Laparoscopic cholecystectomy in patients with sickle cell disease was found to be safe without preoperative blood transfusion.
Keywords: Sickle cell disease, Surgery, Postoperative complications, Blood transfusion
Abstract
Background:
Surgery in patients with sickle cell disease is associated with high morbidity. To reduce this high morbidity, different preoperative transfusion regimens were introduced. However, blood transfusion is associated with problems. This prospective study aims to establish the safety of conducting laparoscopic cholecystectomy without transfusion in sickle cell disease patients.
Methods:
Forty patients (16 males and 24 females; mean age 26.6 years) undergoing laparoscopic cholecystectomy for cholelithiasis were divided into 2 matched groups: Group I “no transfusion” (n=24 patients; 60%) and Group II “transfusion” (n=16; 40%). In Group II, 9 patients (22.5%) received a simple transfusion and 7 (17.5%) a partial exchange transfusion.
Results:
Group II patients had significantly higher levels of Hb-S prior to transfusion. They developed a significantly higher complication rate (25% vs. 0%) and subsequently longer hospital stay (3.9±2 vs. 2.1±1.4). Moreover, there was no significant difference in the complications between the simple transfusion and partial exchange transfusion subgroups.
Conclusion:
Surgery in SCD patients is safe without a preoperative blood transfusion. Moreover, preoperative blood transfusion is associated with significantly higher postoperative complications and longer hospital stay. Hence, a “no transfusion” policy is recommended.
INTRODUCTION
Sickle cell disease (SCD), the most common inherited hematological disease affecting man, is prevalent in the Arabian Gulf countries. The disease is characterized by red cells undergoing sickling due to polymerization of sickle hemoglobin (Hb-S) when deoxygenated, with subsequent loss of flexibility and repeated hemolysis with microvascular occlusion.1 The susceptibility of red cells to sickling correlates well with the concentration of abnormal Hb-S, which results from substitution of valine for glutamic acid in position 6 of the beta-globin chain.1 This state of chronic hemolysis leads to an increased risk of gallstone formation, with a prevalence ranging from 30% to 70%.2–6
Patients with SCD have a higher incidence of postoperative morbidity and mortality than patients without hematologic abnormalities7–13; the risk of postoperative complications in SCD patients ranges from 7% to 32%.14 Preoperative transfusion appears to reduce morbidity and mortality associated with surgery in SCD patients.2,3,4,13 However, a less aggressive transfusion regimen has been successfully practiced7,8,15 and does not carry a greater risk of postoperative complications than aggressive transfusion carries.12 Furthermore, a large United States observational study10 demonstrated a similar rate of complications in both transfused and nontransfused patients undergoing cholecystectomy, and nonrandomized studies11 have shown lower complication rates in nontransfused SCD patients undergoing minor procedures.
The Eastern province of Saudi Arabia has a large local population of patients with SCD. Hence, since 2006 we have used a standardized approach to the perioperative management of SCD patients undergoing laparoscopic cholecystectomy (LC). This study aims at evaluating the perioperative complications and postoperative course of SCD patients undergoing elective LC with or without perioperative blood transfusion.
MATERIALS AND METHODS
From January 2006 to December 2009, all SCD patients who underwent LC for symptomatic gallstones were prospectively included in the study. Patients were divided into 2 groups: Group I was given no transfusion and Group II received a preoperative blood transfusion (simple or partial exchange transfusion). All patients underwent a standardized perioperative management protocol. In brief, all patients were seen in the pre-anesthetic clinic for preoperative evaluation and preparation and admitted 1 day to 2 days before the planned operation for adequate hydration and blood transfusion if it was indicated. Patients with hemoglobin of ≥10g/dL were not transfused. If hemoglobin was <10g/dL, simple blood transfusion with packed red cells was given regardless of Hb-S percentage. Partial exchange transfusion was given only to patients who were considered to have severe SCD based on very high Hb-S, a history of frequent hospital and ICU admissions with sickling crises, and the presence of sickle-related systemic complications. Blood transfusion was usually given 1 day to 2 days before LC. All patients were adequately hydrated the night before the operation through an intravenous line set up in the general surgical ward. Prophylactic antibiotics (one dose of first-generation cephalosporins) were given intravenously with the induction of anesthesia, and an experienced laparoscopic surgeon conducted the surgery. The operative time was estimated from the start of anesthesia until application of dressings. Patients were nursed postoperatively in a general surgical ward and were kept warm, well oxygenated, pain-free, and well hydrated. Incentive spirometry was used in all patients. Early ambulation to guard against DVT was encouraged, and patients were discharged home once fully ambulant and without any postoperative complications. Postoperative complications were closely monitored and recorded.
Statistical Analysis
Data are presented as mean ± SD and range where appropriate. Comparisons between groups were made by using Fisher's exact test, and the one-way ANOVA test. Values of P<.05 were considered statistically significant. All statistical analyses were conducted using SPSS version 16 software.
RESULTS
During the study period, 40 patients (16 males and 24 females) with SCD underwent LC for symptomatic gallstones. The mean age was 26.6 years (range, 6 to 53). Twenty-three had sickling crises, ranging from 1 to 9 (mean, 3.5) episodes per year. Twenty-four (60%) patients received no transfusion (Group I), and 16 (40%) patients were given preoperative blood transfusions (Group II); 9 (22.5%) received a simple transfusion, and 7 (17.5%) received a partial exchange transfusion. The mean preoperative hemoglobin, fetal hemoglobin (Hb-F), and Hb-S for both groups are shown in Table 1. Both groups were comparable with no significant difference in age, male to female ratio, or operation time (Table 1). Group II patients had significantly lower mean preoperative hemoglobin and higher levels of Hb-S prior to transfusion. Group II patients developed a significantly higher complication rate (31.25% vs. 0%) and subsequently longer hospital stay (3.9 ± SD 2 vs. 2.1 ± SD 1.4). Moreover, no significant difference existed in the complications between the simple transfusion and partial exchange transfusion subgroups.
Table 1.
Comparative Parameters Between the 2 Groups
| Parametera | Group I |
Group II |
P Valueb |
|---|---|---|---|
| No transfusion | Transfused group | ||
| Number of patients | 24 | 16 | |
| Age (Mean±SD) (years) | 26.7±13.5 | 26.5±12.5 | .96 |
| No. of males | 8 | 10 | .07 |
| No. of females | 16 | 6 | .07 |
| Preoperative Hb (mean ±SD) | 10.8±1.1 | 9.2±1.4 | .001 |
| Hb-F (mean ±SD) | 11.7±13.7 | 13.2±8.2 | .7 |
| Hb-S (mean ±SD) | 50.6±22.8 | 74.9±10.4 | .005 |
| Operation time | 102.5±33 | 103±45 | .9 |
| (mean ±SD) | |||
| No. of conversion (%) | 1 (4.2) | 1 (6.25) | .8 |
| Postoperative complications | 0 (0) | 5 (31.25) | .02 |
| Postoperative hospital stay (mean ±SD) | 2.1±1.4 | 3.9±2 | .002 |
Hb=hemoglobin; Hb-F=fetal hemoglobin; Hb-S=sickle hemoglobin; SD=standard deviation.
P=.05; Values <.05 are significant.
Two cases (5%) were converted to open, one in each group. One was due to uncontrollable bleeding during simultaneous splenectomy, and the other was due to dense adhesions in a patient with empyema of the gallbladder.
Postoperative Complications
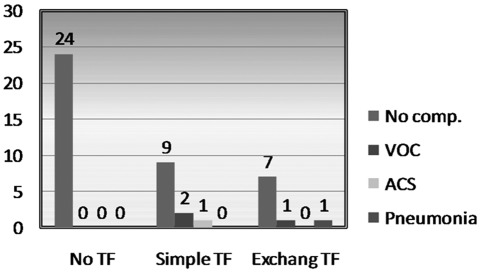
There were 5 (12.5%) postoperative complications; all occurred in Group II patients who received blood transfusions (P<.05) (Figure 1). There were 3 mild vasoocclusive crises (VOC); one patient needed ICU admission for postoperative hypotension (a case of combined LC and splenectomy converted to open due to bleeding), one patient had bronchopneumonia, and one had acute chest syndrome (ACS) and was admitted 2 days after discharge and treated conservatively. No correlation was found between the level of Hb-S and the incidence of complications. Table 2 shows the relation of complications to various parameters. Complications were significant in the transfused Group II (P=.007), very close to significance for the Age group ≥30 years (P=.05), and close to significance for the number of crises, but insignificant for preoperative Hb-S, mean preoperative hemoglobin, and the operative time. The mean postoperative hospital stay was 2.8 days (range, 1 to 8). It was significantly longer for Group II as a result of the higher complication rate.
Figure 1.
Complications in no blood transfusion group (Group I) and transfused (simple or exchange) group (Group II). TF = transfusion group; Comp = complications; VOC = vasooclusive crisis; ACS = acute chest syndrome.
Table 2.
Relations Between Complications and Various Parameters
| Parameters | Complication |
P Value | ||
|---|---|---|---|---|
| No (%) | Yes (%) | |||
| Blood transfusion | No | 24 (60) | 0 (0) | .007 |
| Yes | 16 (30) | 5 (100) | ||
| Age | <30 | 24 (68.6) | 1 (20) | .05 |
| ≥30 | 11 (31.4) | 4 (80) | ||
| Sex | Male | 15 (42.9) | 2 (42.5) | 1.00 |
| Female | 20 (57.1) | 3 (60) | ||
| Number of crisis/year | <5 | 28 (80) | 2 (40) | .09 |
| ≥5 | 7 (20) | 3 (60) | ||
| Preoperative Hb | ||||
| Group I | Mean ± SD | 10.8 ±1.1 | 0 | .82 |
| Group II | Mean ± SD | 9.17±1.56 | 9.36±1.49 | |
| Hb-S | <50 | 13 (37.1) | 0 (0) | .15 |
| ≥50 | 22 (62.9) | 5 (100) | ||
| Operative time | <2 h | 20 (57.1) | 4 (80) | .60 |
| ≥2 h | 15 (42.9) | 1 (20) | ||
aHb=hemoglobin; Hb-S=sickle hemoglobin; SD=standard deviation.
bP<.05 is significant.
DISCUSSION
This study was conducted as a pilot without randomization to see if surgery in SCD patients without preoperative blood transfusion is safe. Blood transfusion is commonly used in the comprehensive management of SCD patients. However, it is not without risk of transfusion-related complications, such as alloimmunization (17% to 30%),14 delayed hemolytic transfusion reaction,16 and hyperhemolysis.17 Therefore, the current advice is to decrease exposure to blood products if at all possible.15 Several preoperative transfusion regimens have been used to reduce the risk of perioperative complications in patients with SCD. They include simple and exchange transfusions before LC. However, at present there is no consensus regarding the value of a preoperative transfusion in these patients.9,12,15,18–20 Exchange transfusion is given to lower the level of Hb-S, usually to 30%, or a simple transfusion of 1 or 2 units of blood to raise the overall hemoglobin level to approximately 10g/dL21 to improve the overall oxygen- carrying capacity. In addition, by lowering the level of Hb-S and the number of sickled red blood cells, whole blood viscosity may fall, reducing the risk of VOC.22 In our study, we have adopted 3 practices for blood transfusion in SCD patients who are undergoing surgery. The majority (60%) had their surgery without any form of transfusion, while the remaining 40% had preoperative transfusion in the form of either a simple transfusion (22.5%) or a partial exchange transfusion (17.5%). Simple “top up” is considered much safer than partial exchange transfusion, and its use has been advocated. It is interesting to find in this study a lack of correlation between the level of Hb-S and the incidence of complications. Such a finding may prompt the conduction of a larger prospective randomized trial. Although clinical and laboratory evidence is available that supports the beneficial effects of dilution of sickle cells on outcome,23–26 it is not certain if there is a threshold for such effects or if the effects are proportionate to the percentage of Hb-S. Also, in this study, there were 5 (12.5%) postoperative complications, all of which occurred in Group II that received blood transfusion with no complication in the larger nontransfused Group I (P<.05). Moreover, there was no significant difference in the complications between the simple transfusion and partial exchange transfusion subgroups.
Unlike this study's findings and that of Vichinsky et al12 who showed no improved prophylactic benefit from an aggressive transfusion regimen compared with conservative transfusion regimen, Haberkern et al9 reported that vaso-occlusive pain episodes were highest in the nontransfused group (mean preoperative Hb-S, 88%) and lowest in the aggressive transfusion group patients (Hb-S, 34%), which may suggest a threshold of Hb-S percentage at which vaso-occlusive events are more likely to occur. Many centers, however, have adopted a more conservative policy following the publication of the study by the preoperative transfusion in the SCD group,12 which showed that simple “top up” transfusion was as safe as aggressive exchange transfusion. Also, a recent national survey of practices in the United Kingdom demonstrated no difference in the rate of postoperative complications in patients who received a transfusion and those who did not, suggesting a growing trend in the United Kingdom to avoid transfusion when possible.15
Some centers, like ours, do not routinely transfuse patients before surgery but rely heavily on the delivery of comprehensive care by a multidisciplinary team to prevent the development of postoperative sickle cell events.8 A retrospective observational study8 that examined outcomes in SCD children undergoing 66 surgical procedures without preoperative transfusion showed only one episode of ACS and no pain crises after 46 minor procedures. It was therefore concluded that any potential benefit from transfusion was low, and the risks of transfusion outweigh the benefits. Hence, transfusion is not justified for minor procedures.8 Also Leff et al20 demonstrated that LC can be performed safely in SCD patients without preoperative transfusion even in patients with hemoglobin levels just above 6g/dL before the operation. In a study that included 369 participants in Saudi Arabia, no advantage was demonstrated for preoperative transfusion over no transfusion in terms of reduced sickle-related complications, perioperative infection, and transfusion-related complications.27 No major complications were reported in either the transfusion or no-transfusion groups, but significantly more overall complications occurred in participants receiving transfusion.27 This argues against routine preoperative blood transfusion in SCD patients who are undergoing surgery.
Transfusions are undertaken less frequently in countries such as Jamaica than in North America, and interestingly, observational studies do not report significantly increased morbidity or mortality in operations without transfusion.28,29 Whether this is due to the setting or clinical differences is unclear. Indeed, the demand for blood transfusions is likely to decline with the introduction of alternative treatments, less invasive surgical techniques like laparoscopic surgery with advanced high-energy devices, and improved anesthetic agents. However, more sound evidence in the form of well-run, multi-center, randomized controlled studies is required to optimize the clinical management regimen for these people.
CONCLUSION
LC can be performed safely without blood transfusion and with no complications. Simple blood transfusion is reserved for patients with hemoglobin <10g/dL who are undergoing major surgery. Transfusion should be avoided in any patient who is undergoing a minor surgical procedure even if preoperative hemoglobin is <10g/dL. Perioperative management pays attention to preventing factors that trigger VOC and ACS, eg, dehydration, acidosis, hypothermia, and infection, by keeping the patient warm, well hydrated, well oxygenated, and pain-free in the perioperative period. The use of pneumatic compression devices, incentive spirometry, and encouragement of early mobilization are also important. We believe this practice is responsible for the low rate of complications in this study.
References:
- 1. Meshikhes AW. Towards safe surgery in patients with sickle cell disease. Saudi Med J. 2007;28(12):1788–1790 [PubMed] [Google Scholar]
- 2. Bhattacharyya N, Wayne AS, Kevy SV, Shamberger RC. Perioperative management for cholecystectomy in sickle cell disease. J Pediatr Surg. 1993;28:72–75 [DOI] [PubMed] [Google Scholar]
- 3. Fullerton MW, Philippart AI, Sarnaik S, Lusher JM. Preoperative exchange transfusion in sickle cell anemia. J Pediatr Surg. 1981;16:297–300 [DOI] [PubMed] [Google Scholar]
- 4. Halvorson DJ, McKie V, McKie K, Ashmore PE, Porubsky ES. Sickle cell disease and tonsillectomy: preoperative management and postoperative complications. Arch Otolaryngol Head Neck Surg. 1997;123:689–692 [DOI] [PubMed] [Google Scholar]
- 5. Mak V, Davies SC. The pulmonary physician in critical care: illustrative case 6: acute chest syndrome of sickle cell anaemia. Thorax. 2003;58:726–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease: life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644 [DOI] [PubMed] [Google Scholar]
- 7. Bischoff RJ, Williamson A, III, Dalali MJ, Rice JC, Kerstein MD. Assessment of the use of transfusion therapy perioperatively in patients with sickle cell hemoglobinopathies. Ann Surg. 1988;207:434–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Griffin TC, Buchanan GR. Elective surgery in children with sickle cell disease without preoperative blood transfusion. J Pediatr Surg. 1993;28:681–685 [DOI] [PubMed] [Google Scholar]
- 9. Haberkern CM, Neumayr LD, Orringer EP, et al. Cholecystectomy in sickle cell anemia patients: perioperative outcome of 364 cases from the National Preoperative Transfusion Study. Preoperative Transfusion in Sickle Cell Disease Study Group Blood. 1997;89:1533–1542 [PubMed] [Google Scholar]
- 10. Koshy M, Weiner SJ, Miller ST, et al. Surgery and anesthesia in sickle cell disease. Cooperative Study of Sickle Cell Diseases. Blood. 1995;86:3676–3684 [PubMed] [Google Scholar]
- 11. Shao SH, Orringer EP. Sickle cell intrahepatic cholestasis: approach to a difficult problem. Am J Gastroenterol. 1995;90:2048–2050 [PubMed] [Google Scholar]
- 12. Vichinsky EP, Haberkern CM, Neumayr L, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group. N Engl J Med. 1995;333:206–213 [DOI] [PubMed] [Google Scholar]
- 13. Ware R, Filston HC, Schultz WH, Kinney TR. Elective cholecystectomy in children with sickle hemoglobinopathies: successful outcome using a preoperative transfusion regimen. Ann Surg. 1988;208:17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davies SC, McWilliam AC, Hewitt PE, Devenish A, Brozovic M. Red cell alloimmunization in sickle cell disease. Br J Haematol. 1986;63:241–245 [DOI] [PubMed] [Google Scholar]
- 15. Buck J, Casbard A, Llewelyn C, Johnson T, Davies S, Williamson L. Preoperative transfusion in sickle cell disease: a survey of practice in England. Eur J Haematol. 2005;75:14–21 [DOI] [PubMed] [Google Scholar]
- 16. Garratty G. Severe reactions associated with transfusion of patients with sickle cell disease. Transfusion. 1997;37:357–361 [DOI] [PubMed] [Google Scholar]
- 17. Cullis JO, Win N, Dudley JM, Kaye T. Posttransfusion hyperhaemolysis in a patient with sickle cell disease: use of steroids and intravenous immunoglobulin to prevent further red cell destruction. Vox Sang. 1995;69:355–357 [DOI] [PubMed] [Google Scholar]
- 18. Bond LR, Hatty SR, Horn ME, Dick M, Meire HB, Bellingham AJ. Gall stones in sickle cell disease in the United Kingdom. Br Med J. 1987;295:234–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Plummer JM, Duncan ND, Mitchell DI, McDonald AH, Reid M, Arthurs M. Laparoscopic cholecystectomy for chronic cholecystitis in Jamaican patients with sickle cell disease: preliminary experience. West Indian Med J. 2006;55:22–24 [DOI] [PubMed] [Google Scholar]
- 20. Leff DR, Kaura T, Agarwal T, Davies SC, Howard J, Chang AC. A nontransfusional perioperative management regimen for patients with sickle cell disease undergoing laparoscopic cholecystectomy. Surg Endosc. 2007;21:1117–1121 [DOI] [PubMed] [Google Scholar]
- 21. Ohene-Frempong K. Indications for red cell transfusion in sickle cell disease. Semin Hematol. 2001;38:5–13 [DOI] [PubMed] [Google Scholar]
- 22. Al-Sammak ZM, Al-Falaki MM, Pasha AA. Assessment of perioperative transfusion therapy and complications in sickle cell disease patients undergoing surgery. MEJ Anesth. 2008;19(5):983–995 [PubMed] [Google Scholar]
- 23. Murphy JR, Wengard M, Brereton W. Rheological studies of Hb SS blood: Influence of hematocrit, hypertonicity, separation of cells, deoxygenation, and mixture with normal cells. J Lab Clin Med. 1976;87:475. [PubMed] [Google Scholar]
- 24. Miller ST, Jensen D, Rao SP. Less intensive long-term transfusion therapy for sickle cell anemia and cerebrovascular accident. J Pediatr. 1992;120:54–57 [DOI] [PubMed] [Google Scholar]
- 25. Janik J, Seeler RA. Perioperative management of children with sickle hemoglobinopathy. J Pediatr Surg. 1980;15:117–120 [DOI] [PubMed] [Google Scholar]
- 26. Anderson R, Cassell M, Mullinax GL, Chaplin H., Jr Effect of normal cell on viscosity of sickle-cell blood: In vitro studies and report of six years’ experience with a prophylactic program of “partial exchange transfusion.” Arch Intern Med. 1963;111:286–294 [DOI] [PubMed] [Google Scholar]
- 27. Al-Jaouni S, Al-Muhayawi SM, Qari MH, Nawas MA, Al-Mazrooa A. Randomized clinical trial to evaluate the safety of avoiding pre-operative transfusion in sickle cell anemia. Bahrain Medical Bulletin. 2006;28(4):164–167 [Google Scholar]
- 28. Homi J, Reynolds J, Skinner A, Hanna W, Serjeant G. General anaesthesia in sickle cell disease. Br Med J. 1979;1(6178):1599–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thame JR, Hambleton IR, Serjeant GR. RBC Transfusion experience in the Jamaican cohort study. Transfusion. 2001;41(5):596–601 [DOI] [PubMed] [Google Scholar]