Conservative surgery can be proposed for cases of urinary tract endometriosis associated with postoperative amenorrhea; however, there is a risk of concomitant colorectal surgery and postoperative complications.
Keywords: Deep infiltrating endometriosis, Bladder, Ureter, Anastomotic fistula, Postoperative complications
Abstract
Objectives:
To report the outcomes of surgical management of urinary tract endometriosis and discuss the choice between conservative and radical surgery.
Materials and Methods:
We reviewed data concerning women managed for ureteral or bladder deep infiltrating endometriosis in 5 surgical departments participating in the CIRENDO prospective database. Preoperative data, surgical procedure data, and postoperative outcomes were analyzed.
Results:
Data from 30 women pooled in the database showed 15 women presenting with ureteral endometriosis, 14 women with bladder nodules, and 1 with both types of lesions. Ureterolysis was performed in 14 cases; the ureter was satisfactorily freed in 10 of these. In 4 women over 40 years old, who were undergoing definitive amenorrhea, moderate postoperative ureteral stenosis was tolerated and later improved in 3 cases, while the fourth underwent secondary ureteral resection and ureterocystoneostomy. Primary ureterectomy was carried out in 4 women. Two cases of intrinsic ureteral endometriosis were found in 5 ureter specimens. Four complications were related to surgical procedures on ureteral nodules, and 2 complications followed the removal of bladder endometriosis. Delayed postoperative outcomes were favorable with a significant improvement in painful symptoms and an absence of unpleasant urinary complaints, except for one patient with prolonged bladder denervation.
Conclusion:
Conservative surgery, in association with postoperative amenorrhea, can be proposed in a majority of cases of urinary tract endometriosis. Although the outcomes are generally favorable, the risk of postoperative complications should not be overlooked, as surgery tends to be performed in conjunction with other complex procedures such as colorectal surgery.
INTRODUCTION
Endometriosis is characterized by the presence of active endometrial tissue outside the uterine cavity.1 It has an estimated prevalence of up to 15% in women of reproductive age2 and has considerable economic implications both for the patient and the National Health System.3 Three types of endometriosis have been described: peritoneal superficial endometriosis, ovarian endometriomas, and deep infiltrating endometriosis (DIE). The latter usually involves the uterosacral ligaments (USL), the rectovaginal space (RVS), the upper third of the posterior vaginal wall, the bowel, and the urinary tract.4
Urinary tract DIE may be found in up to 6% of women presenting with pelvic endometriosis and may involve either the bladder or ureters.5 The management of urinary endometriosis, as well as that of other localizations of DIE, is not based on high-level evidence data, but rather on case-series reported by surgical teams working in tertiary referral centers. Because the vast majority of authors are surgeons, these studies tend to conclude that surgical management is curative and can be performed successfully by experienced multidisciplinary teams. The association of medical treatment is often recommended, either pre- or postoperatively. Most case-series provide very good outcomes after short- and midterm follow-up, giving rise to the general opinion that outcomes of surgical removal of urinary tract DIE are favorable in a majority of cases.6,7 However, the choice between ureterolysis and segmental resection in the treatment of ureteral endometriosis remains controversial.6–10
The aim of our study was to report the outcomes of surgical management of urinary DIE in women managed by various surgical teams participating in the CIRENDO prospective database and to focus on the choice of surgical strategy and the circumstances in which specific complications occurred.
METHODS
A retrospective review was performed of data from the CIRENDO database, including patients managed for endometriosis in 11 surgical departments situated in the North-West of France and on Reunion Island. CIRENDO (the North-West Inter Regional Female Cohort for Patients with Endometriosis) is a prospective cohort, financed by the G4 Group (The University Hospitals of Rouen, Lille, Amiens, and Caen) and coordinated by one of the authors (H.R.). The CIRENDO Study Group includes gynecologic surgeons from the 4 University Hospital Departments of Gynecology previously mentioned, 3 public hospitals (Belvédère Mont Saint-Aignan, Elbeuf, and Dieppe), 3 private clinics in Normandy (Europe, Mathilde, Saint- Hilaire clinics), and most recently the department of gynecologic surgery at Saint Pierre Regional Hospital on Reunion Island. Patient inclusion in the cohort began in 2005 at the University Hospital of Rouen (women managed for DIE only) and in June 2009 in the other centers (women with all endometriosis localizations). A clinical research technician carries out data recording, contact, and follow-up. Information is obtained using self-questionnaires (1 preoperative and 2 postoperative at 12 and 36 months), and surgical and histological records. The questionnaires are identical to those used for the Endometriosis database of Auvergne, (coordinated by Prof Michel Canis, Clermont Ferrand, France), allowing the pooling of data from the 2 databases. To date, data from more than 420 women with histologically confirmed endometriosis have been recorded in the CIRENDO database.
We selected from the database all women for whom the surgeon answered “yes” to the question “Did you carry out a urologic surgical procedure?” After a thorough review of surgical reports, only women who had benefited from cystectomy (either full-thickness or up to the mucosa), ureteral segmental resection or ureterolysis were included. Women managed by this latter procedure were only included if the ureter was completely surrounded by a fibrous endometriotic ring, resulting in either extrinsic compression of the ureteral wall or intrinsic involvement of the ureteral muscularis or mucosa,7 in line with Nezhat et al's definition of “ureteral endometriosis” as being any situation where endometriosis causes compression or distortion of the anatomy of the ureter, even when hydronephrosis is not yet present.7
The preoperative data and detailed information on surgical procedures were taken from the CIRENDO database, and information about preoperative workup and postoperative outcomes were checked from the medical records of each patient. Delayed postoperative outcomes were evaluated using CIRENDO standardized self-questionnaires and the standardized MHU (Measurement of Urinary Handicap) questionnaire.11
The preoperative workup included MRI (Magnetic Resonance Imaging), cystoscopy, pelvic and abdominal ultrasonography, CT (computed tomography) scan or intravenous pyelography, and renal scintigraphy (Table 1). Several patients also underwent endorectal ultrasonography or CT scan with virtual colonoscopy depending on the localizations (Table 1).
Table 1.
Patient Characteristics (N=30)
| Ureteral Endometriosis N=16 (53%) | Bladder Endometriosis N=15 (50%) | |
|---|---|---|
| Age (yrs; mean±SD) | 38.3±6.2 | 33.4±6.1 |
| Parity | ||
| Nulliparous | 4 (25) | 5 (33.3) |
| Para ≥1 | 12 (75) | 10 (66.7) |
| Associated localizations | ||
| Sigmoid colon | 6 (37.5) | 5 (33.3) |
| Rectum | 13 (81.3) | 8 (53.3) |
| Rectovaginal space | 3 (18.8) | 5 (33.3) |
| Ovaries | 4 (25) | 4 (26.7) |
| Uterosacral ligaments | 16 (100) | 4 (26.7) |
| Small bowel | 1 (6.3) | 4 (26.7) |
| Diaphragm | 2 (12.5) | 2 (13.3) |
| Appendix | 1 (6.3) | 1 (6.7) |
| Pain evaluation using 10-points analog rating scale* | ||
| Dysmenorrhea | 8.2±1.8 | 8.2±1.5 |
| Deep dyspareunia | 6.6±2.3 | 6.8±2.3 |
| Non menstrual pain | 7±2.6 | 6.8±2.3 |
| History of painful symptoms (years) | ||
| Dysmenorrhea | 12.7±9.9 | 7.8±5.7 |
| Deep dyspareunia | 9.1±6.3 | 6.2±1.6 |
| Nonmenstrual pain | 9.7±3.5 | 2.9±3.3 |
| Fertility | ||
| No infertility | 12 (75) | 10 (66.7) |
| Primary infertility | 4 (25) | 4 (25) |
| Secondary infertility | 0 | 1 (6.7) |
| Ureteral endometriosis | ||
| Left | 10 (62.5) | 0 |
| Right | 4 (25) | 0 |
| Bilateral | 2 (12.5) | 1 (6,7) |
| Bladder endometriosis | ||
| Supratrigonal | 1 (6.3) | 13 (86.7) |
| Trigonal | 0 | 2 (13,3) |
| Previous surgery for endometriosis | 9 (56.3) | 8 (53.3) |
| Preoperative workup | ||
| MR imaging | 16 (100) | 15 (100) |
| Pelvic and abdominal ultrasonography | 11 (68.8) | 8 (53.3) |
| Pyelography | 7 (43.8) | 10 (66.7) |
| Computed tomography + IV contrast | 1 (6.3) | 2 (13.3) |
| Endorectal ultrasonography | 14 (87.6) | 10 (66.7) |
| Computed tomography + virtual colonoscopy | 4 (25) | 2 (13.3) |
| Renal scintigraphy | 1 (6.3) | 0 |
| Cystoscopy ± ureteral stent insertion | 7 (43.8) | 10 (66.7) |
Surgical procedures were performed by surgeons experienced in laparoscopic management of DIE, assisted by urological surgeons. Double-J ureteral stents were either pre- or intraoperatively inserted, and decisions regarding surgical procedures on the urinary tract were based on intraoperative findings. Ureterolysis was considered satisfactory when the fibrous ring surrounding the ureter was completely removed to a point where the remaining ureter appeared distally normal. When ureterolysis was insufficient for the removal of ureter stenosis, ureteral resection was immediately performed with either end-to-end ureteral anastomosis or ureterocystoneostomy. For women over 40 years old undergoing definitive therapeutic amenorrhea, ureterolysis was performed with careful follow-up of the postoperative resolution of ureteral stenosis.
In cases of DIE of the bladder, full-thickness excision of the nodule and adjacent bladder wall was carried out, followed by immediate single-layer suture using 3-0 PDS, The depth of the excision into the myometrium was dependant on surgeon procedure and the patient's intention to conceive. In women undergoing cystectomy, cystography was performed before a decision was made about the removal of bladder catheterization at day 8. Ureteral stents were usually removed after 1 (bladder endometriosis) to 6 weeks (ureteral endometriosis). Postoperative medical treatment to induce prolonged amenorrhea was recommended in patients who did not intend to conceive.
RESULTS
According to inclusion criteria, from January 2006 to June 2010, 30 women were eligible for the study: 26 were managed at Rouen University Hospital and 4 at the centers Saint Hilaire, Europe, Elbeuf, and Saint Pierre. Patient characteristics and preoperative workup data are presented in Table 1.
Table 2 presents the surgical procedures performed in relation to the different localizations of endometriosis. Due to bilateral localizations in 2 women, 18 procedures on ureteral nodules were recorded. Ureterolysis was performed in 14 nodules, and the ureter was satisfactorily freed in 10 cases (Figure 1). In the remaining 4, moderate ureteral stenosis was tolerated, 3 of which showed postoperative improvement in both pyelon and ureter diameter. As regards the fourth case, persistent stenosis of the ureter and dilatation of the pyelon justified performing ureteral resection and ureterocystoneostomy 5 months later. Four women (25%) underwent primary segmental resection of the ureter with immediate end-to-end ureteral anastomosis. An intraoperative complication was recorded in only one woman, managed for enlarged DIE of both the rectum and the right ureter. Lateral dissection led to deep hemorrhage originating in the obturator vein, laparoconversion was performed and hemostasis was achieved by prolonged compression of the vein. Despite blood loss requiring intraoperative blood transfusion, postoperative outcomes were favorable.
Table 2.
Surgical Procedures Performed in Patients with Urinary Endometriosis (N=30)
| Ureteral Endometriosis N=16 (53%) | Bladder Endometriosis N=15 (50%) | |
|---|---|---|
| ASFr-classification (intra-operative finding) | ||
| Stage I | 0 | 1 (6.7) |
| Stage II | 0 | 3 (20) |
| Stage III | 6 (37.5) | 1 (6.7) |
| Stage IV | 10 (62.5) | 10 (66.7) |
| Urinary tract surgical procedures | ||
| Ureterolysis | 13 (81.3) | 2 (13.3) |
| Ureterectomy + end-to-end ureteral anastomosis | 3 (18.8) | 1 (6.7) |
| Ureterectomy + ureteroneocystotomy | 2* (12.5) | 1** (6.7) |
| Nephrectomy | 0 | 0 |
| Partial cystectomy | 1 (6.3) | 15 (100) |
| Full-thickness | 1 (6.3) | 13 (86.7) |
| Without opening the bladder | 0 | 2 (13.3) |
| Digestive tract surgical procedures | ||
| Rectal shaving | 5 (31.3) | 2 (13.3) |
| Colorectal resection + colorectal anastomosis | 1 (6.3) | 5 (33.3) |
| Temporary stoma | 1 (6.3) | 5 (33.3) |
| Small bowel resection | 1 (6.3) | 1 (6.7) |
| Appendectomy | 0 | 1 (6.7) |
| Omentoplasty | 2 (12.5) | 1 (6.7) |
| Other surgical procedures | ||
| Excision of rectovaginal nodules | 4 (25) | 3 (20) |
| Ovarian endometrioma vaporization using plasma energy | 1 (6.3) | 1 (6.7) |
| Ovarian endometrioma cystectomy | 3 (18.8) | 3 (20) |
| Total hysterectomy | 3 (18.8) | 2 (13.3) |
| Operative time (min) | 281.9±98.6 | 260.3±116.7 |
| Surgical route | ||
| Laparoscopy | 15 (93.8) | 12 (80) |
| Laparotomy | 1 (6.3) | 3 (20) |
| Intraoperative complications | ||
| Hemorrhage requiring laparoconversion | 1 (6.3) | 0 |
| Inadvertent ureteral section | 0 | 1** (6.7) |
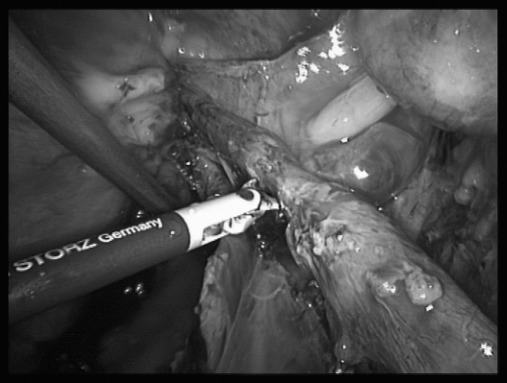
Figure 1.
Right ureteral stenosis due to deep infiltrating endometriosis whose management included simple ureterolysis and postoperative amenorrhea, resulting in progressive resolution of both pyelic dilatation and painful symptoms.
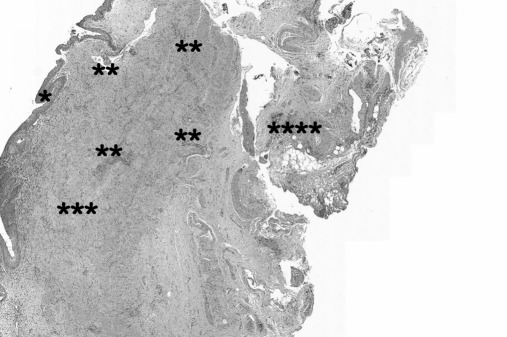
Among the 5 specimens of ureters removed, intrinsic ureteral endometriosis was histologically revealed in only 2 cases (40%; Figure 2).
Figure 2.
Histological findings in intrinsic ureteral endometriosis: *urothelium; **intrinsic endometriosis foci; ***ureteral muscular layer; ****ureteral adventitial sheath.
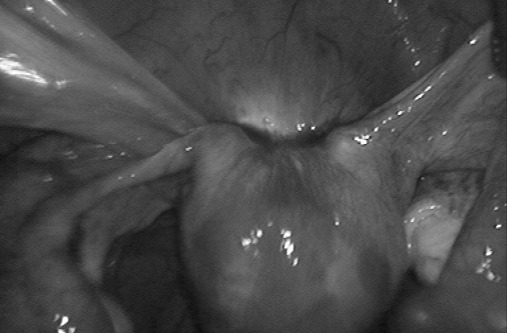
Fifteen women presented with DIE of the bladder (Figure 3) and underwent full-thickness excision of the nodule (86.7%; Figure 4) or excision of the bladder wall up to the submucosal layer (13.3%). A severe intraoperative complication was recorded in one woman who presented with an enlarged bladder nodule, associated with DIE of the rectovaginal space. During the dissection of the left edge of the bladder nodule, the left ureter was sectioned close to the uterine isthmus. Laparoconversion with ureterocystoneostomy was carried out with a favorable outcome.
Figure 3.
Bladder endometriotic (adenomyotic) nodule managed by full-thickness excision.
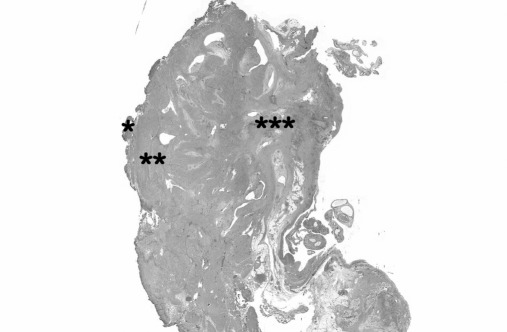
Figure 4.
Histological findings in bladder endometriosis: *urothelium; **bladder muscular layer; ***endometriotic (adenomyotic) foci.
After a mean follow-up of 24.9 months (SD 17.8, range 5 to 60), no patient showed recurrent urinary tract endometriotic lesions, and no patient was lost to follow-up.
In 16 women managed for ureteral nodules, 4 complications were noted (25%). One patient who underwent ureterolysis with a Harmonic scalpel had delayed ureteric fistula due to secondary thermal necrosis that was treated using a pig-tailed ureteric stent, followed by ureterocystoneostomy. A second patient managed by segmental resection for intrinsic endometriosis was admitted 7 days after surgery with pyelonephritis that was successfully treated with antibiotherapy. The third complication occurred in a 33-year-old woman managed for enlarged DIE of both the rectum and ureter, where removal was followed by bladder atony, requiring 5 bladder catheterizations per day over 6 months. The fourth patient was managed for DIE involving the rectum, responsible for extrinsic stenosis of the ureter, and underwent enlarged hysterectomy, rectal shaving, and segmental ureteric resection with end-to-end anastomosis. Because the Double-J ureteral stent had been inserted 6 months previously, an extensive calcified crust had developed, leading to intraoperative spreading of microlithiasis into the pyelon, above the anastomosis. These circumstances were responsible for rapid postoperative obliteration of the stent and anastomosis fistula, requiring secondary ureterocystoneostomy.
In 15 patients managed for bladder endometriosis, 2 severe complications were recorded (13.3%). A 28-year-old woman undergoing full-thickness excision of the dome nodule presented with a fistula between the bladder and anterior vaginal wall, requiring reintervention 5 weeks later, with a favorable outcome. Another woman with DIE of the bowel and an endometriotic nodule arising on the bladder trigon underwent full-thickness removal of the bladder nodule, total hysterectomy, and segmental resection of the sigmoid colon. Subsequent prolonged bladder atony required 4 to 5 bladder catheterizations per day over a 15-month period.
Delayed postoperative outcomes were favorable with a significant improvement in painful symptoms and the absence of unpleasant urinary complaints, with the exception of one woman with prolonged bladder denervation (Table 3). A complete relief of pelvic pain and dyspareunia, or a major improvement in the intensity of pain was recorded. Not earlier than 6 months after surgery, the assessment of urinary function was performed using MHU scores, and revealed satisfactory outcomes. Among 9 women who intended to become pregnant, 5 became pregnant spontaneously and one following an IVF procedure. The remaining 3 women who failed to conceive were found to have an impaired ovarian reserve as suggested by abnormal levels of anti-müllerian hormone (AMH). With the exception of one patient with bladder denervation, all women would recommend the surgical management to a friend who had the same disease (96%).
Table 3.
Delayed Postoperative Outcomes
| Ureteral Endometriosis N=16 (53%) | Bladder Endometriosis N=15 (50%) | |
|---|---|---|
| Presence of painful symptoms | ||
| Dysmenorrhea | 3 (18.8) | 7 (46.7) |
| Deep dyspareunia | 5 (31.6) | 6 (40) |
| Non menstrual pain | 5 (31.6) | 7 (46.7) |
| Pain evaluation using 10-points analog rating scale* | ||
| Dysmenorrhea | 1.6±3.4 | 2.1±2.8 |
| Deep dyspareunia | 2.5±3.9 | 2.5±3.5 |
| Non menstrual pain | 2.3±3.7 | 2.3±3.1 |
| Fertility | ||
| Intention to become pregnant after surgery | 2 (12.5) | 7 (46.7) |
| Pregnancy | 2 | 4 |
| Delivery | 1 | 2 |
| MHU (urinary handicap measurement) | ||
| - urinary incontinence score | 1.93±2.3 | 1.2±1.7 |
| - urinary imperiosity score | 1.5±1.9 | 2.2±1.8 |
| - Pollakiuria score | 1.7±1.8 | 1.6±1.7 |
| - dysuria score | 0.7±1.3 | 0.8±1.4 |
| Endometriosis surgery required due to recurrent complaints | 0 | 0 |
| Recovery from endometriosis related pain | 0 | 0 |
| Recovery from postoperative complications or additional surgical procedures | **1 (6.3) | **2 (13.3) |
| Medical treatment | ||
| GnRH analogs | 5 (31.6) | 8 (53.3) |
| Progestins | 7 (43.8) | 4 (26.7) |
| Contraceptive pills | 1 (6.3) | 3 (20) |
| Levonorgestrel intrauterine device | 0 | 0 |
| Implanon® | 0 | 0 |
| Assisted reproductive techniques | ||
| Ovarian stimulation | 2 (12.5) | 3 (20) |
| Intrauterine insemination | 0 | 0 |
| IVF | 1 (6.3) | 2 (13.3) |
DISCUSSION
Our case-series shows that surgical management of DIE of the urinary tract may successfully be performed by trained surgeons, with specific postoperative complications occurring in up to 25% of patients. Although most authors agree that surgical management of urinary tract DIE leads to favorable outcomes in a majority of cases,7 we believe that patients should be aware of the associated additional surgical procedures, eg, insertion of ureteral stents or ureteroneocystotomy, and the risk of postoperative complications, such as inadvertent bladder denervation or vaginal fistulae.
Ureteral endometriosis is most commonly unilateral, involving a small segment of distal left ureter and often represented by a per-ureteric ring.7,12,13 It may be intrinsic or extrinsic, depending on whether the uroepithelium and submucosal layer of the ureteral wall are infiltrated.7 Extrinsic compression of the ureter appears to be more common than intrinsic disease14; however, several authors have suggested that the rate of occurrence of the latter is probably underestimated by surgeons who perform ureterolysis compared to those who tend to carry out ureteral resection.9 Ureteral involvement is usually due to the lateral spreading of deep endometriosis primarily infiltrating rectovaginal space or uterosacral ligaments, and is associated in two-thirds of cases with ipsilateral ovarian endometriomas.7 Progressive ureteral stenosis may be responsible for the destruction of the renal parenchyma and gradual kidney function loss, which can be preoperatively estimated by scintigraphy.15,16
Bladder endometriosis usually presents as an adenomyotic nodule originating from the anterior uterine wall and peritoneum, progressing forward towards the bladder, which it covers at the submucosal and mucosal layer. Both are infiltrated and pushed into the bladder cavity, subsequently becoming visible on cystoscopy. Bladder DIE may be responsible for various painful or unpleasant symptoms, such as suprapubic pain, dysuria, hematuria, and cyclic urgency.7,17 Most authors believe the complete removal of bladder endometriosis to be relatively simple, although it can be difficult to intraoperatively identify the posterior limit of the nodule against the myometrium, which must be preserved in women who desire to conceive. MRI examination usually shows the anterior wall of the uterus wrapping around the adenomyotic nodule, so that complete nodule excision requires the hollowing out of the nodule in the myometrium; and although skilled surgeons in a majority of cases can achieve complete removal of bladder endometriosis, that of the uterus may remain incomplete.
In some women presenting with hydronephrosis, the insertion of a Double-J ureteral stent is performed before referral to a tertiary reference center. If the delay between the Double-J insertion and the surgical management of the ureteral or bladder DIE exceeds several weeks, a calcification process may develop around the stent. Because the stent is usually replaced during the second surgical procedure, manipulation may break the calcified crust leading to microlithiasis of the urinary tract. In these circumstances, there is a risk of ureteral stent obliteration followed by urine overpressure in the urinary tract and anastomosis fistulae. We recommend, based on experience, ruling out the risk of microlithiasis before performing ureteral resection.
Despite the complexity of surgical procedures and the severity of postoperative complications, delayed outcomes are favorable in a large majority of patients, as shown by the MHU assessment and improvement in pelvic pain. The rate of pregnancy is also satisfactory, particularly as failure to conceive is generally due to factors other than ureteral or bladder nodules. After a follow-up varying from 5 to 60 months, 96% of women expressed satisfaction with postoperative results and would recommend the surgical treatment to a friend suffering from the same disease.
Despite numerous research papers previously published in the literature, there is as yet no definitive answer to the question “Which surgical procedure should be recommended for women presenting with ureteral endometriosis?” Studies show that in general 2 surgical approaches are used, resection of the ureteral segment, and ureterolysis, which frees the ureter from extrinsic compression. Most surgeons are familiar with both techniques, though the reasons that guide their choice remain unclear particularly as recommendations on the surgical management of urinary tract DIE tend to reflect experience or personal convictions based on scarce evidence. Thus, some authors9 recommend radical resection to reduce the risk of recurrences as much as possible, while other authors10 reserve ureterectomy for only limited cases and believe conservative surgery to be the management of choice in relieving ureteral obstruction and removing adenomyosis or endometriosis, even in patients with moderate or severe pyelic dilatation.
In our opinion, this debate much resembles that concerning the management of deep endometriosis infiltrating the rectum in which the 2 general convictions about DIE, separate surgeons worldwide into 2 distinct categories. The ”carcinologic” approach is adopted by surgeons who strongly believe that the radical removal of all endometriotic foci is the most effective way to prevent the risk of recurrences, much as radical surgery does in cancer. Thus ureteral resection should be performed to avoid leaving intrinsic implants in place.9 Conversely, other authors have proposed an approach to DIE that focuses more on the woman's needs and the resolution of complaints, than on prior lesion excision.18 This approach becomes possible where medical treatment is associated with surgery and administered long term, ie, for years and not only for months18 and suggests that the surgical approach in DIE should primarily focus on the relief of urinary symptoms, hydronephrosis, and pelvic pain, rather than on mandatory “carcinologic” resection of lesions. We believe that the latter symptom-guided approach with conservative management could provide a valid therapeutic option in a majority of cases, especially in women who no longer intend to conceive and who receive long-term medical treatment.19 Furthermore, in our case-series, 3 of 5 ureter specimens were free of any endometriotic intrinsic implants, a result which is comparable to other results previously reported in the literature,9,20 and which suggests that in half the number of cases, the prevention of ureteral recurrences cannot be justification alone for performing a ureterectomy.
Randomized studies comparing a radical to conservative surgical approach are not available on this topic; therefore, case-series or retrospective comparative studies would be welcome in providing information that could be pooled in systematic reviews. Because the prevalence of urinary tract endometriosis is relatively low, the only way to assess effective treatment is through a large randomized study involving surgical teams worldwide, skilled in both radical and conservative approaches, where outcomes are measured not only in terms of recurrence rate but also the risk of postoperative complications.
Contributor Information
Francisc Rozsnyai, Department of Gynecology and Obstetrics, University Hospital, TârguMure, Romania..
Horace Roman, Department of Gynecology and Obstetrics, Rouen University Hospital-Charles Nicolle, Rouen, France.; Research Group 4308 “Spermatogenesis and Male Gamete Quality,” IHU Rouen Normandy - IFRMP23, Reproductive Biology Laboratory, Rouen University Hospital, Rouen, France.
Benoit Resch, Department of Gynecology and Obstetrics, Rouen University Hospital-Charles Nicolle, Rouen, France..
Fabrice Dugardin, Department of Urology, Rouen University Hospital-Charles Nicolle, Rouen, France..
Juan Berrocal, Clinique de l’Europe, Rouen, France..
Gérôme Descargues, Clinique Saint Hilaire, Rouen, France..
Remi Schmied, Department of Gynecology and Obstetrics, General Hospital Elbeuf, France..
Malik Boukerrou, Department of Gynecology and Obstetrics, Saint Pierre General Regional Hospital, Reunion Island, France..
Loïc Marpeau, Department of Gynecology and Obstetrics, Rouen University Hospital-Charles Nicolle, Rouen, France..
References:
- 1. Antonelli A, Simeone C, Zani D, et al. Clinical aspects and surgical treatment of urinary tract endometriosis: our experience with 31 cases. Eur Urol. 2006;49:1093–1097 [DOI] [PubMed] [Google Scholar]
- 2. Hemmings R, Rivard M, Olive Dl, Poliquin-Fleury J, Gagne D, Hugo P, Gosselin D. Evaluation of risk factors associated with endometriosis. Fertil Steril. 2004;81:1513–1521 [DOI] [PubMed] [Google Scholar]
- 3. Gao X, Outley J, Botteman M, Spalding J, Simon JA, Pashos CL. Economic burden of endometriosis. Fertil Steril. 2006;86:1561–1572 [DOI] [PubMed] [Google Scholar]
- 4. Chapron C, Chopin N, Borghese B, et al. Deeply infiltrating endometriosis: Pathogenic implications of the anatomical distribution. Hum Reprod. 2006;21:1839–1845 [DOI] [PubMed] [Google Scholar]
- 5. Nehzat CH, Malik S, Osias J, Nezhat F, Nezhat C. Laparoscopic management of 15 patients with infiltrating endometriosis of the bladder and a case of primary intravesical endometrioid adenosarcoma. Fertil Steril. 2002;78:872–875 [DOI] [PubMed] [Google Scholar]
- 6. Nezhat CR, Nezhat F, Admon D, Seidman D, Nezhat CH. Laparoscopic management of genitourinary endometriosis. J Am Assoc Gynecol Laparosc. 1994;1:S25. [PubMed] [Google Scholar]
- 7. Bosev D, Nicoll LM, Bhagan L, et al. Laparoscopic management of ureteral endometriosis: the Stanford University Hospital experience with 96 consecutive cases. J Urol. 2009;182:2748–2752 [DOI] [PubMed] [Google Scholar]
- 8. Nezhat CH, Malik S, Nezhat F, Nezhat C. Laparoscopic ureteroneocystostomy and vesicopsoas hitch for infiltrative endometriosis. JSLS. 2004;8:3–7 [PMC free article] [PubMed] [Google Scholar]
- 9. Chapron C, Chiodo I, Leconte M, et al. Severe ureteral endometriosis: the intrinsic type is not so rare after complete surgical exeresis of deep endometriotic lesions. Fertil Steril. 2010;93:2115–2120 [DOI] [PubMed] [Google Scholar]
- 10. Donnez J, Nisolle M, Squifflet J. Ureteral endometriosis: a complication of rectovaginal endometriotic (adenomyotic) nodules. Fertil Steril. 2002;77:32–37 [DOI] [PubMed] [Google Scholar]
- 11. Amarenco G, Kerdraon J, Perrigot M. Echelle d’évaluation du handicap pelvien : mesure du handicap urinaire (MHU). In: Pélissier J, Coster P, Lopez S, Marés P, ed. Rééducation vésico-sphinctérienne et ano-rectale. Paris: Masson; 1992;498–504 [Google Scholar]
- 12. Vercellini P, Pisacreta A, Pesole A, Vicentini S, Stellato G, Crosignani PG. Is ureteral endometriosis an asymetric disease? BJOG. 2000;107:559–561 [DOI] [PubMed] [Google Scholar]
- 13. Ghezzi F, Cromi A, Bergamini V, Serati M, Sacco A, Meuller MD. Outcome of laparoscopic ureterolysis for ureteral endometriosis. Fertil Steril. 2006;86:418–422 [DOI] [PubMed] [Google Scholar]
- 14. Umar SA, Maclennan GT, Cheng L. Endometriosis of the ureter. J Urol. 2008;179:2412. [DOI] [PubMed] [Google Scholar]
- 15. Collinet P, Marcelli F, Villers A, Regis C, Lucot JP, Cosson M, Vinatier D. Management of endometriosis of the urinary tract. Gynecol Obstet Fertil. 2006;34:347–352 [DOI] [PubMed] [Google Scholar]
- 16. Seracchioli R, Mabrouk M, Manuzzi L, et al. Importance of retroperitoneal ureteric evaluation in cases of deep infiltrating endometriosis. J Minim Invasive Gynecol. 2008;15:435–439 [DOI] [PubMed] [Google Scholar]
- 17. Abrao MS, Dias JA, Jr., Bellelis P, Podgaec S, Bautzer CR, Gromatsky C. Endometriosis of the ureter and bladder are not associated diseases. Fertil Steril. 2009;91:1662–1667 [DOI] [PubMed] [Google Scholar]
- 18. Vercellini P, Crosignani PG, Abbiati A, Somigliana E, Vigano P, Fedele L. The effect of surgery for symptomatic endometriosis: the other side of the story. Hum Reprod Update. 2009;15:177–188 [DOI] [PubMed] [Google Scholar]
- 19. Roman H, Vassilieff M, Gourcerol G, et al. Surgical management of deep infiltrating endometriosis of the rectum: pleading for a symptom-guided approach. Hum Reprod. 2010; doi: 10.1093/humrep/deq332 [DOI] [PubMed] [Google Scholar]
- 20. Mereu L, Gagliardi ML, Clarizia R, Mainardi P, Landi S, Minelli L. Laparoscopic management of ureteral endometriosis in cases of moderate-severe hydroureteronephrosis. Fertil Steril. 2010;93:46–51 [DOI] [PubMed] [Google Scholar]