Abstract
While mitochondrial dysfunction is a pathological process that occurs after acute kidney injury (AKI), the state of mitochondrial homeostasis during the injury and recovery phases of AKI remains unclear. We examined markers of mitochondrial homeostasis in two nonlethal rodent AKI models. Myoglobinuric AKI was induced by glycerol injection into rats, and mice were subjected to ischemic AKI. Animals in both models had elevated serum creatinine, indicative of renal dysfunction, 24 h after injury which partially recovered over 144 h postinjury. Markers of proximal tubule function/injury, including neutrophil gelatinase-associated lipocalin and urine glucose, did not recover during this same period. The persistent pathological state was confirmed by sustained caspase 3 cleavage and evidence of tubule dilation and brush-border damage. Respiratory proteins NDUFB8, ATP synthase β, cytochrome c oxidase subunit I (COX I), and COX IV were decreased in both injury models and did not recover by 144 h. Immunohistochemical analysis confirmed that COX IV protein was progressively lost in proximal tubules of the kidney cortex after ischemia-reperfusion (I/R). Expression of mitochondrial fission protein Drp1 was elevated after injury in both models, whereas the fusion protein Mfn2 was elevated after glycerol injury but decreased after I/R AKI. LC3-I/II expression revealed that autophagy increased in both injury models at the later time points. Markers of mitochondrial biogenesis, such as PGC-1α and PRC, were elevated in both models. These findings reveal that there is persistent disruption of mitochondrial homeostasis and sustained tubular damage after AKI, even in the presence of mitochondrial recovery signals and improved glomerular filtration.
Keywords: mitochondria, ischemia, rhabdomyolysis, proximal tubules
acute kidney injury (AKI) is an increasingly prevalent, complex clinical disorder. Intrinsic AKI, produced from direct damage to the kidney, may arise in a number of ways, including drug/toxicant exposure or ischemia (5, 7, 31). Although numerous mechanisms of AKI are observed clinically and in experimental models, it is generally recognized as a multifactorial injury with overlapping elements as opposed to a single-component injury.
Renal ischemia-reperfusion (I/R) is a common cause of AKI. An ischemic insult occurs when there is reduced blood flow to the kidney and may occur after drug or toxicant exposure, vascular diseases, sepsis, or blood volume depletion and hypotension (2, 31). The pathophysiology of ischemic AKI is comprised of both microvascular and tubular components. The microvascular injury is characterized by increased vasoconstriction and decreased vasodilation, endothelial and smooth muscle cell damage, and leukocyte infiltration (2, 6, 29).
Rhabdomyolysis is a condition in which heme proteins, released in the form of myoglobin from muscle cells or hemoglobin from erythrocytes, produce secondary organ toxicity, predominantly AKI (3, 22). Although the mechanism of myoglobinuric-induced AKI is not entirely known, a number of factors including ischemic injury resulting from vasoconstriction and blood volume depletion as blood pools at the site of muscle injury, direct tubule toxicity from iron influx and hydroxyl radical formation, and tubular obstruction contributes to the disease process (3, 24, 25).
Subcellular injury to the tubular epithelium is a primary component of AKI, and although different injuries may manifest by particular mechanisms, common pathogenic elements are observed in multiple injury models. Mitochondrial damage is a major contributor to the lethal and sublethal tubular cell injury observed in the disease progression of AKI (10, 13, 15, 16, 23, 37). Increased production of reactive oxygen species and nitric oxide, formed prominently within the mitochondria, as well as compromised antioxidant mechanisms following ischemic periods make the mitochondria particularly susceptible (24, 25, 28, 43). Additionally, elevations in intracellular and mitochondrial Ca2+ and Fe3+ may contribute to the central role of the mitochondria in the disease process (8, 16). Subsequent disruption of mitochondrial respiratory complexes, membrane depolarization, ATP depletion, lipid peroxidation, membrane permeabilization, and release of apoptotic proteins contribute to mitochondrial and cellular injury (1–3, 13, 23).
The dynamic nature of mitochondria lends to dramatic alterations in structural integrity and population following acute toxic challenge. Mitochondrial fragmentation has been observed in models of AKI, and this process contributes to the resulting injury (4). However, the role of mitochondrial fission and fusion following initial injury and during the recovery and maintenance phase is still not completely understood. Mitochondrial biogenesis is initiated after acute organ injuries (27, 41), including cellular models of AKI (26). There is an immediate induction of the transcriptional co-activator peroxisome proliferator-activated receptor γ co-activator 1-α (PGC-1α) in experimental models of stroke, liver damage, heart failure, and neuromuscular disorders in response to the increased energy demand in such tissues (27, 36, 38, 41). PGC-1α is a primary regulator of mitochondrial biogenesis and is known to associate with transcription factors responsible for mitochondrial gene expression, including the nuclear respiratory factors (NRFs) and mitochondrial transcription factor A (Tfam) (40). There is significantly less known about other members of the PGC-1 family, PGC-1β and PGC-1-related co-activator (PRC), in acute injury models or in mitochondrial biogenesis, and the role of mitochondrial biogenesis during the acute injury phase and throughout sustained injury and recovery has not been fully established.
In this study, we asked how mitochondria would initially respond to an acute injury to the kidney and during recovery of function. There is evidence that the health of this organelle is a critical determinant in both injury progression and recovery in AKI, and there is a need to obtain a more complete understanding of mitochondria in this type of injury. We assessed several mitochondrial parameters in two rodent AKI models; examined expression of respiratory proteins, fission and fusion processes, and autophagy; and determined mitochondrial biogenesis at initiation of injury and throughout recovery of renal function. Previous studies examined each of these functions in various AKI models during onset of injury; however, this is the first study, to our knowledge, that encompasses multiple markers of mitochondrial homeostasis throughout an extended injury/recovery period in two distinct AKI models.
EXPERIMENTAL PROCEDURES
Glycerol model of myoglobinuric AKI.
Male Sprague-Dawley rats, 180–200 g, were injected with an equally divided hypertonic glycerol solution (50% glycerol/H2O, 10 ml/kg im) into the muscle of each hindlimb as previously described (42). Renal function was monitored as described below starting at 24 h postinjection until rats were euthanized at 24, 72, or 144 h after injections, at which time kidneys were harvested and snap-frozen for molecular analysis. All procedures involving animals were performed with approval from the Institutional Animal Care and Use Committee (IACUC) in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
I/R model of AKI.
Eight-week-old male C57BL6 mice weighing 25–30 g were subjected to bilateral renal pedicle ligation as described previously (44). Briefly, renal artery and vein were isolated and blood flow was occluded with a vascular clamp for 20 min, and mice were euthanized at 24, 72, or 144 h after procedure, at which time kidneys were harvested for molecular analysis. All procedures involving animals were performed with approval from the IACUC in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Assessing renal function.
Tail vein blood was collected from rats at various time points after glycerol injection, and serum was used to measure serum creatinine levels. For mice, blood was collected by retro-orbital eye bleed. Creatinine levels were measured using a Quantichrom Creatinine Assay Kit (BioAssay Systems, Hayward, CA) according to the manufacturer's protocol. Urine was collected from rats housed in metabolic cages overnight (16 h) at various time points throughout the study. Urine samples were used to determine creatinine levels (Quantichrom Creatinine Assay Kit, BioAssay Sytems), glucose (Quantichrom Glucose Assay Kit, BioAssay Systems), and neutrophil gelatinase-associated lipocalin (NGAL) levels (NGAL ELISA Kit, BioPorto) according to the manufacturers' instructions.
Immunoblot analysis.
Renal cortical tissue was lysed in RIPA buffer containing cocktail protease and phosphatase inhibitors. Total protein content was measured by the BCA assay. Fifty micrograms of total protein were loaded into SDS-PAGE gels and immunoblots were performed as previously described (26). Antibodies used for immunoblot studies were obtained from the following vendors: caspase 3 (Enzo Life Sciences), GAPDH (Fitzgerald Antibodies), ATP synthase β and LC3 (Abcam), COX I and NDUFB8 (MitoSciences), Drp1, NRF-1, and Tfam (Santa Cruz Biotechnology), Mfn2 (Sigma), and PGC-1α (EMD Biosciences).
mRNA analysis.
Total RNA was isolated from renal cortical tissue with TRIzol (Invitrogen) according to the manufacturer's protocol and cDNA was synthesized using RevertAid First Strand cDNA Synthesis Kit (Fermentas). PCR reactions were performed using 3 μl diluted cDNA product as described previously (12). Primer sets used for PCR are listed in Table 1.
Table 1.
Primer sequences used for qRT-PCR experiments
| Gene | Sequence |
|---|---|
| NDUFB8 | FW: GGC GAT CCC AAC AAA GAA CC |
| REV: TTT CTA GGA TTG AAG GAG TC | |
| ATP synthaseβ | FW: GAG ACC AAG AAG GTC AAG ATG |
| REV: GAA GGG ATT CGG CCC AAT GCA G | |
| ND6 | FW: AGG GCT TGG GTT GAT TGT TAG |
| REV: TGC CAT CGC AGT AGT ATA CCC | |
| COX I | FW: TAA TGT AAT CGT CAC CGC ACA |
| REV: ATG TCA GGA GCC CCA ATT ATC | |
| Drp1 | FW: CTG ACG CTT GTG GAT TTA CC |
| REV: CCC TTC CCA TCA ATA CAT CC | |
| Mfn2 | FW: ACG AGC AAT GGG AAG AGC AC |
| REV: TCC ATC AGC ACG AGG TCA TC | |
| PGC-1α | FW: AGG AAA TCC GAG CTG AGC TGA ACA |
| REV: GCA AGA AGG CGA CAC ATC GAA CAA | |
| PGC-1β | FW: TGG AAA GCC CCT GTG AGA GT |
| REV: TTG TAT GGA GGT GTG GTG GG | |
| PRC | FW: AGG AAA CTC AGG CAG CAT TG |
| REV: GGC CCT GGA TTT AGG AGA TT | |
| NRF-1 | FW: TCG GGC ATT TAT CCC AGA GAT GCT |
| REV: TAC GAG ATG GGC TAT GCT GTG TGT | |
| NRF-2α | FW: AGG TGA CGA GAT GGG CTG C |
| REV: CGT TGT CCC CAT TTT TGC G | |
| Tfam | FW: GAG CCG AAT CAT CCT TTG C |
| REV: GAG CCG AAT CAT CCT TTG C | |
| Tubulin | FW: CTC TCT GTC GAT TAC GGC AAG |
| REV: TGG TGA GGA TGG AGT TGT AGG |
COX I, cytochrome c oxidase subunit I; PGC-1α, peroxisome proliferator-activated receptor γ co-activator 1-α; PRC, PGC-1-related co-activator; NRF-1, nuclear respiratory factor-1; Tfam, mitochondrial transcription factor A; FW, forward; REV, reverse.
Immunohistochemistry.
Paraffin-embedded sections were cleared in xylenes and then rehydrated in a graded ethanol wash. Antigen unmasking was performed by boiling sections in citrate buffer for 10 min followed by cooling at room temperature for 30 min. Endogenous peroxidase activity was quenched by incubating sections in 3% H2O2 for 10 min. Sections were then blocked in 10% normal goat serum for 1 h, followed by COX IV (Abcam, 1:700 dilution) antibody incubation for 1 h. Sections were then incubated in biotinylated anti-rabbit secondary antibody for 30 min followed by horseradish peroxidase-linked avidin-biotin complex reagent (Vectastain) for 30 min. Finally, antibody detection was visualized by DAB peroxidase substrate developer (Vectastain), counterstained with hematoxylin, mounted, and coverslipped. Images were acquired with a Nikon microscope under control of QCapture imaging software. Low-magnification images are at ×10 and high-magnification images were captured at ×40.
ATP measurement.
Renal cortical tissue ATP was measured as previously described (35, 38). ATP was extracted from flash-frozen kidney cortex with 0.4 M HClO4, and ATP levels were determined using an ATP bioluminescence assay kit (Roche) and normalized to protein concentration.
Statistical analysis.
Graphs represent a sample size of three to six for each group. Data were analyzed by ANOVA based on ranks followed by the Mann-Whitney rank-sum test for individual group comparisons of nonparametric data.
RESULTS
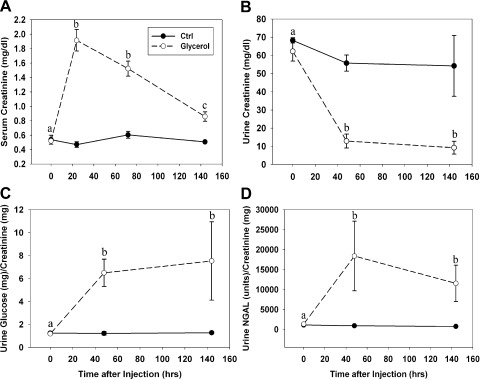
Rats exposed to intramuscular injection of glycerol exhibited kidney injury within 24 h as determined by an increase in serum creatinine (SCr) levels (Fig. 1A). At time of injection (t = 0 h), control and glycerol rats had similar SCr levels. SCr in glycerol-treated rats increased about fourfold at 24 h and then gradually decreased at 72 and 144 h without reaching control levels.
Fig. 1.
Renal dysfunction after glycerol-induced myoglobinuria. A: serum creatinine was maximal 24 h after injection and partially recovered between 24 and 144 h after injury without returning to normal levels. B: urine creatinine was reduced 48 h after injury and remained decreased at 144 h. Urine glucose (C) and neutrophil gelatinase-associated lipocalin (NGAL; D) were elevated 48 h after glycerol injection and remained elevated at 144 h. Different superscripts above data points are significantly different from one another (P < 0.05).
Although SCr measurements demonstrated functional recovery between 24 and 144 h after glycerol treatment, urinary measurements reveal sustained renal injury. Urinary creatinine concentrations were reduced 85% at 48 h after glycerol injection and remained decreased at 144 h after injury (Fig. 1B). Because glucose is freely filtered by the glomerulus and nearly 100% reabsorbed in the proximal tubule (39), urinary glucose is a marker of proximal tubular function. Urine glucose levels increased about sixfold at 48 h and remained at this level at 144 h after glycerol injection (Fig. 1C). NGAL is expressed in low abundance in proximal tubular cells, is upregulated and excreted into the urine after injury, and is now used as a biomarker of AKI (20). NGAL increased 40-fold at 48 h and remained elevated at 144 h following glycerol injection (Fig. 1D). Overall, these results reveal partial recovery of glomerular function (e.g., glomerular filtration) following AKI, but persistent proximal tubular dysfunction.
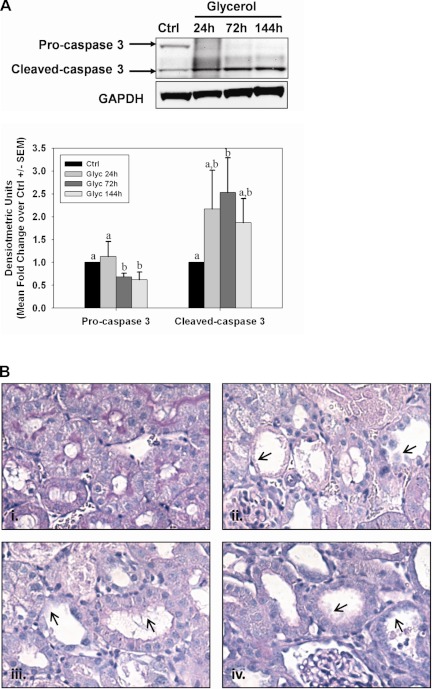
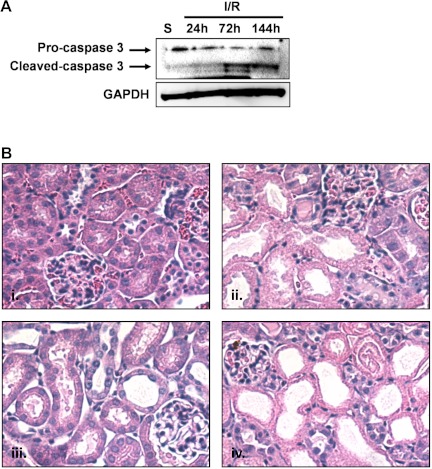
Apoptosis is known to be a primary mechanism of cell death in models of AKI. To evaluate apoptosis in our AKI models, we performed caspase-3 immunoblot analysis on renal cortical tissue collected at 24, 72, and 144 h after glycerol-induced AKI (Fig. 2A). Cleaved caspase 3 expression increased and remained elevated throughout the experimental period following glycerol injection, suggesting continued activation of apoptotic signaling. Additionally, histological evidence revealed persistent tubule dysfunction. Tissue structure was examined following Periodic acid-Schiff (PAS) staining, and it revealed proximal tubule dilation and brush-border damage at 24 h after glycerol that was sustained throughout the study (Fig. 2B).
Fig. 2.
Persistent tubule pathology after glycerol-induced acute kidney injury (AKI). A: activation of caspase 3 was observed by the presence of a caspase 3 cleavage fragment at 24, 72, and 144 h after glycerol injection. Bars with different superscripts are significantly different from one another (P < 0.05). B: periodic acid-Schiff (PAS) staining in control rats (i) and 24 h (ii), 72 h (iii), or 144 h (iv) after glycerol injection at ×40 magnification. Arrowheads indicate dilated tubules and brush-border damage after injury.
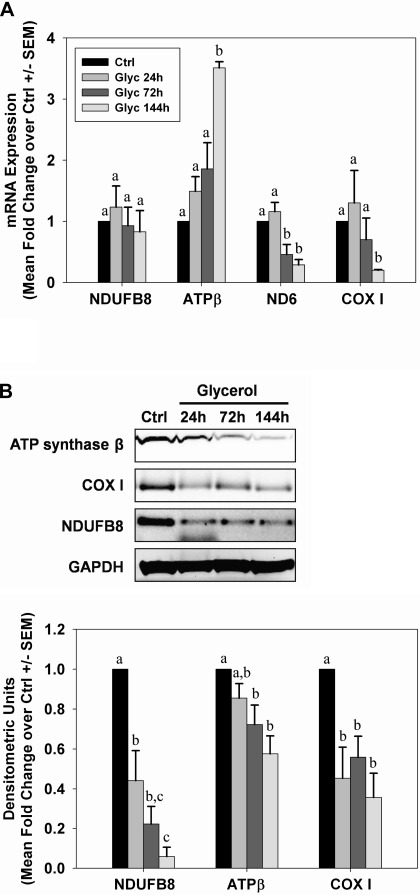
Transcript and protein levels of several mitochondrial respiratory genes were examined over time after AKI in renal cortical lysates. After glycerol-induced AKI, mRNA for the mitochondrial-encoded genes NADH-ubiquinone oxidoreductase chain 6 (ND6) and cytochrome c oxidase subunit I (COX I) was decreased between 72 and 144 h after injection (Fig. 3A). In contrast, mRNA expression for the nuclear-encoded mitochondrial protein NDUFB8 did not change at any time after injury, and expression of ATP synthase β increased 144 h after glycerol injection. Similar to mRNA levels, COX I protein decreased after injury, although this was seen much earlier than mRNA, and the decrease was maintained until 144 h postinjection (Fig. 3B). In contrast to the results observed for NDUFB8 and ATP synthase β mRNA, protein levels for these were reduced early after injury and remained decreased throughout the study period.
Fig. 3.
Sustained depletion of mitochondrial proteins after glycerol-mediated AKI. A: mRNA from control and glycerol rats was analyzed by qRT-PCR for expression of nuclear-encoded respiratory genes NDUFB8 and ATP synthase β and the mitochondrial-encoded genes ND6 and COX I at 24, 72, and 144 h after injury. B: expression of mitochondrial respiratory proteins from kidneys of control and glycerol rats was examined by immunoblot analysis. Bars with different superscripts are significantly different from one another (P < 0.05).
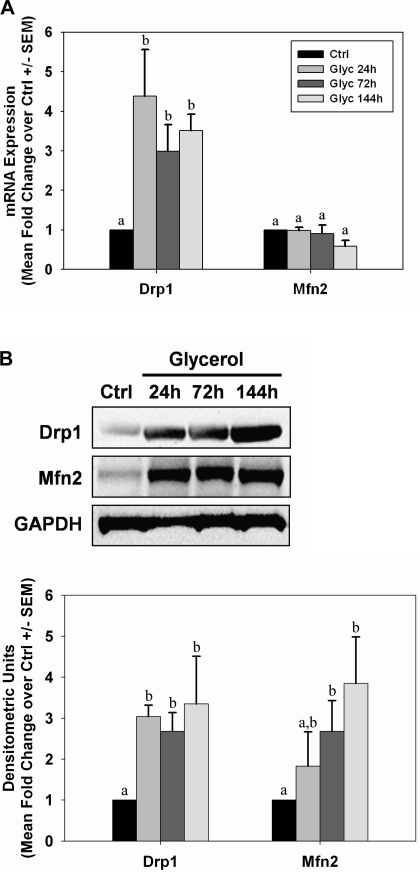
Because alterations in mitochondrial fission/fusion proteins can change in AKI models (4), we examined markers of fission/fusion over time in the glycerol model. Twenty-four hours after glycerol injection, renal dynamin-related protein (Drp1) mRNA was elevated approximately fourfold over control rats and remained at this level through 144 h (Fig. 4A). Correspondingly, Drp1 protein levels were also elevated early and remained elevated through 144 h (Fig. 4B). No changes were observed in mitofusin 2 (Mfn2) mRNA levels (Fig. 4A); however, Mfn2 protein increased 24 h after injection and remained elevated until 144 h (Fig. 4B).
Fig. 4.
Alterations in mitochondrial fission and fusion proteins after glycerol-mediated AKI. mRNA (A) and protein (B) expressions from kidneys of control (Ctrl) and glycerol (Glyc) rats were analyzed by qRT-PCR and immunoblot analysis for expression of Drp1 and Mfn2 at 24, 72, and 144 h after injury. Bars with different superscripts are significantly different from one another (P < 0.05).
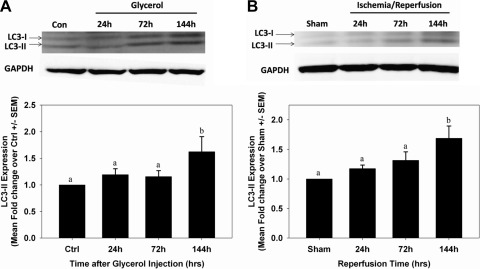
Autophagy has been observed in multiple acute injury models, including AKI (17), and has been intimately linked with induction of Drp1 expression and mitochondrial fission (34). Thus, we examined microtubule-associated protein light chain 3 (LC3)-I/II expression over time following glycerol. An increase in the autophagic marker LC3-II was not observed until 144 h (Fig. 5A). These results provide evidence that autophagy occurs late in the course of AKI.
Fig. 5.
Induction of autophagy after AKI. LC3-I/II protein expression was measured by immunoblot analysis in control and glycerol-treated rats (A) and sham and ischemia-reperfusion (I/R) mice (B). Bars with different superscripts are significantly different from one another (P < 0.05).
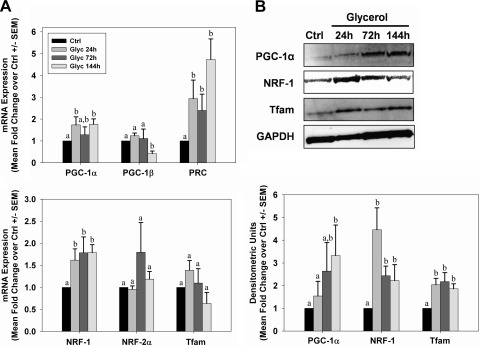
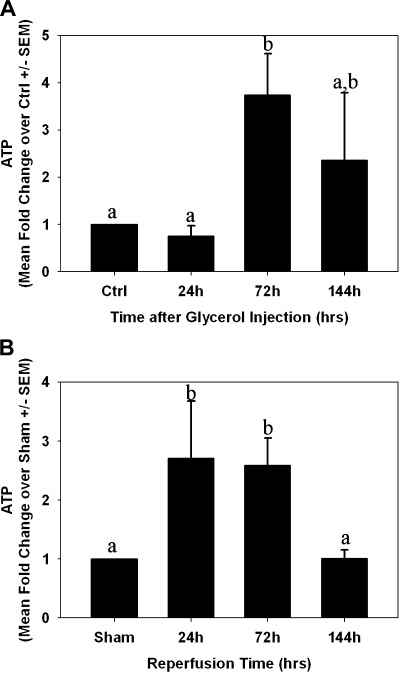
Initiation of mitochondrial biogenesis occurs in acute injury models (26, 27, 36, 41). We assessed expression of several known mediators of mitochondrial biogenesis by PCR and immunoblot analysis. PGC-1α, PRC, and NRF-1 mRNA levels were elevated within 24 h after glycerol injection, and they remained elevated throughout 144 h (Fig. 6A). PGC-1β decreased at 144 h and no changes were observed in NRF-2α or Tfam mRNA. Correspondingly, there was an elevation in the biogenic proteins PGC-1α, NRF-1, and Tfam after injury (Fig. 6B), and the elevation was sustained throughout the study period. Consistent with expression of biogenic proteins, but in contrast to reduced respiratory proteins, tissue ATP levels did not change at 24 h, increased at 72 h, and was not different from controls at 144 h (Fig. 7A).
Fig. 6.
Mitochondrial biogenesis after glycerol-mediated AKI. A: kidneys from control and glycerol-treated rats were analyzed for expression of genes associated with mitochondrial biogenesis by qRT-PCR. B: peroxisome proliferator-activated receptor γ co-activator 1-α (PGC-1α), nuclear respiratory factors (NRF-1), and mitochondrial transcription factor A (Tfam) protein expressions were examined in kidneys of control and glycerol-treated rats by immunoblot analysis. Bars with different superscripts are significantly different from one another (P < 0.05).
Fig. 7.
ATP levels after AKI. ATP was measured in flash-frozen kidney cortex from control/sham animals and at 24, 72, and 144 h after glycerol (A)- or I/R (B)-induced AKI. Bars with different superscripts are significantly different from one another (P < 0.05).
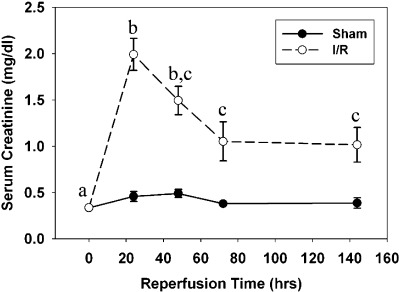
To determine whether the observed changes following myoglobinuric AKI were specific to the species and/or injury model used, we examined mitochondrial homeostasis markers in a mouse I/R injury model. Following a similar pattern to the rat myoglobinuria model, mice subjected to renal I/R exhibited kidney injury within 24 h after reperfusion, with a sevenfold rise in SCr levels at 24 h (Fig. 8). Recovery of SCr levels occurred over time but did not reach control levels at 144 h.
Fig. 8.
Kidney dysfunction after I/R injury. Serum creatinine levels were significantly elevated 24 h after reperfusion, and then slowly decreased between 24 and 144 h without returning to normal levels. Different superscripts above data points are significantly different from one another (P < 0.05).
Persistent apoptosis was also observed following I/R injury, where accumulation of cleaved caspase 3 was noted at 72 and 144 h (Fig. 9A). Histological evidence also revealed persistent tubule damage with proximal tubule dilation and brush-border damage at 24 h after I/R and that was sustained throughout the study (Fig. 9B).
Fig. 9.
Persistent tubule pathology after I/R AKI. A: activation of caspase 3 was observed by the presence of a caspase 3 cleavage fragment at 72 and 144 h after reperfusion. B: hematoxylin and eosin (H&E) staining in sham mice (i) and 24 h (ii), 72 h (iii), or 144 h (iv) after I/R at ×40 magnification.
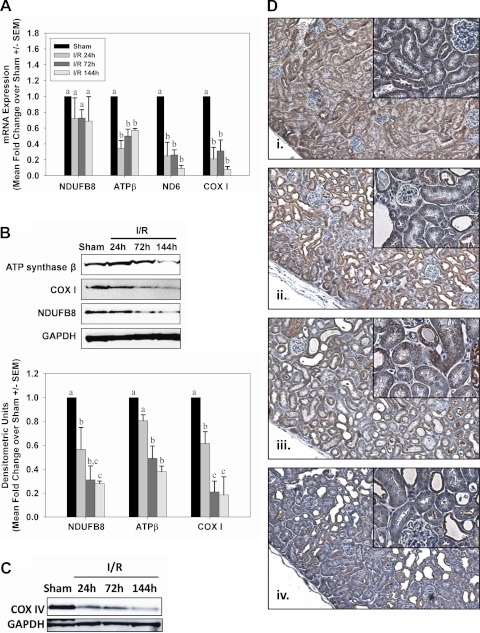
Twenty-four hours after I/R injury renal ND6, COX I, and ATP synthase β mRNA levels decreased and remained decreased at 72 and 144 h after injury (Fig. 10A). Immunoblot analysis on renal cortical lysates revealed that NDUFB8, ATP synthase β, and COX I protein levels decreased after I/R injury and did not recover over 144 h, similar to what was observed in the myoglobinuria model (Fig. 10B).
Fig. 10.
Sustained depletion of mitochondrial proteins after I/R AKI. A: mRNA from sham and I/R mice was analyzed by qRT-PCR for expression of nuclear-encoded respiratory genes NDUFB8 and ATP synthase β and the mitochondrial-encoded genes ND6 and COX I at 24, 72, and 144 h after injury. B: expression of mitochondrial respiratory proteins from kidneys of sham and I/R mice was examined by immunoblot analysis. Bars with different superscripts are significantly different from one another (P < 0.05). C: immunoblot analysis confirmed reduced COX IV protein expression in kidney cortex from mice 24, 72, and 144 h after I/R. D: COX IV immunohistochemistry (brown stain) in sham mice (i) or 24 h (ii), 72 h (iii), or 144 h (iv) after reperfusion in I/R mice, with hematoxylin counterstain. Low-magnification images were captured at ×10 and higher-magnification insets were captured at ×40.
Immunohistochemical analysis confirmed decreases in mitochondrial protein expression in proximal tubules of the kidney cortex. COX IV, which followed the same expression pattern as other respiratory proteins (Fig. 10, B and C), was localized throughout the kidney cortex, particularly the proximal tubule in sham animals (Fig. 10Di). Twenty-four hours after I/R, COX IV immunoreactivity was less intense and more diffuse, although most proximal tubules were still positive for COX IV protein expression (Fig. 10Dii). Immunoreactivity of COX IV within the kidney cortex, particularly with proximal tubules, became progressively less intense 72 and 144 h after injury (Fig. 10Diii, iv, inset).
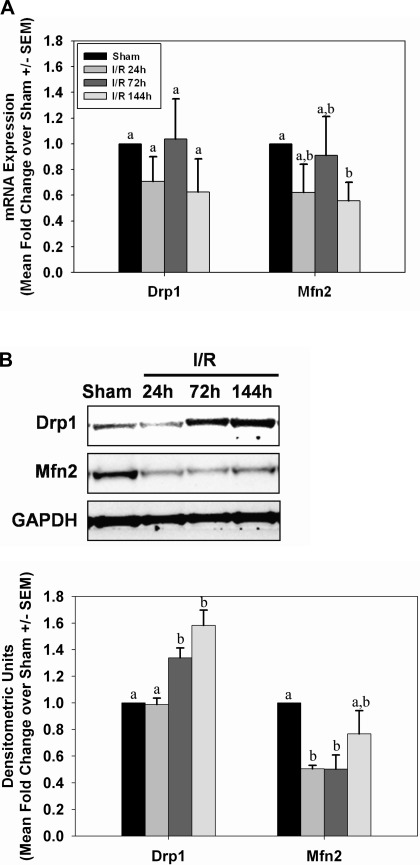
No changes in Drp1 or Mfn2 mRNA were detected in kidneys of mice subjected to I/R injury (Fig. 11A). However, immunoblot analysis revealed an increase in Drp1 protein at 72 and 144 h postreperfusion (Fig. 11B). Mfn2 protein decreased within 24 h and remained decreased at 72 h after I/R injury.
Fig. 11.
Alterations in mitochondrial fission and fusion proteins after I/R AKI. mRNA (A) and protein (B) expressions from kidneys of sham and I/R mice were analyzed by qRT-PCR and immunoblot analysis of Drp1 and Mfn2 at 24, 72, and 144 h after injury. Bars with different superscripts are significantly different from one another (P < 0.05).
Autophagy was measured by LC3-I/II expression over time after I/R (Fig. 5B). Similar to the findings observed in the myoglobinuric model, LC3-II expression increased at 144 h after reperfusion, again suggesting autophagy was active late in the injury/recovery process (Fig. 5B).
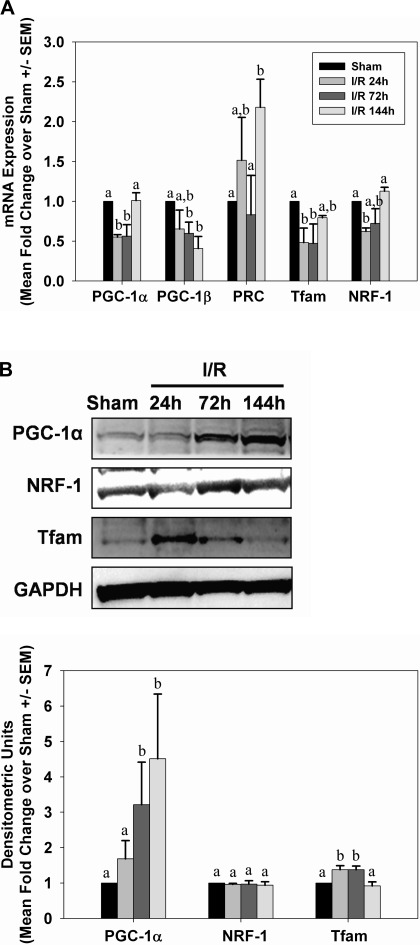
Following I/R injury, only PRC mRNA was elevated and sustained throughout the study period (Fig. 12A). PGC-1α, PGC-1β, Tfam, and NRF-1 mRNA tended to decrease slightly immediately after injury. In contrast to mRNA expression, protein levels of PGC-1α and Tfam increased after injury, but NRF-1 protein levels did not change (Fig. 12B). Similar to what was observed in the myoglobinuria model, tissue ATP levels were elevated 24 and 72 h after I/R injury and returned to control levels at 144 h (Fig. 7B). ATP was increased 24 and 72 h after reperfusion, but back to preinjury level at 144 h.
Fig. 12.
Mitochondrial biogenesis after I/R AKI. A: kidneys from sham and I/R mice were analyzed for mRNA expression of genes associated with mitochondrial biogenesis by qRT-PCR. B: PGC-1α, NRF-1, and Tfam protein expressions were examined by immunoblot analysis in kidneys from sham and I/R mice. Bars with different superscripts are significantly different from one another (P < 0.05).
DISCUSSION
In this study, we examined mitochondrial homeostasis in two different rodent AKI models. Previous studies have illustrated a central role of the mitochondria in acute injuries and provided evidence that the health of this organelle is a primary determinant in both the pathogenesis and recovery of organ function. However, many questions need to be answered in regards to duration of mitochondrial dysfunction, mechanisms of mitochondrial recovery, and influence of mitochondrial biogenesis on restoration of renal function. In the current study, we begin to address these questions by evaluating mitochondrial respiratory gene and protein expression, fusion and fission processes, autophagy, and biogenesis immediately after injury and during a 1-wk recovery period.
In the glycerol model, the levels of mitochondrial proteins (e.g., NDUFB8, ATP synthase β, COXI) decreased and remained decreased throughout the partial recovery of glomerular function. The loss of mitochondrial proteins would result in disruption of mitochondrial function previously demonstrated by Nath et al. (23) when they showed that renal mitochondrial respiration is disrupted early after glycerol injection (within 3 h) and is further diminished at 24 h after injury. The results presented here are consistent with the findings by Nath et al. and would further suggest that respiratory disruption persists as long as 144 h after injury. While mRNA for the mitochondrial-encoded genes ND6 and COX I was decreased after injury, transcript levels for the nuclear-encoded mitochondrial proteins NDUFB8 and ATP synthase β either did not change (NDUFB8) or were elevated after injury (ATP synthase β). This finding would suggest differential regulation of transcription occurring in the nucleus vs. the mitochondria following injury-induced mitochondrial protein degradation.
The upregulation of the mitochondrial biogenesis signaling molecules PGC-1α, PRC, and NRF-1 provides evidence of active transcription of nuclear-encoded mitochondrial proteins, whereas persistent pathological signals or damage may prevent active transcription of the mitochondrial genome. Similar to the glycerol model, mitochondrial proteins were decreased and remained decreased throughout the partial recovery of glomerular function after I/R. Immunohistochemical analysis following I/R confirmed the loss of COX IV in cortical proximal tubules after injury. However, following I/R-induced AKI, transcript levels of both nuclear- and mitochondrial-encoded proteins were depressed early after injury and throughout the recovery phase. These findings reveal differences in mitochondrial recovery signals in response to different inducers of AKI and that active transcription of mitochondrial-encoded genes is inhibited even in the presence of elevated PGC-1α and other biogenic factors.
Our results are consistent with previous reports of induction of PGC-1α and mitochondrial biogenesis in acute organ injuries (26, 36, 38, 41) and following oxidant injury in renal proximal tubular cells (25). Little information is available concerning the roles of other PGC-1 family members, PGC-1β and PRC, in mitochondrial biogenesis and following acute injury. PGC-1β mRNA expression did not change after glycerol or I/R injury; however, PRC mRNA was robustly elevated immediately after glycerol and I/R injury and remained elevated throughout the 1-wk recovery period. There were also elevations in NRF-1 and Tfam mRNA and/or protein, which are critical regulators of mitochondrial biogenesis (40). In summary, mitochondrial biogenesis is a component of the recovery phase after AKI, and PGC-1α actively participates in response to mitochondrial injury. The importance of the prominent response in PRC following AKI remains to be determined.
Initially, the observation that electron transport chain proteins were severely depleted early after injury and did not recover while SCr levels decreased toward control levels was inconsistent with our expectations that mitochondrial proteins would be restored as glomerular function recovered. The loss of COX IV in the proximal tubules and the loss of other mitochondrial proteins in the renal cortex were consistent with the sustained decrease in proximal tubular function; however, a direct causal relationship between loss of mitochondrial proteins and proximal tubular dysfunction cannot be established in the current study.
Although mitochondrial electron transport chain proteins remained decreased throughout the study period, ATP levels paralleled PGC-1α and did not decrease but increased above control levels. Previous studies demonstrated that ATP is reduced dramatically during ischemia, but is mostly recovered 24 h after reperfusion (14, 30, 33). Similarly, ATP is decreased within 1 h following glycerol-induced AKI (42). In both models, ATP was elevated at 72 h after injury and trended back to control levels at 144 h. The mechanism or source of higher than normal ATP after injury is still unclear, particularly under conditions of extended mitochondrial disruption and reduced oxidative phosphorylation proteins. Elevated nucleotide pools have been reported in plasma and red blood cells in human and rodent renal failure studies (9, 21) and it has been suggested that this may be the result of under excretion in the urine or from salvage pathways due to excessive accumulation of inorganic phosphate. Additionally, increased gluconeogenesis has been reported in proximal tubules isolated from rat kidneys 1–3 days after I/R that may compensate for an increased energy demand (18). It is not known whether any of these mechanisms contribute to the elevated renal ATP levels observed in the current study.
Following AKI, there were marked changes in mitochondrial fission and fusion protein expression. In the myoglobinuria model, both Drp1 and Mfn2 protein expression were elevated after injury and remained elevated throughout the recovery period. However, increased Drp1 protein levels were correlated with increased Drp1 mRNA while Mfn2 protein levels were not associated with increased Mfn2 mRNA, suggesting an altered posttranscriptional regulation. In contrast, in the I/R model elevated Drp1 protein expression was delayed until the recovery phase without any change in Drp1 mRNA expression throughout the study, suggesting there may be changes in protein degradation after I/R. Mfn2 protein levels decreased after injury and during recovery without a change in Mfn2 mRNA levels. These results reveal differences between the two AKI models with respect to mitochondrial fusion protein expression; however, fission protein Drp1 was elevated after injury in both models, suggesting that mitochondrial fission may be a more general process in AKI, whereas fusion protein expression may be more specifically regulated.
Induction of Drp1 protein and mitochondrial fragmentation has been reported previously in I/R AKI models and this process is a major contributor to injury progression. Brooks et al. (4) demonstrated that by inhibiting Drp1 either pharmacologically or by molecular techniques attenuated mitochondrial fragmentation, cytochrome c release, apoptosis, and kidney injury in both cellular and animal models of AKI. Both of the AKI models used in the current study are consistent with the results obtained by Brooks et al. and others, which demonstrated induction of Drp1 is correlated with caspase 3 cleavage and apoptosis (11, 19, 32). However, the current study also demonstrates alterations in the mitochondrial fusion protein Mfn2 following AKI, and the contribution of this protein to mitochondrial fragmentation and injury progression has not been fully evaluated and warrants additional attention in future studies.
Autophagy has been reported in a number of acute injury models, including AKI (17, 23). Induction of autophagy is thought to be crucial in the removal of damaged proteins and organelles (i.e., mitochondria) after injury, and blocking this response may prevent efficient cellular and organ recovery (17). Additionally, autophagy has been linked with changes in mitochondrial dynamics, which include selective mitochondrial fission, fusion, and sequestration in the course of removal of damaged mitochondria (34). It is interesting that this response was not upregulated until later in the recovery phase (72–144 h) in both models. The findings in the current manuscript contrast a previous study that demonstrated the presence of mitochondria in autophagic vacuoles 3 h after glycerol injection (23). The differences between the studies may simply be the result of differences in the sensitivity of the autophagy markers and/or the relative amount of autophagy occurring at a given time point. Widespread autophagy may not occur until later in the injury/recovery process, which was detectable by changes in LC3-II protein expression at 144 h after injection.
There is an overwhelming need to develop new treatment strategies for AKI as there are currently no methods to improve renal function, but rather only procedures to prevent further damage and to maintain functional output, such as dialysis. Mitochondria undergo significant alterations following AKI and influence the pathophysiology as well as recovery of organ function during and after injury. We demonstrated that persistent mitochondrial dysfunction occurs within damaged proximal tubules after AKI and may contribute to the sustained injury observed within these structures. This phenomenon occurs even in the process of active repair signals and during improved glomerular function. Additionally, persistent mitochondrial dysfunction may also lead to chronic deficiencies in cell and organ function similar to disorders of the heart, brain, and kidney that are known to be associated with mitochondrial disease. As such, strategic development of methods to improve mitochondrial functions, i.e., mitochondrial biogenesis, following injury may offer unique therapeutic targets for the treatment of AKI.
GRANTS
This study was supported by National Institutes of Health (NIH) Grant GM 084147, the NIH/National Institute of Environmental Health Sciences Training Program in Environmental Stress Signaling T32ES012878-05 and NIH/National Heart, Lung, and Blood Institute Training to Improve Cardiovascular Therapies T32HL007260-34, and by the Biomedical Laboratory Research and Development Program of the Department of Veterans Affairs. Animal facilities were funded by NIH Grant C06 RR-015455. This publication was supported, in part, by the South Carolina Clinical and Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina CTSA, NIH/NCRR Grant UL1RR029882.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
The contents of this manuscript do not represent the views of the Department of Veteran Affairs or the United States Government.
AUTHOR CONTRIBUTIONS
Author contributions: J.A.F. and R.G.S. conception and design of research; J.A.F. performed experiments; J.A.F. and R.G.S. analyzed data; J.A.F. and R.G.S. interpreted results of experiments; J.A.F. prepared figures; J.A.F. drafted manuscript; J.A.F. and R.G.S. edited and revised manuscript; J.A.F. and R.G.S. approved final version of manuscript.
REFERENCES
- 1. Bonventre JV. Mechanisms of ischemic acute renal failure. Kidney Int 43: 1160–1178, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med 361: 62–72, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119: 1275–1285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cameron JS. Allergic interstitial nephritis: clinical features and pathogenesis. Q J Med 66: 97–115, 1988 [PubMed] [Google Scholar]
- 6. Conger JD, Weil JV. Abnormal vascular function following ischemia-reperfusion injury. J Investig Med 43: 431–442, 1995 [PubMed] [Google Scholar]
- 7. Cooper K, Bennett WM. Nephrotoxicity of common drugs used in clinical practice. Arch Intern Med 147: 1213–1218, 1987 [PubMed] [Google Scholar]
- 8. Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J 341: 233–249, 1999 [PMC free article] [PubMed] [Google Scholar]
- 9. Dean BM, Sensi M, Perrett D. Elevation of rat erythrocyte nucleotide levels following acute renal failure induced by glycerol or mercuric chloride. Nephron 22: 538–543, 1978 [DOI] [PubMed] [Google Scholar]
- 10. Feldkamp T, Kribben A, Weinberg JM. Assessment of mitochondrial membrane potential in proximal tubules after hypoxia-reoxygenation. Am J Physiol Renal Physiol 288: F1092–F1102, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1: 515–525, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Funk JA, Odejinmi S, Schnellmann RG. SRT1720 induces mitochondrial biogenesis and rescues mitochondrial function after oxidant injury in renal proximal tubule cells. J Pharmacol Exp Ther 333: 593–601, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hall AM, Unwin RJ. The not so “mighty chondrion”: emergence of renal diseases due to mitochondrial dysfunction. Nephron Physiol 105: p1–10, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Irazu CE, Ruidera E, Singh I, Orak JK, Fitts CT, Rajagopalan PR. Effect of ischemia and 24 hour reperfusion on ATP synthesis in the rat kidney. J Exp Pathol 4: 29–36, 1989 [PubMed] [Google Scholar]
- 15. Jassem W, Fuggle SV, Rela M, Koo DD, Heaton ND. The role of mitochondria in ischemia/reperfusion injury. Transplantation 73: 493–499, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Jassem W, Heaton ND. The role of mitochondria in ischemia/reperfusion injury in organ transplantation. Kidney Int 66: 514–517, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Kimura T, Takabatake Y, Takahashi A, Kaimori JY, Matsui I, Namba T, Kitamura H, Niimura F, Matsusaka T, Soga T, Rakugi H, Isaka Y. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol 22: 902–913, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kondou I, Nakada J, Hishinuma H, Masuda F, Machida T, Endou H. Alterations of gluconeogenesis by ischemic renal injury in rats. Ren Fail 14: 479–483, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell 15: 5001–5011, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Moss AH, Solomons CC, Alfrey AC. Elevated plasma adenine nucleotide levels in chronic renal failure and their possible significance. Proc Clin Dial Transplant Forum 9: 184–188, 1979 [PubMed] [Google Scholar]
- 22. Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest 90: 267–270, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nath KA, Grande JP, Croatt AJ, Likely S, Hebbel RP, Enright H. Intracellular targets in heme protein-induced renal injury. Kidney Int 53: 100–111, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Plotnikov EY, Kazachenko AV, Vyssokikh MY, Vasileva AK, Tcvirkun DV, Isaev NK, Kirpatovsky VI, Zorov DB. The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney Int 72: 1493–1502, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Plotnikov EY, Chupyrkina AA, Pevzner IB, Isaev NK, Zorov DB. Myoglobin causes oxidative stress, increase of NO production and dysfunction of kidney's mitochondria. Biochim Biophys Acta 1792: 796–803, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Rasbach KA, Schnellmann RG. Signaling of mitochondrial biogenesis following oxidant injury. J Biol Chem 282: 2355–2362, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Rimbaud S, Garnier A, Ventura-Clapier R. Mitochondrial biogenesis in cardiac pathophysiology. Pharmacol Rep 61: 131–138, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Shah SV, Walker PD. Evidence suggesting a role for hydroxyl radical in glycerol-induced acute renal failure. Am J Physiol Renal Fluid Electrolyte Physiol 255: F438–F443, 1988 [DOI] [PubMed] [Google Scholar]
- 29. Solez K, Kramer EC, Fox JA, Heptinstall RH. Medullary plasma flow and intravascular leukocyte accumulation in acute renal failure. Kidney Int 6: 24–37, 1974 [DOI] [PubMed] [Google Scholar]
- 30. Stromski ME, Cooper K, Thulin G, Gaudio KM, Siegel NJ, Shulman RG. Chemical and functional correlates of postischemic renal ATP levels. Proc Natl Acad Sci USA 83: 6142–6145, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med 334: 1448–1460, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Tian C, Murrin LC, Zheng JC. Mitochondrial fragmentation is involved in methamphetamine-induced cell death in rat hippocampal neural progenitor cells. PLos One 4: e5546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Trifillis AL, Kahng MW, Cowley RA, Trump BF. Metabolic studies of postischemic acute renal failure in the rat. Exp Mol Pathol 40: 155–168, 1984 [DOI] [PubMed] [Google Scholar]
- 34. Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27: 433–446, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vives-Bauza C, Yang L, Manfredi G. Assay of mitochondrial ATP synthesis in animal cells and tissues. Methods Cell Biol 80: 155–171, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Wang H, Peiris TH, Mowery A, Le Lay J, Gao Y, Greenbaum LE. CCAAT/enhancer binding protein-beta is a transcriptional regulator of peroxisome-proliferator-activated receptor-gamma coactivator-1alpha in the regenerating liver. Mol Endocrinol 22: 1596–1605, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci USA 97: 2826–2831, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wenz T, Diaz F, Spiegelman BM, Moraes CT. Activation of the PPAR/PGC-1alpha pathway prevents a bioenergetic deficit and effectively improves a mitochondrial myopathy phenotype. Cell Metab 8: 249–256, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39. Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med 261: 32–43, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Yin W, Signore AP, Iwai M, Cao G, Gao Y, Chen J. Rapidly increased neuronal mitochondrial biogenesis after hypoxic-ischemic brain injury. Stroke 39: 3057–3063, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zager RA, Foerder C, Bredl C. The influence of mannitol on myoglobinuric acute renal failure: functional, biochemical, and morphological assessments. J Am Soc Nephrol 2: 848–855, 1991 [DOI] [PubMed] [Google Scholar]
- 43. Zager RA. Mitochondrial free radical production induces lipid peroxidation during myohemoglobinuria. Kidney Int 49: 741–751, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Zhuang S, Lu B, Daubert RA, Chavin KD, Wang L, Schnellmann RG. Suramin promotes recovery from renal ischemia/reperfusion injury in mice. Kidney Int 75: 304–311, 2009 [DOI] [PubMed] [Google Scholar]