Abstract
Purpose
Sapacitabine is an oral deoxycytidine nucleoside analog with a unique mechanism of action that is different from cytarabine.
Patients and Methods
To define the dose-limiting toxicities (DLT) and maximum-tolerated dose (MTD) of sapacitabine given orally twice daily for 7 days every 3 to 4 weeks, or twice daily for 3 days for 2 weeks (days 1 through 3 and days 8 through 10) every 3 to 4 weeks, in refractory-relapse acute leukemia and myelodysplastic syndrome (MDS). A total of 47 patients were treated in the phase I study that used a classical 3 + 3 design. Sapacitabine was escalated from 75 to 375 mg twice daily for 7 days (n = 35) and from 375 to 475 mg twice daily for 3 days on days 1 through 3 and days 8 through 10.
Results
The DLTs with both schedules were gastrointestinal. The MTDs were 375 mg twice daily for 7 days and 425 mg twice daily for 3 days on days 1 through 3 and days 8 through 10. The recommended phase II single-agent dose schedules were 325 mg twice daily for 7 days and 425 mg twice daily for 3 days on days 1 through 3 and days 8 through 10. Responses were observed in 13 patients (28%); four were complete responses, and nine were marrow complete responses.
Conclusion
Sapacitabine is a new, safely administered, oral deoxycytidine analog that has encouraging activity in leukemia and MDS. Phase II studies are ongoing.
INTRODUCTION
Despite recent progress in acute myeloid leukemia (AML), modern combination-chemotherapy regimens result in complete response (CR) rates of 40% to 70% and long-term event-free survival rates of only 20% to 50%, depending on patient ages, comorbid conditions, and leukemia karyotypes.1,2 Similar results are noted in adult acute lymphocytic leukemia (ALL), for which long-term event-free survival rates are only 30% to 40%.3,4 In myelodysplastic syndrome (MDS), several recent studies with azacitidine, decitabine, and lenalidomide have shown encouraging results.5–7 However, the median survival remains short, in the range of 2 to 3 years. These findings indicate the need to discover new strategies that might improve the outcomes in leukemia and MDS. Nucleoside analogs represent a major group of antitumor cytotoxic agents. Several agents in this class have shown activity in leukemia and MDS. These include deoxyadenosine analogs, such as fludarabine, deoxycoformycin, chlorodeoxyadenosine, and clofarabine,8,9 or cytosine nucleoside analogs, such as cytarabine,1,2 azacitidine, and decitabine.
2′-C-Cyano-2′-deoxy-β-D-arabino-pentofur anosylcytosine (CNDAC) is a rationally designed analog of deoxycytidine with a unique mechanism of action.10 On phosphorylation to the triphosphate and incorporation into DNA, replication is not inhibited at cytotoxic concentrations, unlike cytarabine, fludarabine, or clofarabine. Rather, after additional polymerization, the strong electrophilic properties of the cyano group cause a rearrangement of the nucleotide to a form that lacks a 3′-hydroxyl moiety.11,12 This results in a single-strand break that is repaired to only a small extent by the transcription-coupled nucleotide excision pathway.13 On a subsequent round of DNA replication, unrepaired single-strand breaks are converted to double-strand breaks, which leads to cell death.14
During the discovery phase, many derivatives of CNDAC were studied. Sapacitabine (previously designated CS-682), an N4-palmitoyl derivative of CNDAC, was chosen for clinical development because of its broad range of antitumor activity in preclinical studies.11 The palmitoyl side chain on CNDAC allows for improved oral absorption of sapacitabine and protects the N4 amino group from deamination, which is a major route of inactivation for this class of molecule.11 After oral administration, sapacitabine is converted to CNDAC by amidases and esterases in the gastrointestinal tract, plasma, and liver, and it becomes bioavailable15,16 at concentrations that reduce clonogenic survival of cells in vitro.13
CNDAC has shown positive preclinical activity in a broad spectrum of human tumor cells, including leukemic cell lines. The phase I studies of single-agent sapacitabine in solid tumors identified myelosuppression as the dose-limiting toxicity (DLT).15,16 This is generally a favorable feature for agents that ultimately demonstrate activity in leukemia at three-fold to 20-fold of the maximum-tolerated doses (MTDs) observed in solid tumors. These findings, together with the oral bioavailability of sapacitabine, led to the interest in developing it for hematologic malignancies.
PATIENTS AND METHODS
Patient Eligibility
Adults with relapsed or refractory acute leukemia, MDS, or untreated disease who were unwilling to proceed with conventional systemic chemotherapy were eligible. Other eligibility criteria included age of 18 years or older; Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2; adequate hepatic (ie, bilirubin ≤ 1.5 mg/dL; ALT ≤ 2.5 × upper limit of normal [ULN] or ≤ five times ULN if hepatic abnormality related to leukemia) and renal functions (ie, creatinine ≤ 2.0 mg/dL or creatinine clearance > 60 mL/min); no prior chemotherapy, radiation therapy, or investigational anticancer therapy in the previous 2 weeks; absence of CNS involvement by leukemia; and absence of uncontrolled intercurrent illnesses, including infections, cardiac conditions, or other organ dysfunctions. All patients signed an informed consent form according to institutional guidelines and in compliance with the Declaration of Helsinki.
Study Design and Treatment Plan
This study used a classical 3 + 3, phase I design. The starting dosage of sapacitabine was 75 mg orally twice daily (to total 150 mg daily) for 7 consecutive days every 3 to 4 weeks on the basis of safety data from phase I trials in solid tumors. The timing of the subsequent cycles of treatment varied depending on the disease status and toxicities. The dose was escalated to 100, 125, 175, 225, 275, 325, and 375 mg orally twice daily. The dose level of 100 mg was entered with only one patient and was skipped to 125 mg because of the safety data that emerged from a phase I trial in solid tumors. After the DLT and MTD were defined with the twice daily dosage schedule for 7 days, a second dosing schedule of twice daily oral sapacitabine for 3 consecutive days weekly for 2 weeks, every 3 to 4 weeks, was implemented. Dose levels evaluated on this second dosing schedule were 375, 425, and 475 mg twice daily.
DLT and Dose Escalation Rules
A DLT was defined as the occurrence of any clinically significant and drug-related, grades 3 to 4, nonhematologic toxicity despite maximum supportive care, or prolonged pancytopenia in the presence of a hypocellular bone marrow (ie, cellularity 5% or fewer without evidence of leukemia) that lasted longer than 42 days from the start of therapy. Three patients were entered at each dose level. If no DLT was observed, patients were treated at the next-higher dose level. If one DLT was observed, three more patients were treated at that dose level. MTD was defined as the dose that produced grades 3 to 4 toxicity in at least two of six patients treated. The recommended dose for future phase II studies would be the dose level immediately before the MTD. The duration of therapy was indefinite as long as there was benefit to the patient.
Response and Toxicity Criteria and Statistical Methods
Response criteria were standard.17,18 Complete remission (CR) in patients with AML or ALL was defined as normalization of the blood and bone marrow with 5% or fewer blasts in normocellular or hypercellular bone marrow, a granulocyte count of 109/L or greater, and a platelet count of 100 × 109/L or greater. A partial response (PR) was defined as the same as CR but with at least a 50% decrease to 6% to 25% marrow blasts. A CR without platelet count recovery (CRp) was defined as the same as CR but without platelets recovery to 100 × 109/L or greater. A marrow CR (CRi) was defined as the same as CR but without granulocytes recovery to 109/L and/or platelets recovery to 100 × 109/L or greater. Response in MDS was defined according to the modified International Working Group (IWG) criteria.18
Toxicity was graded on a scale of 0 to 5 by using the National Cancer Institute Common Terminology Criteria of Adverse Events (NCI-CTCAE) version 3.0.19 The 4-week mortality (from start of sapacitabine) was calculated as a combined measure of the drug efficacy and safety, regardless of a causal relationship to the study drug. Survival and response durations were analyzed by the Kaplan-Meier method.
Clinical Pharmacology
Patients who consented to participate in pharmacokinetic (PK) and pharmacodynamic studies were instructed to fast (ie, no food or drink except water) after midnight before the morning of day 1 of cycle 1. The dose of sapacitabine was administered about 1 hour before breakfast. Peripheral blood samples were taken predose (time 0) and at 1, 2, 4, 6, 8, and 24 hours after the dose of sapacitabine was given. The 24-hour time point for day 1 was obtained in the morning of day 2 before the patient took the second dose of sapacitabine. All blood samples (10 mL) were collected in green stopper vacutainer tubes coated with heparin and containing 400 μmol/L tetrahydrouridine (THU), the latter of which was added to inhibit deamination of CNDAC by cytidine deaminase (CDA). Immediately after collection, the blood samples were chilled in an ice-water bath, and plasma was separated from peripheral-blood mononuclear cells (PBMCs) by centrifugation at 4°C. Plasma samples were stored at −70°C, and cell pellets were processed additionally for pharmacodynamic analysis.
Plasma Pharmacology
Plasmas samples were spiked with radioactive-labeled sapacitabine (carbon-13 nitrogen-15 [13C15N4]) and CNDAC (carbon-13 nitrogen15 [13C15N4]), as the internal standards and levels of sapacitabine and CNDAC were determined by a validated high-performance liquid chromatography and tandem mass spectrometric detection (LC/MS/MS) assay. The lower limits of quantification (LOQs) for sapacitabine and CNDAC were 0.2 ng/mL and 1 ng/mL, respectively. Actual sampling times were used in all PK analyses. The maximum plasma concentration (Cmax) and time to Cmax (Tmax) were taken directly from the data. Because of the lack of a log-linear decay during the 8-hour sampling period, no estimate could be made for the elimination rate constant, λz, and half-life (t1/2). Area under the curve from zero to the final sample with a concentration at LOQ or greater (AUC0-t) was calculated by using the linear trapezoidal method. All PK calculations were done and all individual patient plasma concentration–time graphs were prepared by using SAS for Windows version 9.1.3 (SAS Institute, Cary, NC). Graphs of mean plasma concentration versus time were prepared by using SigmaPlot for Windows version 11.0.
Cellular Pharmacology
Cell pellets collected from blood samples were washed with phosphate-buffered saline (PBS). PBMCs were isolated by Ficoll-Hypaque density-gradient centrifugation, were washed with PBS, and were counted with an electronic particle counter. Approximately 1 to 2 × 107 cells were used to extract nucleotides, and the nucleotides were separated by strong anion-exchange high-performance liquid chromatography and were quantitated by using ultraviolet absorbance with reference to standards.20 The remaining cells, if available, were incubated with 1.0 μCi hydrogen-3–thymidine for 30 minutes to determine DNA synthesis in triplicates of 2 × 106 cells.
RESULTS
Patient Characteristics and Treatment Administration
A total of 47 patients were treated. The characteristics are listed in Table 1. The median age was 65 years (range, 35 to 90 years); 17 patients (36%) were women. Forty-two patients had AML, one had ALL, and four had MDS (Table 1). Seven patients with AML had received no prior chemotherapy. The median number of treatment cycles was three (range, one to 16 cycles). Five patients completed six or more treatment cycles, including one patient who completed 16 cycles.
Table 1.
Baseline Demographic and Clinical Characteristics of the Study Group
| Characteristic | Patients (N = 47) |
|
|---|---|---|
| No. | % | |
| Diagnosis | ||
| AML | 42 | 89 |
| MDS | 4 | 9 |
| ALL | 1 | 2 |
| ECOG performance status | ||
| 0 | 9 | 19 |
| 1 | 33 | 70 |
| 2 | 5 | 11 |
| Treatment status* | ||
| Frontline | 7 | 17 |
| Salvage 1 | 7 | 17 |
| Salvage 2 | 15 | 36 |
| Salvage 3 | 4 | 9 |
| Salvage 4 or more | 10 | 23 |
| Age, years | ||
| Median | 65 | |
| Range | 35-90 | |
| Prior intensive chemotherapy* | 32 | 74 |
| Karyotype | ||
| Diploid metaphases | 12 | 26 |
| Chromosome 5 or 7 abnormalities | 14 | 30 |
| Non-complex abnormalities (< 3) | 7 | 15 |
| 8± other | 5 | 11 |
| Complex abnormalities (≥ 3) | 5 | 11 |
| >Insufficient metaphases | 4 | 9 |
| Hemoglobin, g/dL | ||
| Median | 9.6 | |
| Range | 7.7-14.3 | |
| WBC, ×109/L | ||
| Median | 3.0 | |
| Range | 0.3-119.5 | |
| Platelets, ×109/L | ||
| Median | 32 | |
| Range | 5-353 | |
| Marrow blasts, % | ||
| Median | 40 | |
| Range | 1-88 | |
| Lactic dehydrogenase, U/L | ||
| Median | 564 | |
| Range | 277-3,134 | |
Abbreviations: AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; ALL, acute lymphocytic leukemia; ECOG, Eastern Cooperative Oncology Group.
Acute leukemia only (n = 43).
DLTs and MTDs
The number of patients treated at each dose level and the observed DLTs are listed in Table 2. Drug-associated gastrointestinal adverse effects were dose limiting at the sapacitabine dose level of 375 mg orally twice daily for 7 days; this level included one drug-related death as a result of neutropenic colitis. The dose schedule of sapacitabine chosen for phase II studies was 325 mg orally twice daily for 7 days; this schedule could be given frequently (eg, every 3 to 4 weeks), in the outpatient setting, with minimal extramedullary (gastrointestinal) or medullary toxicity that may provide better control of the leukemia. After the completion of investigation of the 7-day schedule, patients received sapacitabine at 375, 425, and 475 mg orally twice daily for 3 days, weekly for 2 weeks (days 1 through 3 and days 8 through 10). Again, gastrointestinal toxicity was dose limiting at the sapacitabine dose of 475 mg orally twice daily. Although only one patient experienced the DLT of grade 3 diarrhea at this dose level, a decision was made not to expand this dose level because of significant thrombocytopenia observed in another patient who had a decrease in platelet count from normal to less than 10 × 109/L during the first treatment cycle. The phase II recommended dose with this schedule was 425 mg orally twice daily on days 1 to 3 weekly for 2 weeks.
Table 2.
Dose Escalations and Dose-Limiting Toxicities
| Dose level, mg | No. of Patients | Dose-Limiting Toxicity |
|---|---|---|
| Twice daily × 7 days | 35 | |
| 75 | 3 | None |
| 100 | 1 | None |
| 125 | 3 | None |
| 175 | 3 | None |
| 225 | 3 | None |
| 275 | 6 | Diarrhea and neutropenic colitis, grade 3* |
| 325† | 9 | Diarrhea, grade 3* |
| 375 | 7 | Abdominal pain and small-bowel obstruction, grade 3*; neutropenic colitis, death* |
| Twice daily for 3 d/wk × 2 weeks | 12 | |
| 375 | 3 | 0 |
| 425† | 6 | 0 |
| 475 | 3 | Diarrhea, grade 3† |
No. of patients = 1.
Recommended phase II dose.
Safety and Tolerability
The most common drug-related adverse events (Table 3) were constitutional (fatigue), gastrointestinal (anorexia, abdominal pain, diarrhea, nausea, and vomiting), and hematologic (neutropenia and thrombocytopenia). Myelosuppression-associated complications in the first course included febrile episodes in 14 patients (30%). These consisted of fever of unknown origin in nine patients (19%) and infections in five patients (11%). Two deaths occurred within 4 weeks of start of therapy. The first death was a 75-year-old man with AML who was treated at the dose level of 375 mg twice daily for 7 days every 21 days. On day 12 of treatment cycle 1, he was admitted to the hospital with abdominal pains and distention, nonbloody diarrhea, and neutropenic fever. No peritoneal signs were present by physical exam. Abdominal CT scan showed no bowel obstruction or free air, but there were slightly prominent loops of small bowel with significant bowel wall thickening. The clinical suspicion was neutropenic colitis. The patient was treated with broad-spectrum antibiotics and bowel rest. Despite aggressive treatment, he developed hypotension, pulmonary insufficiency, and acidosis, and he died on day 22. The death was considered definitely related to the study drug. The second death occurred in a 51-year-old man with refractory AML who died on day 27 with persistent AML, pneumonia, and multiorgan failure.
Table 3.
Patients With Drug-Related Toxicity by Dosing Schedule
| Toxicity | No. of Patients by Dosage Regimen and Toxicity Grade |
|||
|---|---|---|---|---|
| Twice Daily × 7 Days (n = 35) |
Twice Daily × 3 d/wk × 2 Weeks (n = 12) |
|||
| Grades 1-2 | Grades 3-4 | Grades 1-2 | Grades 3-4 | |
| Hematologic | ||||
| Anemia | 0 | 1 | 1 | 0 |
| Leukopenia | 1 | 6 | 0 | 7 |
| Neutropenia | 0 | 5 | 0 | 4 |
| Thrombocytopenia | 1 | 1 | 1 | 7 |
| Nonhematologic | ||||
| Abdominal pain | 4 | 1 | 0 | 0 |
| Constipation | 2 | 0 | 0 | 0 |
| Diarrhea | 9 | 1 | 1 | 1 |
| Nausea | 13 | 0 | 6 | 0 |
| Neutropenic colitis | 0 | 2* | 0 | 0 |
| Small bowel obstruction | 0 | 1 | 0 | 0 |
| Stomatitis | 2 | 0 | 1 | 0 |
| Vomiting | 5 | 0 | 2 | 0 |
| Oral mucosal petechiae | 0 | 0 | 1 | 0 |
| Mucosal hemorrhage | 0 | 0 | 1 | 0 |
| Mucosal inflammation | 1 | 0 | 0 | 0 |
| Asthenia | 2 | 0 | 0 | 0 |
| Fatigue | 6 | 0 | 4 | 0 |
| Increase in ALT | 1 | 0 | 0 | 0 |
| Increase in alkaline phosphatase | 1 | 0 | 0 | 0 |
| Anorexia | 2 | 0 | 1 | 0 |
| Decreased in appetite | 1 | 0 | 0 | 0 |
| Dysgeusia | 0 | 0 | 1 | 0 |
| Dyspnea | 1 | 0 | 0 | 0 |
| Hiccups | 1 | 0 | 0 | 0 |
| Alopecia | 8 | 0 | 1 | 0 |
| Petechiae | 1 | 0 | 1 | 0 |
One patient died as a result of neutropenic colitis.
PKs
PK data were available from 10 patients who received doses of 125 mg (n = 2), 325 mg (n = 4), 375 mg (n = 1), 425 mg (n = 2), and 475 mg (n = 1) twice daily (Table 4). The median Tmax of plasma sapacitabine for all courses was 120 minutes (range, 60 to 360 minutes), and it was similar for CNDAC (median, 120 minutes; range, 60 to 480 minutes). There was no apparent dose-related change in the individual patient plasma concentrations of sapacitabine or CNDAC on either day 1 or day 8. Similarly, there was no apparent relationship between either Cmax or AUC0-t and dose for either analyte. Although there was an apparent trend toward an increase in Cmax and AUC0-t for sapacitabine, the relationships were not significant.
Table 4.
Summary of Pharmacokinetic Parameters for Sapacitabine and CNDAC
| Parameter | Dose (mg) |
||||
|---|---|---|---|---|---|
| 125 | 325 | 375 | 425 | 475 | |
| Sapacitabine* | |||||
| Cycle 1, day 1 | 3.16 | 35.10 | 14.90 | 35.30 | 2.77 |
| Cmax, ng/mL | 2.00 | 2.14 | 15.60 | ||
| 5.79 | |||||
| 27.50 | |||||
| Tmax, minutes | 250 | 135 | 119 | 360 | 240 |
| 380 | 65 | 120 | |||
| 65 | |||||
| 120 | |||||
| AUC(0-t), min × ng/mL | 755.5 | 16,463 | 1,671 | 9,160 | 536 |
| 406.7 | 181.5 | 2,629 | |||
| 903 | |||||
| 3,529 | |||||
| Cycle 1, day 8 | |||||
| Cmax, ng/mL | 5.43 | 8.88 | |||
| Tmax, minutes | 240 | 360 | |||
| AUC(0-t), min × ng/mL | 774.7 | 3,437 | |||
| CNDAC* | |||||
| Cycle 1, day 1 | 15.5 | 19.8 | 6.76 | 67.3 | 11.6 |
| Cmax, ng/mL | 8.23 | 2.88 | 34.6 | ||
| 9.98 | |||||
| 63.7 | |||||
| Tmax, minutes | 250 | 135 | 119 | 480 | 240 |
| 65 | 65 | 120 | |||
| 120 | |||||
| 120 | |||||
| AUC(0-t), min × ng/mL | 3,738 | 3,100 | 463.4 | 18,315 | 1,493 |
| 1,998 | 242.9 | 5,325 | |||
| 1,647 | |||||
| 12,169 | |||||
| Cycle 1, day 8 | |||||
| Cmax, ng/mL | 4.65 | 16.9 | |||
| Tmax, min | 240 | 120 | |||
| AUC(0-t), min × ng/mL | 709.1 | 4,490 | |||
NOTE. N = 10 patients.
Abbreviations: CNDAC, 2′-C-Cyano-2′-deoxy-β-d -arabino-pentofuranosylcytosine; Cmax, maximum plasma concentration; Tmax, time to maximum plasma concentration; AUC(0-t), area under the curve from zero to the final sample with a concentration.
Each value represents an individual patient.
Adequate leukemia blasts for nucleotide pool analyses throughout the sampling time course were recovered from five patients (125 mg, n = 2; 325 mg, n = 2; 475 mg, n = 1). In no case was the level of CNDAC triphosphate quantifiable, likely because the plasma Cmax concentrations of CNDAC in the range 33 to 250 nmol/L would not generate triphosphate concentrations detectable by ultraviolet absorbance20 There was no consistent inhibition of DNA synthesis in leukemia blasts observed in the ex vivo assays.
Response
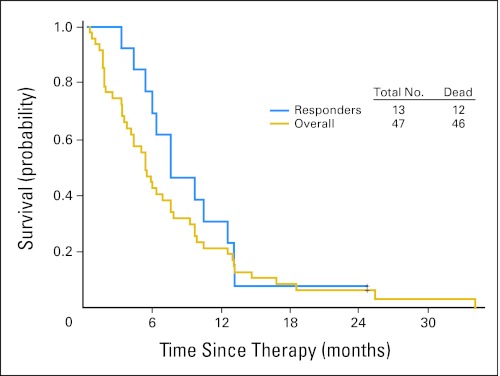
Overall, 13 patients (28%) achieved objective responses: four had CR, two had CRp, and seven had CRi. Several patients had extensive prior therapy. The details of the patients are listed in Table 5. Two patients with AML had experienced relapsed leukemia cutis with numerous skin nodules. Both patients achieved a significant reduction in the number of skin nodules, and one patient had complete resolution of all skin nodules. In addition to our observations in patients with objective responses, we observed prolonged stable disease in one patient. This patient had AML in second relapse after standard induction chemotherapy. The baseline bone marrow had 34% blasts with an absolute granulocyte count of 0.25 × 109/L. This patient received a total of 16 cycles of treatment and had a reduction in bone marrow blast to as low as 6% and normalization of granulocyte count greater than 109/L. In addition, 20 patients (43%) had at least 1-log reduction of peripheral blasts (n = 12) and/or at least 50% reduction of marrow blasts (n = 8). Two patients died in the first 4 weeks of therapy. The estimated 4-week mortality for the total study group was only 4%. Survivals of the overall group and of responders are shown in Figure 1.
Table 5.
Characteristics of Responders
| Patient | Age (years) | Diagnosis | Salvage Status | CG | Prior Treatment | Response to Prior Treatment | CRD1 | Last CRD | Sapacitabine Twice Daily × 7 or Days 1-3 Each Week × 2 Weeks (mg/m2) | Response Type | Duration of Response (months) | Patient | % BM Blasts |
PLTs (× 109/L) |
% PB |
PMN (× 109/L) |
Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pretransplantation | Post-Transplantation | Pretherapy | Post-Therapy | Pretherapy | Post-Therapy | Pretherapy | Post-Therapy | ||||||||||||||
| 14 | 59 | MDS→EMD | S2 | IM | HDAC | NR | 0 | 4 | 225 × 7 | CR | ≥ 3.9 | 14 | 1 | 1 | 72 | 82 | 0 | 0 | 2.5 | 1.1 | CR of EMD; off therapy because of concurrent disease |
| Allo SCT | CR | ||||||||||||||||||||
| DLI | CR | ||||||||||||||||||||
| 32 | 66 | CMML→AML | S3 | del 13q* | Decitabine | CR | 1 | 0 | 325 × 7 | CRp | 2.2 | 32 | 50 | 3 | 38 | 44 | 8 | 0 | 6.6 | 1.6 | |
| clofarabine (for MDS) | NR | ||||||||||||||||||||
| 41 | 57 | AML | S1 | Diploid | IA | PR | 0 | 425 × 3/wk × 2 | CR | ≥ 1 | 41 | 21 | 3 | 261 | > 100 | 0 | 0 | 2.3 | > 1.0 | Off for SCT | |
| 45 | 85 | AML | S5 | abn 5/7† | DA | CR | 22 | 84 | 475 × 3/wk × 3 | CR | ≥1 | 45 | 40 | 2 | 41 | 120 | 0 | 0 | 0.5 | 1.5 | Off for consolidation therapy |
| DA | CR | ||||||||||||||||||||
| FIA | CR | ||||||||||||||||||||
| FAM | CR | ||||||||||||||||||||
| 12 | 75 | MDS-RAEB | S1 | Diploid | Azacitidine | Unknown | 225 × 7 | BM CR | 0.5 | 12 | 8 | 4 | 31 | 51 | 4 | 0 | 0.1 | 0.17 | |||
| 13 | 79 | MDS-CMML | S1 | Diploid | DAC | CR | 15 | 225 × 7 | CR | 3.3 | 13 | 17 | 2 | 324 | 372 | 2 | 0 | 0.28 | 1.3 | ||
| 6 | 82 | AML | S2 | Complex | DAC | NR | 0 | 0 | 125 × 7 | CRp | 2.1 | 6 | 77 | 2 | 45 | 83 | 66 | 0 | 2.4 | 3.2 | |
| GO | NR | ||||||||||||||||||||
| 9 | 53 | MDS→ | Diploid | IA/tipinarnib | CR | 3 | — | 175 × 7 | BM CR | 2 | 9 | 18 | 1 | 20 | 14 | 1 | 0 | 0.7 | 0.6 | ||
| AML | S3 | CDDO | NR | ||||||||||||||||||
| 10 | 56 | AML | S1 | IM | IA | CR | 1 | 175 × 7 | BM CR | ≥ 1.9 | 10 | 14 | 2 | 30 | 42 | 8 | 0 | 1.2 | 0.18 | Off for cord SCT | |
| 15 | 73 | MDS-CMML | S1 | Other (noncomplex) | DAC | CR | 12 | 275 × 7 | BM CR | 2 | 15 | 9 | 5 | 28 | 4 | 0 | 0 | 3.3 | 0.03 | ||
| 17 | 90 | MDS→AML | F | Other (noncomplex) | — | — | 275 × 7 | BM CR | 2.5 | 17 | 40 | 5 | 27 | 32 | 8 | 0 | 0.04 | 0.34 | |||
| 22 | 69 | Treatment related AML | F | Complex | — | — | 325 × 7 | BM CR | 0.5 | 22 | 33 | 2 | 24 | 14 | 5 | 0 | 12.2 | 0.4 | |||
| 23 | 55 | AML/(+EMD) | S4 | Diploid | IA | CR | 3 | — | 325 × 7 | BM CR | 1.5 | 23 | 10 | 4 | 151 | 52 | 0 | 0 | 3.4 | 1.0 | > 50% reduction in EMD |
| IA | CR | ||||||||||||||||||||
| Flud/AraC | NR | ||||||||||||||||||||
Abbreviations: CG, cytogenetics; CRD1, duration of first complete response; CRDlast, duration of last complete response; BM, bone marrow; PLTs, platelets; PB, peripheral blood; PMN, granulocytes; MDS, myelodysplastic syndrome; EMD, extramedullary disease (leukemic cutis); S2, second salvage; IM, insufficient metaphases; HDAC, high-dose cytarabine; NR, no response; CR, complete response; AlloSCT, allogeneic stem-cell transplantation; DLI, donor lymphocyte infusions; CMML, chronic myelomonocytic leukemia; AML, acute myeloid leukemia; S1, first salvage; IA, idarubacin and cytarabine; CDDO, 2-cyano-3, 12 dioxoolean-1, 9-dien-28-oic acid; PR, partial response; SCT, stem-cell transplantation; DA, daunorubicin and cytarabine; FIA, fludarabine, idarubacin, cytarabine; FAM, fludarabine, cytarabine, mitoxantrone; RAEB, refractory anemia with excess blasts; DAC, decitabine; CRp, complete response without platelet recovery; GO, gemtuzumab ozogamicin; S3, third salvage; F, front-line; S4, fourth salvage; Flud, fludarabine; AraC, cytarabine.
del13q represents deletion in the long arm of chromosome 13.
abn 5/7 represents chromosome 5 or 7 abnormalities.
Fig 1.
Survival overall and in responders.
DISCUSSION
Sapacitabine is an oral prodrug of CNDAC, a rationally designed analog of deoxycytidine. Despite its structure and chemical similarity to cytarabine, it has unique properties in causing single-strand DNA breaks because of the presence of a cyano group within the deoxyribose moiety of the molecule. As myelosuppression was the DLT in solid tumor studies, we evaluated sapacitabine in a phase I study in acute leukemias and MDS. We defined the new extramedullary DLT to be gastrointestinal. Two phase II dose schedules were recommended for future studies: 325 mg orally twice daily for 7 days every 3 to 4 weeks and 425 mg orally twice daily on days 1, 2, 3 weekly for 2 weeks every 3 to 4 weeks. Although the plasma levels of sapacitabine and CNDAC were highly variable and were not dose proportional, it appears that the sapacitabine dose could be titrated to reach MTD by an increment of 100 mg/d in patients with leukemias and MDS. PK data were available for 10 patients entered into five dose levels that ranged between 125 and 475 mg. Given this relatively small cohort and the inherent variability of oral administration, it is not surprising that a significant dose-response relationship was not evident. Additional clinical studies are presently administering sapacitabine in different schedules at doses within this range and will address more appropriately the sapacitabine dose-response relationship.
The drug was well tolerated and showed encouraging antileukemic activity in the setting of poor-prognosis AML and MDS. Among 47 patients, 13 achieved objective responses, and an additional 20 patients had significant reductions of their blasts. Objective responses also were observed in extramedullary disease (ie, leukemic cutis).
The prognosis of patients with AML remains poor. Overall, only 25% to 30% of patients with newly diagnosed disease are cured. The prognosis is significantly worse in elderly patients with AML and in patients who have AML with poor karyotypes, for whom the cure rates are less than 10%. In elderly patients with AML, standard intensive chemotherapy is associated with a high mortality rate (ie, 30% to 50% in the first 6 to 8 weeks).3 Such patients may benefit from novel agents, like sapacitabine, that provide antileukemic efficacy and ease of administration (oral outpatient therapy) without the concurrent high mortality.
Although survival of patients with MDS has improved with the introduction of hypomethylating agents, the prognosis remains poor. The median survival of patients with higher-risk MDS treated with azacitidine is only 20 to 25 months. Once patients with MDS experience failure on therapy with hypomethylating agents, the prognosis is poor; the median survival after failure of hypomethylating-agent therapy is only 4 to 6 months.21 Therefore, effective anti-MDS agents are needed for this orphan indication. Randomized studies of sapacitabine to evaluate different dose schedules are ongoing in elderly patients with newly diagnosed or first-relapsed AML and in patients with MDS who have failed hypomethylating agents.
In conclusion, sapacitabine has demonstrated a reasonable safety profile in this phase I study and has encouraging antileukemic activity. Current studies are evaluating its role as a single agent in elderly patients with AML and in MDS. The development of combinations of sapacitabine with other antileukemic agents is warranted.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Simon R. Green, Cyclacel Pharmaceuticals (U); Judy H. Chiao, Cyclacel Pharmaceuticals (C) Consultant or Advisory Role: None Stock Ownership: Judy H. Chiao, Cyclacel Pharmaceuticals Honoraria: None Research Funding: Hagop Kantarjian, Cyclacel Pharmaceuticals Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Hagop Kantarjian, Guillermo Garcia-Manero, Susan O'Brien, Judy H. Chiao, Jorge Cortes, William Plunkett
Administrative support: Robert Westwood, Simon R. Green
Provision of study materials or patients: Stefan Faderl, Farhad Ravandi, Judy H. Chiao
Collection and assembly of data: Hagop Kantarjian, Farhad Ravandi, Judy H. Chiao, Patricia A. Boone
Data analysis and interpretation: Hagop Kantarjian, Guillermo Garcia-Manero, Judy H. Chiao, William Plunkett
Manuscript writing: Hagop Kantarjian, Judy H. Chiao, William Plunkett
Final approval of manuscript: Hagop Kantarjian, Guillermo Garcia-Manero, Susan O'Brien, Stefan Faderl, Farhad Ravandi, Robert Westwood, Judy H. Chiao, Jorge Cortes, William Plunkett
REFERENCES
- 1.Ravandi F, Burnett A, Agura E, et al. Progress in the treatment of acute myeloid leukemia. Cancer. 2007;110:1900–1910. doi: 10.1002/cncr.23000. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian H, O'Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: Predictive prognostic models for outcome. Cancer. 2006;106:1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 3.Kantarjian H, Thomas D, O'Brien S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101:2788–2801. doi: 10.1002/cncr.20668. [DOI] [PubMed] [Google Scholar]
- 4.Gökbuget N, Hoelzer D, Arnold R, et al. Treatment of adult ALL according to protocols of the German Multicenter Study Group for Adult ALL (GMALL) Hematol Oncol Clin North Am. 2000;14:1307–1325. doi: 10.1016/s0889-8588(05)70188-x. [DOI] [PubMed] [Google Scholar]
- 5.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: Results of a phase III randomized study. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 7.List A, Dewald G, Bennett J, et al. Myelodysplastic Syndrome-003 study: Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 8.Keating M, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 9.Kantarjian H, Gandhi V, Cortes J, et al. Phase 2 clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood. 2003;102:2379–2386. doi: 10.1182/blood-2003-03-0925. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda A, Nakajima Y, Azuma A, et al. Nucleosides and nucleotides: 100. 2′-C-cyano2′-deoxy-1-β-arabinofuranosyl-cytosine (CNDAC): Design of a potential mechanism-based DNA-strand-breaking anti-neoplastic nucleoside. J Med Chem. 1991;34:2917–2919. doi: 10.1021/jm00113a034. [DOI] [PubMed] [Google Scholar]
- 11.Hanaoka K, Suzuki M, Kobayashi T, et al. Antitumor activity and novel DNA self-strand-breaking mechanism of CNDAC (1-(2′-C-cyano-2-deoxy-β-D-arabino-pentofuranosyl) cytosine) and its N4-palmitoyl derivative (CS_682) Int J Cancer. 1999;82:226–236. doi: 10.1002/(sici)1097-0215(19990719)82:2<226::aid-ijc13>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 12.Azuma A, Huang P, Matsuda A, et al. 2′-C-cyano-2-deoxy-β-D-arabino-pentafuranosyl cytosine: A novel anticancer nucleoside analog that causes both DNA strand breaks and G2 arrest. Mol Pharmacol. 2001;59:725–731. doi: 10.1124/mol.59.4.725. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Liu X, Matsuda A, Plunkett W. Repair of 2′-C-cyano2′-deoxy-1-β-D-arabinofuranosyl-cytosine-induced DNA single-strand breaks by transcription-coupled nucleotide excision repair. Cancer Res. 2008;68:3881–3889. doi: 10.1158/0008-5472.CAN-07-6885. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Wang Y, Chubb S, et al. Impact of DNA repair proteins on cell survival in response to damage induced by the DNA self-strand-breaking nucleoside analogue CNDAC. Proc. Am Assn Cancer Res. 2008;49:607. abstr. [Google Scholar]
- 15.Delaunoit T, Burch PA, Reid JM, et al. A phase I clinical and pharmacokinetic study of CS-682 administered orally in advanced malignant solid tumors. Invest New Drugs. 2006;24:327–333. doi: 10.1007/s10637-006-5392-0. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert J, Carducci MA, Baker SD, et al. A phase I study of the oral antimetabolite, CS-682, administered once daily 5 days per week in patient with refractory solid tumor malignancies. Invest New Drugs. 2006;24:499–508. doi: 10.1007/s10637-006-8219-0. [DOI] [PubMed] [Google Scholar]
- 17.Cheson BD, Cassileth PA, Head DR, et al. Report of the National Cancer Institute–sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol. 1990;8:813–819. doi: 10.1200/JCO.1990.8.5.813. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0 (CTCAE) http://ctep.cancer.gov/forms/CTCAEv3.pdf.
- 20.Azuma A, Huang P, Matusda A, et al. Cellular pharmacokinetic and pharmacodynamics of the deoxycytidine analog 2′-C-cyano-2′-deoxy-1-β-D-arabino-pentouranosylcytosine. Biochem Pharmacol. 2001;61:1497–1507. doi: 10.1016/s0006-2952(01)00617-7. [DOI] [PubMed] [Google Scholar]
- 21.Jabbour E, Garcia-Manero G, Shan J, et al. Outcome of patients with myelodysplastic syndrome and chronic myelomonocytic leukemia post –decitabine failure. Blood. 2008;112 abstr 1659. [Google Scholar]