSF-1 in injured TEC that foster the expansion of aberrant Mø (M1 phenotype) that mediate defective renal repair and non-resolving inflammation, thereby hastening the onset of lupus nephritis in MRL-Faslpr mice.
Growing evidence suggests transient injury may trigger defective repair and lupus in a variety of tissues including the kidney. For example, transient, low level UVB-exposure to the skin induces CSF-1 in keratinocytes, thereby recruiting Mø that mediated inflammation leading to repair in lupus-resistant mice, but triggering cutaneous (discoid) lupus in MRL-Faslpr mice (36). As UVB incites similar levels of CSF-1 in keratinocytes, this suggests that the leukocytes in the skin responding to CSF-1 in lupus-resistant mice mediate healing, while those in MRL-Faslpr mice mediate destruction. In fact, aberrant Mø responding to CSF-1 after sunlight-exposure may explain the mechanism by which sunlight triggers Mø-mediated CSF-1 dependent cutaneous lupus in lupus-susceptible patients. Thus, transient injury leading to defective repair, non-resolving inflammation and early-onset lupus in the kidney and skin in MRL-Faslpr mice are apparently analogous. Moreover, our studies indicate that defective repair is not unique to MRL-Faslpr mice, but is a feature of other lupus-susceptible mice including the Sle123 and MRL-++. This is consistent with tape stripping, another form of transient injury, leading to repair in lupus-resistant mice and conversely, inciting chronic skin lesions with features of cutaneous lupus in lupus-susceptible NZB × NZW F1 mice (37). Taken together, transient injury in lupus-susceptible mice leads to non-resolving inflammation and early-onset lupus.
The MRL background, and not the absence of Fas, is linked to defective repair after I/R. We show that renal repair is defective in pre-clinical MRL-++ mice (Fas intact) and this defect is more pronounced in pre-clinical MRL-++ mice that are aged closer to the onset of clinical lupus nephritis. As defective repair is less pronounced in age-matched (6 wks), pre-clinical MRL-++ compared with MRL-Faslpr mice, our experiments differ from those indicating that blocking Fas ligand in lupus-resistant mice (B6) attenuates injury (38). However, as the MRL background is central to defective renal repair after I/R, it is possible that the impact of signaling through Fas is masked or altered in the context of the MRL background. Moreover, early-onset lupus is evident in MRL-++, albeit to a lesser degree than in MRL-Faslpr mice, as some features (increased glomerular and perivascular pathology and loss of renal function), but not others (serum IgG), are triggered in MRL-++ mice after I/R. In support of our findings, a more traumatic injury, severe skin burn in MRL-++ mice results in early-onset cutaneous lupus, an increase in serum IgGs and systemic disease (39) 1–2 mo post trauma. Thus, a more traumatic injury or extending the time after I/R may lead to more pronounced early-onset lupus nephritis. However, our findings are consistent with systemic lupus being less severe in MRL-++ than MRL-Faslpr mice (33). Finally, although reports indicate that healing in MRL-++ mice is complete and scar-less in some tissues (skin, heart) (40, 41), our data, along with other reports (42, 43), challenges this concept. Thus, defective renal repair leading to early-onset lupus nephritis is linked to the MRL background.
There is ample evidence to suggest that Mø in lupus-susceptible mice are aberrant. Mø in MRL-Faslpr mice hyper-proliferate to CSF-1 (5), display enhanced MHC Ia expression (6), have a broad-range phagocytic defect including diminished clearance of red blood cells, yeast, and apoptotic cells epithelial cells (unpublished data) and T cells (25), aberrantly express cytokines (44–46) and abnormal Rho-dependent cytoskeletal organization (47). We now report that these aberrant Mø fail to repair renal injury. We detected a shift in the dominance of M1 Mø at the initiation of renal injury to M2 Mø during repair after I/R in lupus-resistant mice. This is consistent with the switch from a pro-inflammatory (M1) to a reparative (M2) phenotype during the transition from tubule injury to tubule repair (20). We now report that non-resolving inflammation and early-onset lupus is mediated by the failure of a predominance of M1 Mø to shift towards M2 Mø in the kidney. As Mø from MRL-Faslpr mice proliferate more robustly to CSF-1 than lupus-resistant mice, CSF-1 generated by injured tubules expand the M1 rather than M2 Mø, and thereby foster destructive inflammation leading to early-onset lupus nephritis. This defective shift from M1 to M2 may be a general feature of intra-renal Mø in lupus-susceptible hosts following injury, as intra-renal Mø in Sle 123 mice respond similarly after renal injury. Moreover, abnormal mononuclear phagocytes have been identified in other models of lupus nephritis. In particular, mononuclear phagocytes with an aberrant activation profile contribute to tissue damage by mediating local intra-renal inflammation in lupus-susceptible NZB/W mice (48). We appreciate that the role of M1 and M2 Mø highlighted in our study may be only one facet of a more widespread disturbance of innate immunity in lupus-susceptible mice and individuals. This hypothesis is currently being studied.
While intra-renal Mø are distinct in lupus nephritis, their phenotypes are not consistent in all models. We report a skewing towards M1 (F4/80low), rather than M2 (F4/80hi) phenotypes in MRL-Faslpr and Sle 123 mice during lupus nephritis incited by I/R and in spontaneous lupus nephritis in MRL-Faslpr mice. In contrast, intra-renal Mø in an induced model of lupus nephritis (activation lymphocyte derived DNA [ALD-DNA]) polarize Mø toward an M2b phenotype (49). As M2b Mø are induced by exposure to immune complexes or TLR (19), this may suggest that the pathogenesis of lupus nephritis in MRL-Faslpr and Sle 123 mice versus ALD-DNA induced lupus nephritis differ. Moreover, Mø in the kidney during lupus nephritis in NZB/W mice acquire a high expression of F4/80 (48). As lupus nephritis may be triggered by a variety of mechanisms, it is plausible that dissimilar Mø phenotypes reflect differences within patient populations with distinct mechanisms. Moreover, recognizing that the methods employed for phenotyping Mø are not precisely standardized, we are not able to make exacting comparisons with studies from other laboratories. Indeed, a standardized, detailed comparison of intra-renal Mø phenotypes in multiple mouse models of lupus nephritis and human lupus nephritis will likely uncover a stratification of the pathogenesis leading to novel therapeutic strategies for this illness.
Taken together, we speculate that non-resolving inflammation triggering lupus nephritis results from aberrant Mø, poised to destroy, rather than repair injured kidneys. To more fully probe into the mechanism responsible for escalating destructive, rather than reparative Mø after renal injury, we are exploring whether defective switching is a result of inherently abnormal Mø polarization in lupus-susceptible mice, or one facet of a broader defect in innate immunity. However, we wish to point out that it is also possible that destructive Mø persist because other triggers continually provoke non-resolving inflammation. We anticipate that future studies directed towards clarifying this “chicken and egg” problem will elucidate the pathogenesis, and thereby provide novel therapeutic approaches for human lupus nephritis.
Supplementary Material
Acknowledgements
The authors wish to acknowledge Dr. E.R. Stanley for providing the TgC mice (FVB background) that we backcrossed onto the MRL-Faslpr background.
Grant support: This work was supported by NIH grants DK 36149 (VRK), the Alliance for Lupus Research (VRK), the National Kidney Foundation (YI), the Sweden-America Foundation (EB), the Sixten Gamzéus Stiftelse (EB), the Stiftelsen Olle Engkvist Byggmästare (EB), the Deutsche Forschungsgemeinschaft ME-3194/1-1 (JM) and NIH grant R01-A058150 (LM).
Nonstandard abbreviations used
- TEC
tubular epithelial cell
- I/R
ischemia/reperfusion injury
- Mø
macrophage
- CSF-1R
CSF-1 receptor
- TNF-α
tumor necrosis factor-α
- BM
bone marrow
- B6 mice
C57BL/6 mice
- MRL-Faslpr mice
MRL/MpJ-Faslpr/Faslpr mice
- MRL-++ mice
MRL/MpJ mice
- Sle 123 mice
B6.Sle1Sle2Sle3Sle5 mice
References
- 1.Theofilopoulos AN, Dixon FJ. Eito-pathogenesis of murine SLE. Immunol Rev. 1981;55:179–216. doi: 10.1111/j.1600-065x.1981.tb00343.x. [DOI] [PubMed] [Google Scholar]
- 2.Kelley VR, Wuthrich RP. Cytokines in the pathogenesis of systemic lupus erythematosus. Semin Nephrol. 1999;19:57–66. [PubMed] [Google Scholar]
- 3.Kikawada E, Lenda DM, Kelley VR. IL-12 Deficiency in MRL-Fas(lpr) Mice Delays Nephritis and Intrarenal IFN-gamma Expression, and Diminishes Systemic Pathology. J Immunol. 2003;170:3915–3925. doi: 10.4049/jimmunol.170.7.3915. [DOI] [PubMed] [Google Scholar]
- 4.Bloom RD, Florquin S, Singer GG, Brennan DC, Kelley VR. Colony stimulating factor-1 in the induction of lupus nephritis. Kidney Int. 1993;43:1000–1009. doi: 10.1038/ki.1993.141. [DOI] [PubMed] [Google Scholar]
- 5.Moore KJ, Naito T, Martin C, Kelley VR. Enhanced response of macrophages to CSF-1 in autoimmune mice: a gene transfer strategy. J Immunol. 1996;157:433–440. [PubMed] [Google Scholar]
- 6.Kelley VE, Roths JB. Increase in macrophage Ia expression in autoimmune mice: role of the Ipr gene. J Immunol. 1982;129:923–925. [PubMed] [Google Scholar]
- 7.Yui MA, Brissette WH, Brennan DC, Wuthrich RP, Rubin-Kelley VE. Increased macrophage colony-stimulating factor in neonatal and adult autoimmune MRL-lpr mice. Am J Pathol. 1991;139:255–261. [PMC free article] [PubMed] [Google Scholar]
- 8.Tesch GH, Maifert S, Schwarting A, Rollins BJ, Kelley VR. Monocyte chemoattractant protein 1-dependent leukocytic infiltrates are responsible for autoimmune disease in MRL-Fas(lpr) mice. J Exp Med. 1999;190:1813–1824. doi: 10.1084/jem.190.12.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiffer L, Bethunaickan R, Ramanujam M, Huang W, Schiffer M, Tao H, Madaio MP, Bottinger EP, Davidson A. Activated renal macrophages are markers of disease onset and disease remission in lupus nephritis. J Immunol. 2008;180:1938–1947. doi: 10.4049/jimmunol.180.3.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menke J, Rabacal WA, Byrne KT, Iwata Y, Schwartz MM, Stanley ER, Schwarting A, Kelley VR. Circulating CSF-1 Promotes Monocyte and Macrophage Phenotypes that Enhance Lupus Nephritis. J Am Soc Nephrol. 2009;20:2581–2592. doi: 10.1681/ASN.2009050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenda DM, Stanley ER, Kelley VR. Negative role of colony-stimulating factor-1 in macrophage, T cell, and B cell mediated autoimmune disease in MRL-Fas(lpr) mice. J Immunol. 2004;173:4744–4754. doi: 10.4049/jimmunol.173.7.4744. [DOI] [PubMed] [Google Scholar]
- 12.Naito T, Yokoyama H, Moore KJ, Dranoff G, Mulligan RC, Kelley VR. Macrophage growth factors introduced into the kidney initiate renal injury. Mol Med. 1996;2:297–312. [PMC free article] [PubMed] [Google Scholar]
- 13.Lenda D, Kikawada E, Stanley ER, Kelley VR. Reduced Mø Recruitment, Proliferation, and Activation in CSF-1 Deficient Mice, Results in Decreased Tubular Apoptosis During Renal Inflammation. J Immunol. 2004 doi: 10.4049/jimmunol.170.6.3254. [DOI] [PubMed] [Google Scholar]
- 14.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Okusa MD. Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Semin Nephrol. 2010;30:268–277. doi: 10.1016/j.semnephrol.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humphreys BD, Czerniak S, Dirocco DP, Hasnain W, Cheema R, Bonventre JV. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci U S A. 2011;108:9226–9231. doi: 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menke J, Iwata Y, Rabacal WA, Basu R, Yeung YG, Humphreys BD, Wada T, Schwarting A, Stanley ER, Kelley VR. CSF-1 signals directly to renal tubular epithelial cells to mediate repair in mice. J Clin Invest. 2009;119:2330–2342. doi: 10.1172/JCI39087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 19.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsunawaki S, Sporn M, Ding A, Nathan C. Deactivation of macrophages by transforming growth factor-beta. Nature. 1988;334:260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 23.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fadok VA, Bratton DL, Guthrie L, Henson PM. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol. 2001;166:6847–6854. doi: 10.4049/jimmunol.166.11.6847. [DOI] [PubMed] [Google Scholar]
- 25.Potter PK, Cortes-Hernandez J, Quartier P, Botto M, Walport MJ. Lupus-prone mice have an abnormal response to thioglycolate and an impaired clearance of apoptotic cells. J Immunol. 2003;170:3223–3232. doi: 10.4049/jimmunol.170.6.3223. [DOI] [PubMed] [Google Scholar]
- 26.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Jang MH, Herber DM, Jiang X, Nandi S, Dai XM, Zeller G, Stanley ER, Kelley VR. Distinct in vivo roles of colony-stimulating factor-1 isoforms in renal inflammation. J Immunol. 2006;177:4055–4063. doi: 10.4049/jimmunol.177.6.4055. [DOI] [PubMed] [Google Scholar]
- 28.Wuthrich RP, Glimcher LH, Yui MA, Jevnikar AM, Dumas SE, Kelley VE. MHC class II, antigen presentation and tumor necrosis factor in renal tubular epithelial cells. Kidney Int. 1990;37:783–792. doi: 10.1038/ki.1990.46. [DOI] [PubMed] [Google Scholar]
- 29.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 30.Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem. 2004;279:52282–52292. doi: 10.1074/jbc.M407629200. [DOI] [PubMed] [Google Scholar]
- 31.Morel L, Croker BP, Blenman KR, Mohan C, Huang G, Gilkeson G, Wakeland EK. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc Natl Acad Sci U S A. 2000;97:6670–6675. doi: 10.1073/pnas.97.12.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Z, Duan B, Croker BP, Morel L. STAT4 deficiency reduces autoantibody production and glomerulonephritis in a mouse model of lupus. Clin Immunol. 2006;120:189–198. doi: 10.1016/j.clim.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Kelley VE, Roths JB. Interaction of mutant lpr gene with background strain influences renal disease. Clin Immunol Immunopathol. 1985;37:220–229. doi: 10.1016/0090-1229(85)90153-9. [DOI] [PubMed] [Google Scholar]
- 34.Izui S, Kelley VE, Masuda K, Yoshida H, Roths JB, Murphy ED. Induction of various autoantibodies by mutant gene lpr in several strains of mice. J Immunol. 1984;133:227–233. [PubMed] [Google Scholar]
- 35.Fujiu K, Manabe I, Nagai R. Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J Clin Invest. 2011;121:3425–3441. doi: 10.1172/JCI57582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menke J, Hsu MY, Byrne KT, Lucas JA, Rabacal WA, Croker BP, Zong XH, Stanley ER, Kelley VR. Sunlight triggers cutaneous lupus through a CSF-1-dependent mechanism in MRL-Fas(lpr) mice. J Immunol. 2008;181:7367–7379. doi: 10.4049/jimmunol.181.10.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guiducci C, Tripodo C, Gong M, Sangaletti S, Colombo MP, Coffman RL, Barrat FJ. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med. 2010;207:2931–2942. doi: 10.1084/jem.20101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ko GJ, Jang HR, Huang Y, Womer KL, Liu M, Higbee E, Xiao Z, Yagita H, Racusen L, Hamad AR, et al. Blocking Fas ligand on leukocytes attenuates kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2011;22:732–742. doi: 10.1681/ASN.2010010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anam K, Amare M, Naik S, Szabo KA, Davis TA. Severe tissue trauma triggers the autoimmune state systemic lupus erythematosus in the MRL/++ lupus-prone mouse. Lupus. 2009;18:318–331. doi: 10.1177/0961203308097479. [DOI] [PubMed] [Google Scholar]
- 40.Clark LD, Clark RK, Heber-Katz E. A new murine model for mammalian wound repair and regeneration. Clin Immunol Immunopathol. 1998;88:35–45. doi: 10.1006/clin.1998.4519. [DOI] [PubMed] [Google Scholar]
- 41.Leferovich JM, Bedelbaeva K, Samulewicz S, Zhang XM, Zwas D, Lankford EB, Heber-Katz E. Heart regeneration in adult MRL mice. Proc Natl Acad Sci U S A. 2001;98:9830–9835. doi: 10.1073/pnas.181329398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oh YS, Thomson LE, Fishbein MC, Berman DS, Sharifi B, Chen PS. Scar formation after ischemic myocardial injury in MRL mice. Cardiovasc Pathol. 2004;13:203–206. doi: 10.1016/j.carpath.2004.03.610. [DOI] [PubMed] [Google Scholar]
- 43.Grisel P, Meinhardt A, Lehr HA, Kappenberger L, Barrandon Y, Vassalli G. The MRL mouse repairs both cryogenic and ischemic myocardial infarcts with scar. Cardiovasc Pathol. 2008;17:14–22. doi: 10.1016/j.carpath.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Levine J, Hartwell D, Beller DI. Imbalanced cytokine production by macrophages from autoimmune-prone mice. Immunol Lett. 1991;30:183–192. doi: 10.1016/0165-2478(91)90023-4. [DOI] [PubMed] [Google Scholar]
- 45.Levine JS, Pugh BJ, Hartwell D, Fitzpatrick JM, Marshak-Rothstein A, Beller DI. Interleukin-1 dysregulation is an intrinsic defect in macrophages from MRL autoimmune-prone mice. Eur J Immunol. 1993;23:2951–2958. doi: 10.1002/eji.1830231134. [DOI] [PubMed] [Google Scholar]
- 46.Hartwell DW, Fenton MJ, Levine JS, Beller DI. Aberrant cytokine regulation in macrophages from young autoimmune-prone mice: evidence that the intrinsic defect in MRL macrophage IL-1 expression is transcriptionally controlled. Mol Immunol. 1995;32:743–751. doi: 10.1016/0161-5890(95)00036-e. [DOI] [PubMed] [Google Scholar]
- 47.Longacre A, Koh JS, Hsiao KK, Gilligan H, Fan H, Patel VA, Levine JS. Macrophages from lupus-prone MRL mice are characterized by abnormalities in Rho activity, cytoskeletal organization, and adhesiveness to extracellular matrix proteins. J Leukoc Biol. 2004;76:971–984. doi: 10.1189/jlb.0604346. [DOI] [PubMed] [Google Scholar]
- 48.Bethunaickan R, Berthier CC, Ramanujam M, Sahu R, Zhang W, Sun Y, Bottinger EP, Ivashkiv L, Kretzler M, Davidson A. A unique hybrid renal mononuclear phagocyte activation phenotype in murine systemic lupus erythematosus nephritis. J Immunol. 2011;186:4994–5003. doi: 10.4049/jimmunol.1003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang W, Xu W, Xiong S. Blockade of Notch1 signaling alleviates murine lupus via blunting macrophage activation and M2b polarization. J Immunol. 2010;184:6465–6478. doi: 10.4049/jimmunol.0904016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.