Abstract
This mini-review summarizes and integrates findings from recent meta-analyses and original neuroimaging studies on functional brain abnormalities in dyslexic readers. Surprisingly, there is little empirical support for the standard neuroanatomical model of developmental dyslexia, which localizes the primary phonological decoding deficit in left temporo-parietal (TP) regions. Rather, recent evidence points to a dysfunction of a left hemisphere reading network, which includes occipito-temporal (OT), inferior frontal, and inferior parietal regions.
Keywords: brain, developmental dyslexia, fMRI, meta-analysis, neuroimaging, reading
Introduction
Developmental dyslexia is defined as a specific disorder of reading development. It is characterized by an unexpected impairment in the acquisition of reading skills despite normal intelligence, motivation, and adequate schooling (Ferrer et al., 2010). According to the diagnostic criteria of DSM-IV (American Psychiatric Association, 2000) and ICD-10 (World Health Organization, 2007), performance in reading accuracy and/or fluency, reading comprehension, and/or spelling is substantially below the performance expected from the person's chronological age, intelligence, education, and sensory acuity. In addition, the difficulties significantly interfere with academic achievement or activities in everyday life that require reading skills. In a substantial number of cases, delayed development of oral language skills in early childhood is the first sign of dyslexia. Problems with vocabulary and grammatical structures are often comorbid with reading difficulties and frequently persist into adulthood.
With respect to neurocognitive explanations, a number of competing hypotheses exist but the dominant explanation posits a phonological deficit as proximal cause (e.g., Snowling, 2000; Shaywitz and Shaywitz, 2005; Vellutino and Fletcher, 2005). This deficit is assumed to affect the emergence of phoneme awareness at the beginning of learning to read, which in turn has a negative effect on the acquisition of self-reliant phonological word reading based on serial grapheme-phoneme coding. The phonological decoding difficulty is assumed to secondarily compromise the build-up of the orthographic lexicon, which is required for fast automatic word recognition and correct spelling. Support for this account was provided by the specific difficulty of dyslexic readers to come up with correct readings of pseudowords. However, this difficulty with pseudowords was typically found in English-based studies and does not generalize to more regular orthographies like German (e.g., Landerl et al., 1997; Ziegler et al., 2001). Accordingly, this version of the phonological deficit explanation is difficult to apply to regular orthographies. Wimmer (1993) proposed that, in regular orthographies, the phonological deficit becomes manifest as a speed impairment in the access to both lexical and sublexical phonology. In a similar way, Wolf and Bowers (1999) modified the phonological deficit explanation by positing an additional visual-verbal speed deficit. Their double deficit explanation was backed up by the finding that performance on tasks requiring rapid naming of pictured objects before learning to read predicted later reading skills.
The classical phonological deficit explanation served as a framework for the functional neuroanatomical study of developmental dyslexia. Narrative reviews of the existing research culminated in a standard model positing three left hemisphere regions with abnormal function in dyslexic readers (Pugh et al., 2000; McCandliss and Noble, 2003; Démonet et al., 2004; Sandak et al., 2004; Shaywitz and Shaywitz, 2005; Schlaggar and McCandliss, 2007). The primary phonological word decoding difficulty is seen as resulting from a dysfunction of a left dorsal temporo-parietal (TP) region, including the posterior aspect of the superior temporal gyrus and adjacent parietal regions. The secondary difficulty with fast word recognition—based on stored orthographic word representations—is seen as resulting from a dysfunction of a left ventral occipito-temporal (OT) region including lateral extrastriate, fusiform, and inferior temporal regions. A third abnormality—presumably reflecting compensatory reliance on effortful articulatory processes—is overactivation of the left inferior frontal gyrus (IFG).
Recent insights from functional neuroimaging
This mini-review provides an evaluation of the standard model. It is based on recent meta-analyses and original functional magnetic resonance imaging (fMRI) studies on brain activation abnormalities in dyslexic readers. The main benefit of the meta-analytic approach is that it reveals consistent effects across studies in an unbiased and objective way. Therefore, it may lead to findings that were overlooked in the original studies and in the narrative reviews because they did not seem to be interesting for theoretical reasons. Certainly, synthesizing over heterogeneous studies also entails potential pitfalls. One such problem is the averaging across different languages. However, despite different behavioral manifestations, there is considerable agreement that the neuronal fingerprint of developmental dyslexia is quite common across different languages (Paulesu et al., 2001; Hu et al., 2010). In addition, the meta-analytic results by definition should only show those regions, which are consistently under or overactivated in dyslexic readers in most of the studies. Therefore, the results may be viewed as the lowest common denominator of the original studies. For a discussion of further limitations of the present meta-analyses see Richlan et al. (2011).
Our first attempt to evaluate the standard neuroanatomical model used quantitative, coordinate-based meta-analysis of 17 published functional neuroimaging studies (Richlan et al., 2009). The results lent support to the central role of left hemisphere TP and OT dysfunctions. Specifically, convergent dyslexic underactivation across studies was found in the left inferior parietal lobule (IPL), superior temporal, middle temporal, inferior temporal, and fusiform regions. A novel finding was underactivation in a left IFG region associated with access to lexical and sublexical phonological representations. Dyslexic overactivation was found in the primary motor cortex and in the anterior insula, presumably reflecting compensatory reliance on articulatory-based phonological processes. The meta-analysis included original studies from quite different age levels, which is problematic in relation to the developmental assumption of the standard model. To recapitulate, this model posits an early dysfunction of the left TP region resulting in an additional secondary dysfunction of the left OT region. Therefore, one would expect to find dominance of left TP underactivation in children with dyslexia and both left TP and left OT underactivation in adults with dyslexia.
A second meta-analysis (Richlan et al., 2011) investigated this expectation by dividing the original studies into nine studies with dyslexic children (age means: 9–11 years) and nine studies with dyslexic adults (age means: 18–30 years). Contrary to expectation, left TP underactivation was present in the adult studies but not in the child studies. There was dyslexic underactivation in bilateral IPL regions in the meta-analysis of the child studies but this underactivation was localized superior to the typical TP regions. With respect to left OT dysfunction, widespread underactivation was found for the adult dyslexic readers. In contrast, for dyslexic children left OT underactivation was limited to an anterior portion of the left ventral OT cortex. This finding is in agreement with the notion of an important role of the left OT region in skilled, efficient reading (e.g., Shaywitz et al., 2007). The finding of left OT underactivation in children is compatible with recent evidence in favor of early left OT engagement in normal reading development (Brem et al., 2010), and early failure of such engagement in dyslexia (Maurer et al., 2007; Bach et al., 2012; Raschle et al., 2012). In summary, the findings of the second meta-analysis raised serious doubts on the validity of the standard model. Specifically, there was no support for the critical developmental assumption that the primary and early emerging dysfunction resides in the left TP cortex. Rather, the findings suggest that an early small left OT dysfunction becomes increasingly extended and is later accompanied by a left TP dysfunction.
Further doubts on the critical role of the left TP region in phonological decoding came from an fMRI study with German adolescent and adult dyslexic readers (Wimmer et al., 2010). The participants were visually presented with phonologically and orthographically familiar words (e.g., Taxi), phonologically familiar but orthographically unfamiliar pseudohomophones (e.g., Taksi), and phonologically and orthographically unfamiliar pseudowords (e.g., Tazi). The task required phonological lexical decisions (i.e., Does it sound like an existing word?). From the standard model one would expect dyslexic readers to exhibit underactivation of the left OT cortex in response to familiar words and underactivation of the left TP cortex in response to unfamiliar pseudowords. However, the non-impaired readers exhibited generally little activation in response to pseudohomophones and pseudowords in left TP regions. These unfamiliar letter strings led to marked activation in left hemisphere OT, IFG, and IPL regions. Importantly, the left IPL activation may not be subsumed under the left TP reading circuit because it was localized quite far from the proposed core regions of phonological reading processes in left posterior superior temporal regions. In response to unfamiliar letter strings, dyslexic readers exhibited underactivation in all three major components of the non-impaired activation pattern (left OT, left IFG, left IPL). Dyslexic overactivation was identified in early visual occipital regions, reflecting prolonged visual processing, as well as in a network of premotor/motor and subcortical regions, presumably reflecting increased reliance on silent articulatory processes.
A follow-up study by Richlan et al. (2010) provided further evidence for dyslexic underactivation in left OT, IFG, and IPL regions. The primary novel feature was the introduction of a new experimental manipulation: stimulus length, with short items consisting of 3–5 letters and long items consisting of 6–10 letters. The manipulation of item length for pseudowords was of specific importance as it allowed the localization of brain regions engaged by serial phonological decoding. If indeed the left hemisphere network of OT, IFG, and IPL regions is engaged by reading of both familiar and unfamiliar letter strings, then non-impaired readers should exhibit a length effect for pseudowords but not for words, and dyslexic readers may again exhibit reduced engagement of the non-impaired reading network. The results confirmed these expectations. In response to both familiar and unfamiliar letter strings, non-impaired readers showed activation in the left hemisphere network of OT, IFG, and IPL regions. Furthermore, activation in the left OT and in the left IFG, but not in the left IPL, was specifically sensitive to the length of pseudowords. Apart from the more superior localization of the left IPL cluster, this feature adds evidence against the summarization of this brain region under the phonological left TP reading circuit. As hypothesized, dyslexic readers exhibited marked underactivation in response to pseudowords in left OT, left IFG, and left IPL regions. They also failed to show the increase in activation in response to the length of pseudowords shown by the non-impaired readers. Similar to the previous fMRI study, the dyslexic readers exhibited overactivation in left premotor, subcortical, and cerebellar regions, presumably engaged by slow and effortful articulatory-based reading. Consistent with this interpretation was the finding of a marked length effect for pseudowords in the left premotor region of dyslexic readers.
Evidence for a left temporo-parietal dysfunction?
To recapitulate, the first meta-analysis (Richlan et al., 2009) found evidence for dyslexic underactivation in left TP regions. The second meta-analysis (Richlan et al., 2011), with a separation of child and adult studies, modified this pattern by finding left TP underactivation only for adults but not for children. As pointed out, the latter finding stands in contrast to the central developmental assumption of the standard neuroanatomical model. This model assumes an early occurring primary left TP dysfunction. However, despite this missing support for the developmental assumption, the two meta-analyses—at least for the adult studies—support the standard model by speaking for an important role of core left TP regions for non-impaired reading processes, and a dysfunction of these regions for dyslexic reading processes.
Surprisingly, the findings from the two fMRI studies with young adult German readers question this conclusion. For core components of the left TP reading circuit (posterior superior temporal regions), the non-impaired readers in this study exhibited generally little activation and consequently there could be no reduced activation for the dyslexic readers. The absence of reliable activation observed for non-impaired readers is specifically remarkable as the standard model localizes serial phonological decoding in left TP regions, and the in-scanner tasks presented unfamiliar letter strings in the context of a phonological lexical decision task. As expected from serial phonological decoding of pseudowords, the non-impaired readers of Richlan et al. (2010) exhibited a marked length effect for pseudowords (but not for words) in a left IFG region and a left OT region. Meanwhile, dyslexic readers exhibited a similar specific length effect in left premotor regions. Thus, there were specific regions apparently engaged by serial decoding, but the left TP cortex was not among these regions. However, both of the original studies identified a left IPL region with reading-related activation exhibited by non-impaired readers, and reduced activation exhibited by dyslexic readers. In some formulations of the standard model (e.g., Pugh et al., 2000; Sandak et al., 2004) the left IPL is subsumed under the left TP circuit. However, some aspects differ from the assumption of the standard model that the left TP circuit is mainly engaged by serial phonological decoding: the non-impaired readers of Richlan et al. (2010) did not exhibit a length effect for pseudowords on left IPL activation. Therefore, the reduced left IPL activation of dyslexic readers—different from the standard model—cannot be interpreted as reflecting a dysfunction with respect to the demands of serial phonological decoding. An alternative account of the function of the left IPL was formulated by Shaywitz and Shaywitz (2008), who linked the left IPL to general attentional mechanisms, which may interact with reading processes. Evidence for this interpretation is only beginning to emerge and certainly more studies on the role of the left IPL during reading are required. Interestingly, a recent meta-analysis of fMRI studies with healthy children found that reading, numerical processing, and tasks posing demands on executive functions led to activation of a similar left IPL region (Houdé et al., 2010).
Dysfunction of a left hemisphere reading network
Convergence between the findings of the original studies and the meta-analyses exists with respect to underactivation in a left hemisphere reading network consisting of OT, IFG, and IPL regions. The identification of left IFG underactivation in the two meta-analyses (Richlan et al., 2009, 2011) was a novel finding and stood in contrast to overactivation in a close-by left precentral region. This finding was of particular importance, as in dominant formulations of the standard neuroanatomical model (e.g., Pugh et al., 2000) it is assumed that a reading circuit in anterior frontal language regions exhibits overactivation in dyslexic readers in order to compensate for the dysfunction in posterior language regions. Obviously, the meta-analytic findings speak for a marked distinction within left frontal regions; that is, between a left IFG region and a left precentral region, with dyslexic underactivation in the former and dyslexic overactivation in the latter. The findings from the two fMRI studies (Richlan et al., 2010; Wimmer et al., 2010) provided further evidence for such a distinction. Both studies identified dyslexic underactivation in the left IFG and dyslexic overactivation in left precentral/motor regions. The former is presumably engaged by access to lexical and sublexical phonological representations, and the latter presumably by silent articulatory reading processes. In a series of studies, Heim and colleagues showed that cytoarchitectonic subdivisions of the left IFG differently contribute to lexical access (area 44) and lexical selection (area 45) (Heim et al., 2005, 2009a,b). Dyslexic children (Grande et al., 2011) and adults (Heim et al., 2012) were recently shown to exhibit functional abnormalities in both of these left IFG subdivisions.
For non-impaired readers, reading-related activation of the left IFG was accompanied by activation in left OT and left IPL regions. All three components of this reading network were found to be underactivated in dyslexic readers. Additional findings of the two fMRI studies were that non-impaired readers exhibited very similar activation patterns in the left OT and the left IFG, with marked increases of activation from familiar to unfamiliar letter strings (Wimmer et al., 2010) and from short to long pseudowords (Richlan et al., 2010). The similarity of activation patterns speaks for a striking functional connectivity between left OT and left IFG regions. The modulation of activation in response to reading demands in non-impaired readers was missing in dyslexic readers. This finding is in line with evidence from studies of functional and effective connectivity which reported coupling of left OT and left IFG regions in non-impaired readers and disruption in dyslexic readers (Shaywitz et al., 2003; Cao et al., 2008; van der Mark et al., 2011).
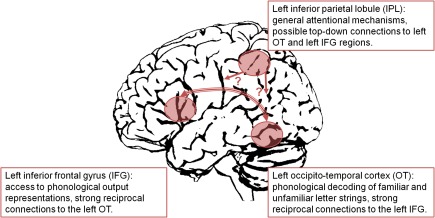
Based on these findings, a model of the impaired left hemisphere reading network in developmental dyslexia including OT, IFG, and IPL regions is illustrated in Figure 1. While there is evidence for strong functional and structural connectivity between left OT and left IFG regions (Catani et al., 2005; Ben-Shachar et al., 2007), connectivity of the left IPL region is less clear. The left OT underactivation was assumed to reflect a deficit in processing of both familiar words and unfamiliar pseudowords. With increasing demands on phonological decoding (i.e., processing of longer pseudowords), the left OT dysfunction of dyslexic readers became even more evident. The same pattern of functional abnormalities was observed for the left IFG. In this region, dyslexic underactivation was interpreted as reflecting a deficit in access to lexical and sublexical phonological representations. When interpreting the left IPL abnormality we can only speculate about possible underlying mechanisms. Assumedly, the function of the left IPL was not specifically related to reading or phonological decoding (as shown by absence of a length effect for pseudowords in non-impaired readers). Rather, it was supposed to be associated with more general mechanisms, as evidenced by engagement in numerical processing and executive functions (Houdé et al., 2010). As suggested by Shaywitz and Shaywitz (2008), the left IPL may be part of a fronto-parietal attention network, which interacts with reading processes via top-down connections. However, the exact mechanisms of this interaction are unclear and should be targeted in future studies, e.g., by means of psychophysiological interactions. Furthermore, one has to keep in mind that the present model is largely based on fMRI studies (with their well-known limitations such as poor temporal resolution). A more comprehensive neuroanatomical model of developmental dyslexia certainly requires the integration of findings from other modalities such as EEG and MEG. However, in contrast to the standard neuroanatomical model (e.g., Pugh et al., 2000), which is based on subjective, narrative review of a limited number of studies, the present model is based on objective, quantitative meta-analysis of a substantially larger number of functional neuroimaging studies on developmental dyslexia. Therefore, it may be viewed as an up-to-date modification of the standard model.
Figure 1.
Dysfunction of a left hemisphere reading network in developmental dyslexia.
There is an interesting developmental question with respect to the model illustrated in Figure 1. In accordance with the phonological deficit explanation, one may assume that a dysfunction of the left frontal component may be specifically critical in the early phase of learning to read, as this component may be engaged by access to phonemes and other sublexical phonological representations. To our knowledge, no evidence for such an early dysfunction of left frontal regions exists. Rather, recent fMRI and EEG studies reported evidence for early left OT engagement in normal reading development and early dysfunction in developmental dyslexia (Maurer et al., 2007; Brem et al., 2010; Bach et al., 2012; Raschle et al., 2012). These findings raise questions about the functional role of the left OT region in reading development. The idea that the left OT functions as a kind of interface area connecting high-level visual representations (or representations from other sensory modalities) to language and conceptual representations (Price and Devlin, 2011) is quite compatible with the finding of an early role of the left OT for learning to read. From this perspective, the aforementioned findings on reduced early left OT engagement in young dyslexic readers may reflect a form of disconnection between visual and phonological representations. This would be consistent with Geschwind's account of dyslexia as a disconnection syndrome (Geschwind, 1965a,b).
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I am grateful to Heinz Wimmer, Martin Kronbichler, Matthias Schurz, Benjamin Gagl, Julia Sophia Crone, and Joe Miller for their support. This research was funded by grants of the Austrian Science Foundation (FWF), grant number P18832-B02, and of the 6th Framework Programme of the European Commission (EC), grant number 018696.
References
- American Psychiatric Association (2000). Diagnostic and Statistical Manual of Mental Disorders – DSM-IV-TR. Washington, DC: American Psychiatric Association [Google Scholar]
- Bach S., Richardson U., Brandeis D., Martin E., Brem S. (2012). Print-specific multimodal brain activation in kindergarten improves prediction of reading skills in second grade. Neuroimage. [Epub ahead of print]. 10.1016/j.neuroimage.2011.07.023 [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M., Dougherty R. F., Wandell B. A. (2007). White matter pathways in reading. Curr. Opin. Neurobiol. 17, 258–270 10.1016/j.conb.2007.03.006 [DOI] [PubMed] [Google Scholar]
- Brem S., Bach S., Kucian K., Guttorm T. K., Martin E., Lyytinen H., Brandeis D., Richardson U. (2010). Brain sensitivity to print emerges when learn letter-speech sound correspondences. Proc. Natl. Acad. Sci. U.S.A. 107, 7939–7944 10.1073/pnas.0904402107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F., Bitan T., Booth J. R. (2008). Effective brain connectivity in children with reading difficulties during phonological processing. Brain Lang. 107, 91–101 10.1016/j.bandl.2007.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Jones D. K., ffytche D. H. (2005). Perisylvian language networks of the human brain. Ann. Neurol. 57, 8–16 10.1002/ana.20319 [DOI] [PubMed] [Google Scholar]
- Démonet J. F., Taylor M. J., Chaix Y. (2004). Developmental dyslexia. Lancet 363, 1451–1460 10.1016/S0140-6736(04)16106-0 [DOI] [PubMed] [Google Scholar]
- Ferrer E., Shaywitz B. A., Holahan J. M., Marchione K., Shaywitz S. E. (2010). Uncoupling of reading and IQ over time: empirical evidence for a definition of dyslexia. Psychol. Sci. 21, 93–101 10.1177/0956797609354084 [DOI] [PubMed] [Google Scholar]
- Geschwind N. (1965a). Disconnexion syndromes in animals and man. I. Brain 88, 237–294 10.1093/brain/88.2.237 [DOI] [PubMed] [Google Scholar]
- Geschwind N. (1965b). Disconnexion syndromes in animals and man. II. Brain 88, 585–644 10.1093/brain/88.3.585 [DOI] [PubMed] [Google Scholar]
- Grande M., Meffert E., Huber W., Amunts K., Heim S. (2011). Word frequency effects in the left IFG in dyslexic and normally reading children during picture naming and reading. Neuroimage 57, 1212–1220 10.1016/j.neuroimage.2011.05.033 [DOI] [PubMed] [Google Scholar]
- Heim S., Alter K., Ischebeck A. K., Amunts K., Eickhoff S. B., Mohlberg H., Zilles K., von Cramon D. Y., Friederici A. D. (2005). The role of the left Brodmann's areas 44 and 45 in reading words and pseudowords. Brain Res. Cogn. Brain Res. 25, 982–993 10.1016/j.cogbrainres.2005.09.022 [DOI] [PubMed] [Google Scholar]
- Heim S., Eickhoff S. B., Amunts K. (2009a). Different roles of cytoarchitectonic BA 44 and BA 45 in phonological and semantic verbal fluency as revealed by dynamic causal modelling. Neuroimage 48, 616–624 10.1016/j.neuroimage.2009.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S., Eickhoff S. B., Ischebeck A. K., Friederici A. D., Stephan K. E., Amunts K. (2009b). Effective connectivity of the left BA 44, BA 45, and inferior temporal gyrus during lexical and phonological decisions identified with DCM. Hum. Brain Mapp. 30, 392–402 10.1002/hbm.20512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S., Wehnelt A., Grande M., Huber W., Amunts K. (2012). Effects of lexicality and word frequency on brain activation in dyslexic readers. Brain Lang. [Epub ahead of print]. 10.1016/j.bandl.2011.12.005 [DOI] [PubMed] [Google Scholar]
- Houdé O., Rossi S., Lubin A., Joliot M. (2010). Mapping numerical processing, reading, and executive functions in the developing brain: an fMRI meta-analysis of 52 studies including 842 children. Dev. Sci. 13, 876–885 10.1111/j.1467-7687.2009.00938.x [DOI] [PubMed] [Google Scholar]
- Hu W., Lee H. L., Zhang Q., Liu T., Geng L. B., Seghier M. L., Shakeshaft C., Twomey T., Green D. W., Yang Y. M., Price C. J. (2010). Developmental dyslexia in Chinese and English populations: dissociating the effect of dyslexia from language differences. Brain 133, 1694–1706 10.1093/brain/awq106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landerl K., Wimmer H., Frith U. (1997). The impact of orthographic consistency on dyslexia: a German-English comparison. Cognition 63, 315–334 10.1016/S0010-0277(97)00005-X [DOI] [PubMed] [Google Scholar]
- Maurer U., Brem S., Bucher K., Kranz F., Benz R., Steinhausen H. C., Brandeis D. (2007). Impaired tuning of a fast occipito-temporal response for print in dyslexic children learning to read. Brain 130, 3200–3210 10.1093/brain/awm193 [DOI] [PubMed] [Google Scholar]
- McCandliss B. D., Noble K. G. (2003). The development of reading impairment: a cognitive neuroscience model. Ment. Retard. Dev. Disabil. Res. Rev. 9, 196–204 10.1002/mrdd.10080 [DOI] [PubMed] [Google Scholar]
- Paulesu E., Démonet J. F., Fazio F., McCrory E., Chanoine V., Brunswick N., Cappa S. F., Cossu G., Habib M., Frith C. D., Frith U. (2001). Dyslexia: cultural diversity and biological unity. Science 291, 2165–2167 10.1126/science.1057179 [DOI] [PubMed] [Google Scholar]
- Price C. J., Devlin J. T. (2011). The interactive account of ventral occipitotemporal contributions to reading. Trends Cogn. Sci. 15, 246–253 10.1016/j.tics.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh K. R., Mencl W. E., Jenner A. R., Katz L., Frost S. J., Lee J. R., Shaywitz S. E., Shaywitz B. A. (2000). Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Ment. Retard. Dev. Disabil. Res. Rev. 6, 207–213 [DOI] [PubMed] [Google Scholar]
- Raschle N. M., Zuk J., Gaab N. (2012). Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset. Proc. Natl. Acad. Sci. U.S.A. 109, 2156–2161 10.1073/pnas.1107721109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F., Kronbichler M., Wimmer H. (2009). Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Hum. Brain Mapp. 30, 3299–3308 10.1002/hbm.20752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F., Kronbichler M., Wimmer H. (2011). Meta-analyzing brain dysfunctions in dyslexic children and adults. Neuroimage 56, 1735–1742 10.1016/j.neuroimage.2011.02.040 [DOI] [PubMed] [Google Scholar]
- Richlan F., Sturm D., Schurz M., Kronbichler M., Ladurner G., Wimmer H. (2010). A common left occipito-temporal dysfunction in developmental dyslexia and acquired letter-by-letter reading? PLoS One 5:e12073 10.1371/journal.pone.0012073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandak R., Mencl W. E., Frost S. J., Pugh K. R. (2004). The neurobiological basis of skilled and impaired reading: recent Findings and new directions. Sci. Stud. Read. 8, 273–292 [Google Scholar]
- Schlaggar B. L., McCandliss B. D. (2007). Development of neural systems for reading. Annu. Rev. Neurosci. 30, 475–503 10.1146/annurev.neuro.28.061604.135645 [DOI] [PubMed] [Google Scholar]
- Shaywitz B. A., Skudlarski P., Holahan J. M., Marchione K. E., Constable R. T., Fulbright R. K., Zelterman D., Lacadie C., Shaywitz S. E. (2007). Age-related changes in reading systems of dyslexic children. Ann. Neurol. 61, 363–370 10.1002/ana.21093 [DOI] [PubMed] [Google Scholar]
- Shaywitz S. E., Shaywitz B. A. (2005). Dyslexia (specific reading disability). Biol. Psychiatry 57, 1301–1309 10.1016/j.biopsych.2005.01.043 [DOI] [PubMed] [Google Scholar]
- Shaywitz S. E., Shaywitz B. A. (2008). Paying attention to reading: the neurobiology of reading and dyslexia. Dev. Psychopathol. 20, 1329–1349 10.1017/S0954579408000631 [DOI] [PubMed] [Google Scholar]
- Shaywitz S. E., Shaywitz B. A., Fulbright R. K., Skudlarski P., Mencl W. E., Constable R. T., Pugh K. R., Holahan J. M., Marchione K. E., Fletcher J. M., Lyon G. R., Gore J. C. (2003). Neural systems for compensation and persistence: young adult outcome of childhood reading disability. Biol. Psychiatry 54, 25–33 10.1016/S0006-3223(02)01836-X [DOI] [PubMed] [Google Scholar]
- Snowling M. J. (2000). Dyslexia. Oxford, UK: Blackwell [Google Scholar]
- van der Mark S., Klaver P., Bucher K., Maurer U., Schulz E., Brem S., Martin E., Brandeis D. (2011). The left occipitotemporal system in reading: disruption of focal fMRI connectivity to left inferior frontal and inferior parietal language areas in children with dyslexia. Neuroimage 54, 2426–2436 10.1016/j.neuroimage.2010.10.002 [DOI] [PubMed] [Google Scholar]
- Vellutino F. R., Fletcher J. M. (2005). “Developmental dyslexia,” in The Science of Reading: A Handbook, eds Snowling M. J., Hulme C. J. (Oxford, UK: Blackwell; ), 362–378 [Google Scholar]
- Wimmer H. (1993). Characteristics of developmental dyslexia in a regular writing system. Appl. Psycholinguist. 14, 1–33 [Google Scholar]
- Wimmer H., Schurz M., Sturm D., Richlan F., Klackl J., Kronbichler M., Ladurner G. (2010). A dual-route perspective on poor reading in a regular orthography: an fMRI study. Cortex 46, 1284–1298 10.1016/j.cortex.2010.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M., Bowers P. (1999). The double-deficit hypothesis for the developmental dyslexias. J. Educ. Psychol. 91, 415–438 [Google Scholar]
- World Health Organization (2007). ICD-10 – International Statistical Classification of Diseases and Related Health Problems. Geneva, Switzerland: World Health Organization; [Google Scholar]
- Ziegler J. C., Perry C., Jacobs A. M., Braun M. (2001). Identical words are read differently in different languages. Psychol. Sci. 12, 379–384 [DOI] [PubMed] [Google Scholar]