In this account, addition of alvimopan to a standard perioperative recovery pathway decreased length of stay and incidence of postoperative ileus for elective laparoscopic colectomy.
Keywords: Alvimopan, Colectomy, Laparoscopic, Pathway
Abstract
Background and Objectives:
Alvimopan, a peripherally acting mu-opioid receptor antagonist, decreased time to gastrointestinal recovery and hospital length of stay in open bowel resection patients in Phase 3 trials. However, the benefit in laparoscopic colectomy patients remains unclear.
Methods:
A retrospective case series review was performed to study addition of alvimopan to a well-established standard perioperative recovery pathway for elective laparoscopic colectomy. The main outcome measures were length of stay and incidence of charted postoperative ileus. Wilcoxon and chi-square tests were used to calculate P values for length of stay and postoperative ileus endpoints, respectively.
Results:
Demographic/baseline characteristics from the 101 alvimopan and 64 pre-alvimopan control patients were generally comparable. Mean length of stay in the alvimopan group was 1.55 days shorter (alvimopan, 2.81±0.95 days; control, 4.36±2.4 days; P<.0001). The proportion of patients with postoperative ileus was lower in the alvimopan group (alvimopan, 2%; control, 20%; P<.0001).
Conclusion:
In this case series, addition of alvimopan to a standard perioperative recovery pathway decreased length of stay and incidence of postoperative ileus for elective uncomplicated laparoscopic colectomy. The improvement in the mean length of stay for patients who receive alvimopan is a step forward in achieving a fast-track surgery model for elective laparoscopic colectomies.
INTRODUCTION
In addition to surgical technique, current surgical therapy is placing emphasis on perioperative multidisciplinary modalities to improve overall patient outcomes. One such outcome variable, the length of stay after abdominal surgery is decreasing. Many patients are experiencing earlier gastrointestinal (GI) recovery and shorter postoperative length of stay (LOS) due to advances in laparoscopy, anesthesia, and implementation of perioperative recovery pathways.1–5
Delayed GI recovery is the most common reason for prolonged LOS after abdominal surgery and has multiple negative effects including nausea, vomiting, inability to tolerate diet progression, abdominal distension, and patient discomfort.6–9 Nausea and vomiting may lead to aspiration and pneumonia, and delayed oral intake may put a patient at higher risk for malnutrition.10 Abdominal distention may lead to decreased respiratory reserve, wound complications, and increased narcotic use leading to further delay in return of bowel function and worsening postoperative outcomes.10 Hospital costs are increased because of prolonged stay and recruitment of resources to manage delayed GI recovery and include radiologic images, parenteral nutrition, and laboratory tests.7,11,12 Furthermore, prolonged hospital stay may also increase a patient's exposure to hospital-acquired pathogens and illnesses.13,14
Gastrointestinal recovery after abdominal surgery is dependent on many variables, including neurologic, inflammatory, and pharmacologic factors.15–18 Therefore, it stands to reason that it would be beneficial to implement a multimodal approach to accelerating GI recovery, thereby shortening LOS after surgery. Accordingly, multiple protocols to reduce the duration of postoperative ileus (POI) have been proposed, with varied success.2–5,14,19 These include various pre-, intra-, and postoperative components in a variety of combinations and permutations. However, despite the research demonstrating that pathway use can accelerate GI recovery and decrease LOS, a recent survey found that approximately 30% of physicians who perform colectomies reside at a hospital with a perioperative care protocol for elective bowel resection that include components to accelerate GI recovery.20
In May 2008, alvimopan (Entereg, Adolor, Exton, PA) a peripherally acting μ-opioid receptor antagonist, received FDA approval for the acceleration of time to upper and lower GI recovery after partial large or small bowel resection with primary anastomosis.21,22 Alvimopan has been shown in multiple studies to accelerate gastrointestinal recovery after laparotomy.23–29 Across Phase 3 trials, in comparison with placebo, alvimopan accelerated GI recovery by 11 hours to 26 hours and decreased postoperative LOS by 0.3 day to 1.3 days after partial bowel resection.30 Patients who received alvimopan also had reduced POI-related morbidity compared with those who received placebo.30,31 In these trials, all patients (both those in the alvimopan and placebo arms) were managed with a standardized postoperative care pathway that included removal of the nasogastric tube by postoperative day 1, early ambulation, and early advancement of diet.30
Because patients in the alvimopan clinical trials underwent open colectomy, the benefit of using alvimopan in patients undergoing laparoscopic colectomy is still unclear. However, a recent observational trial in laparoscopic bowel resection patients revealed that these patients can still experience POI-related morbidity after laparoscopic surgery and that there is room for improvement for GI recovery and time to discharge after laparoscopic bowel resection.32 Indeed, some recently published single-institution/system studies have indicated a potential benefit for alvimopan in patients undergoing laparoscopic bowel resection.33,34 Therefore, based on the drug's mode of action, we propose that in patients for whom opioid-based analgesia was used, alvimopan may help accelerate patient GI recovery; thereby shortening their hospital stay. We designed a retrospective chart review to study the effect of adding alvimopan to a standard perioperative recovery pathway used for elective laparoscopic partial colectomies.
MATERIALS AND METHODS
An institutional review board-approved, retrospective chart review at one community hospital from July 2007 through January 2010 was conducted. Laparoscopic partial colectomies performed over a period of 15 months before and 15 months after the introduction of alvimopan to the practice in November 2008 were retrospectively studied in a community based colorectal surgery practice of 2 colorectal surgeons.
Inclusion criteria for this study consisted of patients who underwent a segmental colectomy with primary anastomosis from July 2007 to January 2010. Exclusion criteria included, emergent cases, conversion from laparoscopic to open surgery, patients admitted to the hospital before their day of surgery, concomitant surgical procedures (resections to organs other than bowel), multiple anastomoses, total colon resection, subtotal colon resection, operations for motility disorders or rectal prolapse, creation or reversal of stomas, and in-patient perioperative complications that extended hospital stay except for POI (ie, other operative or cardiopulmonary complications, urinary tract infections, and others).
Consecutive patients who met the inclusion and exclusion criteria listed above and who would have been candidates to receive alvimopan (based on their preoperative opioid use) in the 15-month period before drug introduction to the practice (controls) were compared with patients who received alvimopan after it was introduced to the practice. To further identify the patients who would have been candidates for alvimopan based on their preoperative opioid use, the patient's medication lists were reviewed and patients were included in the control group arm only if they had not taken narcotics in the 5 days prior to their operation.
A standardized perioperative care pathway for laparoscopic colectomy was used for all patients in this study. Patients received intravenous patient controlled analgesia (PCA) using morphine or hydromorphone (Dilaudid, Purdue Pharma, Canada) with scheduled intravenous ketorolac for 24 hours postoperatively. On postoperative day 1, the PCA was stopped and oral pain medication was started (hydrocodone or equivalent). Pain was controlled with oral pain medication, and all underlying medical conditions were addressed. No patients received nasogastric tubes. Sips of clear liquids began on postoperative day 0 (day of surgery). Additionally, on postoperative day 1, a clear liquid diet was started and if tolerated, advanced to full liquids the same day. On postoperative day 2, patients were advanced to a soft diet as tolerated, regardless of evidence of bowel sounds or return of bowel function, provided the patient did not exhibit signs of worsening distention or increased abdominal pain. Typically, patients were then discharged on a soft diet for 5 days postoperatively. Discharge criteria included all of the following: afebrile, vital signs stable for at least the previous 24 hours, tolerating a soft diet, passage of flatus or bowel movement. Beginning in November of 2008, alvimopan was added to the perioperative pathway and was used per the label guidelines (once preoperatively, twice daily postoperatively for up to 15 in-hospital doses).22
The primary outcome measures were LOS and POI, both of which were collected by review of patient charts. Length of stay was defined as the number of calendar days from day of surgery to the day the patient physically left the hospital, regardless of return to home or a rehabilitation facility. Postoperative ileus, based on our group's clinical experience, is considered as a delay of return to bowel function more than 36 to 48 hours postoperatively that could not be attributed to an organic cause (such as dehydration and electrolyte imbalance), usually accompanied by abdominal distention and nausea. The POI diagnosis was made by the surgeon at the time of the hospital stay and was recorded in the discharge summary. In our study, a patient was classified as having experienced POI only if the diagnosis of POI was present in the discharge summary. Thirty-day readmission and mortality data were also collected.
Wilcoxon tests were used to calculate P values for the comparison of means (LOS, age, body mass index [BMI], American Society of Anesthesiologists [ASA] score), and chi-square tests were used to calculate P values for the comparison of proportions (POI, sex, proportion of hand-assist surgeries, indication for surgery, surgery type). Because the LOS data were not normally distributed and right skewed, quasi-Poisson models were used to model the LOS data; log number of days of LOS were used in the model. Logistic regression was used to estimate the odds ratios for the various coefficients. Logistic regression models were used to model the POI data.
RESULTS
In this study, 165 laparoscopic partial colectomies were identified as eligible for inclusion in this study. The mean age of patients was 62 years, the most common indication for surgery was neoplasm, and the most common operation type was laparoscopic sigmoid colectomy (Table 1). In the control and alvimopan groups, 14% and 10% of laparoscopic surgeries, respectively, were hand-assisted. The proportion of patients who underwent hand-assisted laparoscopic surgery was not significantly different between groups, and the results for LOS were similar to those observed for the full dataset when these patients were excluded from the analysis.
Table 1.
Baseline Patient Demographics and Surgical Characteristics
| Control Group (n=64) | Alvimopan Group (n=101) | |
|---|---|---|
| Female, n (%) | 33 (52) | 72 (61) |
| Mean age ± SD | 62.9±14.2 | 61.9±14.5 |
| BMI ± SD | 27.7±4.65 | 28.3±7.8 |
| ASA score ± SD | 2.4±0.50 | 2.4±0.48 |
| Mean operation duration, minutes ± SD | 173±29 | 154±26a |
| Primary indication for operation, n (%) | ||
| Neoplasm | 44 (69) | 6 (60) |
| Diverticulitis | 19 (30) | 38 (37) |
| Crohn's disease | 0 | 2 (2) |
| Appendicitis | 0 | 1 (1) |
| A-V malformation | 1 (1) | 0 |
| Operation type, n (%) | ||
| Laparoscopic sigmoid colectomy | 26 (41) | 41 (40) |
| Laparoscopic low anterior resection | 19 (30) | 35 (35) |
| Laparoscopic right colectomy | 17 (27) | 20 (20) |
| Laparoscopic left colectomy | 1 (1) | 3 (3) |
| Laparoscopic transverse colectomy | 1 (1) | 1 (1) |
| Operative technique, n (%) | ||
| Straight laparoscopy | 55 (86) | 91 (90) |
| Hand-assisted laparoscopyb | 9 (14) | 10 (10) |
Abbreviations: ASA=American Society of Anesthesiologists; A-V=arteriovenous; BMI=body mass index; SD=standard deviation.
P>.001 based on a Wilcoxon test;
Surgeries were considered hand-assisted if a hand gel port was used at any time during the operation.
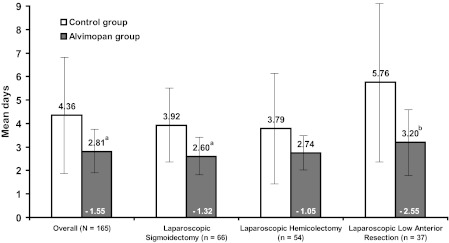
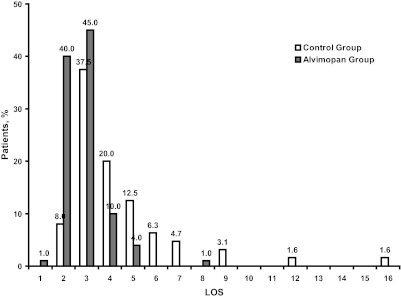
Overall, the mean length of stay in the alvimopan group was 1.55 days shorter than in the control group (alvimopan, 2.81 ± 0.95 days; control, 4.36 ± 2.4 days; P<.0001; Figure 1). The LOS was reduced by approximately 1 day to 2.5 days in the alvimopan group compared with control in the 3 most common types of surgeries. A higher proportion of patients in the alvimopan group were discharged on Days 2 and 3 compared with patients in the control group (Figure 2). Moreover, the outliers for length of stay were significantly shorter for the alvimopan group (1 day to 6 days) compared with the range for the control group (2 days to 16 days). The 2 patients with the longest LOS in the control group both had a diagnosis of POI. Results of the Poisson model for LOS revealed that alvimopan treatment significantly reduced LOS after adjusting for age, sex, BMI, ASA score, operative time, presence of POI, indication for surgery, surgery type, and hand-assistance (P<.001). In addition, the presence of POI significantly increased LOS after adjusting for age, sex, BMI, ASA score, operative time, indication for surgery, surgery type, and hand assistance (P<.001).
Figure 1.
Mean hospital length of stay overall and by operation type. Note: The bars represent the standard deviation associated with each mean. The 3 most common operation types only are graphed. Other operation types included in the overall category were left (n=4) and transverse colectomy (n=2). aP<.001 based on a Wilcoxon test; bP<.05 based on a Wilcoxon test.
Figure 2.
Hospital discharge by day. LOS=length of stay.
Postoperative ileus was noted in 2 of the patients (2%) in the alvimopan group and 13 patients (20%) in the control group (P<.001). Results of the logistic regression model revealed that LOS (P=.003) and operative time (P=.025) were significantly associated with the presence of POI after adjusting for age, sex, BMI, ASA score, and hand assistance. Because some indications for surgery (Crohn's disease, appendicitis, A-V malformation) and surgery types (left colectomy, transverse colectomy) were associated with low incidences of POI and the standard error of estimates were very large because of small Ns, they were not included in the model. However, a Fisher's exact test revealed that there was an association of surgery type (P=.021) but not indication for surgery with POI (P=.348). The surgery type with the largest proportion of patients with POI (24%) was laparoscopic low anterior resection.
Five patients (5.0%) in the alvimopan group and 3 patients (4.7%) in the control group were readmitted 30 days after surgery. In the alvimopan group, patients were readmitted because of pneumonia (1 patient), wound infection (1 patient), Clostridium difficile colitis (1 patient), and anastomotic leak (2 patients, 1 of which required reoperation). In the control group, patients were readmitted because of anemia and dehydration (1 patient), pulmonary embolus (1 patient), and wound infection (1 patient). No patients in either group died in the 30 days after surgery.
DISCUSSION
A renewed interest in the prophylaxis and management of POI has emerged in the past 3 years with the introduction of a new class of drugs that can mitigate the negative effects of opioids on the GI tract after surgery as well as new research on perioperative care pathways that can accelerate GI recovery and shorten hospital LOS.2,3,9,19–21,35–37
Our group follows a standardized perioperative care pathway for laparoscopic colectomy. Our pathway includes preoperative patient counseling to manage patient expectations, the judicial use of early postoperative feeding, and a switch from IV-PCA to oral opioids on postoperative day 1. These pathway components, in addition to the use of minimally invasive surgical technique, help our practice to minimize the expected delay in GI recovery after colectomy.
This study of the effect of adding alvimopan to a standardized perioperative care pathway for laparoscopic colectomy in a clinical practice setting has a number of strengths. The reasonably robust sample includes data from a homogenous group of patients as evidenced by the generally comparable baseline demographics and surgical characteristics. Moreover, the standardized pathway (which was in effect since the beginning of 2007), perioperative care modality, major equipment, and objective hospital discharge criteria remained unchanged throughout the duration of the study. Finally, the 2 operating surgeons contributing data to this study have been in practice for over 2 decades and performing laparoscopic colectomies for more than a decade with consistent case loads. Therefore, the decreased LOS observed in the alvimopan group cannot likely be attributed to a surgeon learning curve over time.
Like many evaluations of “real world” outcomes associated with drug use, this study has limitations. This was a retrospective and un-blinded study. Data were from 2 surgeons in 1 practice and included patients with uncomplicated, elective laparoscopic colectomies only, which may limit the generalization of the results. However, we feel that our pathway and perioperative care modality are common to many surgical practices. Patients were not matched in our study other than based on the inclusion/exclusion criteria and presurgery opioid use. Therefore, it is possible that, although we are reasonably confident that similar groups are being compared, there may be some factors that we did not investigate that could influence LOS and POI in addition to alvimopan use. However, for the baseline criteria that were collected in this study, including body mass index and American Society of Anesthesiologists scores which may be considered as defaults for baseline comorbidity/risk, patients appear similar. Data for narcotic doses and use was not able to be drawn from patient charts; however, perioperative care practices remained unchanged for the duration of this study and therefore narcotic use would have likely remained relatively similar in both cohorts; our pain management protocol (described in the methods) also remained unchanged. Hospital discharge can be influenced by factors in addition to GI recovery such as social support and placement factors. Nevertheless, these factors would presumably be similar in both cohorts in our study. In addition, because a patient was only classified as experiencing POI if the diagnosis of POI was recorded in the chart during the patient's hospitalization, the rate of POI could be underestimated if patients had symptoms of an ileus but no diagnosis in the chart. Furthermore, the operative time in the control group was approximately 20 minutes longer than the operative time in the alvimopan group. Although this is statistically significant, 20 minutes may or may not be clinically meaningful with respect to increasing risk for POI. Indeed, a recent retrospective database review of over 400 segmental laparoscopic colectomies found that increasing operative time did not adversely affect perioperative outcomes, including LOS.38 Finally, hospital cost data were not collected in our study. However, in a recently published study33 of alvimopan use in laparoscopic bowel resection at a regional medical center, a 1.1 day reduction of LOS was associated with a hospital cost savings of $1,368 (this included alvimopan costs). Therefore, it is likely that, based on a day and a half reduction in LOS, cost savings will be realized at our institution as well. Despite these study limitations, we feel this study provides meaningful data regarding alvimopan use in elective laparoscopic partial colectomy patients.
Based on the positive results of this study we have modified our preoperative patient counseling. Previously, we informed all patients undergoing laparoscopic colectomy that the postoperative LOS, barring any complication, could be expected to be between 3 to 5 days. This LOS was corroborated by our results of 4.4 days in the control group of this study. However, based on the mean LOS in the alvimopan group of our study, we have recently shortened the expected LOS for patients to 2 to 4 days. Furthermore, the addition of alvimopan to our pathway appears to yield a more consistent response with respect to our expected LOS for uncomplicated laparoscopic colectomies; fewer patients remain in the hospital beyond 4 days (ie, fewer outliers).
CONCLUSION
The addition to alvimopan to a standardized perioperative care pathway for elective laparoscopic partial colectomies decreased the mean LOS by 1.5 days in our practice. We encourage further studies in other clinical practices and in randomized, controlled trials to corroborate our results.
References:
- 1. Patel NA, Bergamaschi R. Laparoscopy for diverticulitis. Semin Laparosc Surg. December 10(4):177–183, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Gouvas N, Tan E, Windsor A, Xynos E, Tekkis PP. Fast-track vs standard care in colorectal surgery: a meta-analysis update. Int J Colorectal Dis. October 24(10):1119–1131, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Lassen K, Soop M, Nygren J, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. October 144(10):961–969, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Senagore AJ, Stulberg JJ, Byrnes J, Delaney CP. A national comparison of laparoscopic vs. open colectomy using the National Surgical Quality Improvement Project data. Dis Colon Rectum. February 52(2):183–186, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Delaney CP, Chang E, Senagore AJ, Broder M. Clinical outcomes and resource utilization associated with laparoscopic and open colectomy using a large national database. Ann Surg. May 247(5):819–824, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Chang SS, Baumgartner RG, Wells N, Cookson MS, Smith JA., Jr Causes of increased hospital stay after radical cystectomy in a clinical pathway setting. J Urol. January 167(1):208–211, 2002 [PubMed] [Google Scholar]
- 7. Iyer S, Saunders WB, Stemkowski S. Economic burden of postoperative ileus associated with colectomy in the United States. J Manag Care Pharm. Jul-Aug 15(6):485–494, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saclarides TJ. Current choices–good or bad–for the proactive management of postoperative ileus: A surgeon's view. J Perianesth Nurs. April 21(2A Suppl):S7–15, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Story SK, Chamberlain RS. A comprehensive review of evidence-based strategies to prevent and treat postoperative ileus. Dig Surg. 2009;26(4):265–275 [DOI] [PubMed] [Google Scholar]
- 10. Bosio RM, Delaney CP, Senagore AJ. Economic impact of postoperative ileus and prolonged length of stay. Semin Colon Rectal Surg. 2005;16:235–238 [Google Scholar]
- 11. Goldstein JL, Matuszweski K, Delaney CP, et al. The inpatient economic burden of postoperative ileus associated with abdominal surgery in the United States. P T. 2007;32:82–89 [Google Scholar]
- 12. Asgeirsson T, El-Badawi KI, Mahmood A, Barletta J, Luchtefeld M, Senagore AJ. Postoperative ileus: it costs more than you expect. J Am Coll Surg. February 210(2):228–231, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Adams SD, Mercer DW. Fulminant Clostridium difficile colitis. Curr Opin Crit Care. August 13(4):450–455, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Sacar S, Sayin Kutlu S, Turgut H, Cevahir N, Hircin Cenger D, Tekin K. Epidemiology and associated factors for nosocomial methicillin-resistant Staphylococcus aureus infection in a tertiary- care hospital. Epidemiol Infect. May 138(5):697–701, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Boeckxstaens GE, de Jonge WJ. Neuroimmune mechanisms in postoperative ileus. Gut. September 58(9):1300–1311, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Luckey A, Livingston E, Tache Y. Mechanisms and treatment of postoperative ileus. Arch Surg. February 138(2):206–214, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Behm B, Stollman N. Postoperative ileus: etiologies and interventions. Clin Gastroenterol Hepatol. March 1(2):71–80, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Sanger GJ, Holzer P. Endogenous opioids and the gastrointestinal tract. Seminars in Colon and Rectal Surgery. 2005;16:197–199 [Google Scholar]
- 19. Joh YG, Lindsetmo RO, Stulberg J, Obias V, Champagne B, Delaney CP. Standardized postoperative pathway: accelerating recovery after ileostomy closure. Dis Colon Rectum. December 51(12):1786–1789, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Delaney CP, Senagore AJ, Gerkin TM, et al. Association of surgical care practices with length of stay and use of clinical protocols after elective bowel resection: results of a national survey. Am J Surg. March 199(3):299–304, 2010; discussion 304 [DOI] [PubMed] [Google Scholar]
- 21. Adolor_Corporation Adolor and GlaxoSmithKline Announce FDA Approval of Entereg(R) (alvimopan) for the Management of Postoperative Ileus (POI) [Press Release]. May Available at: http://phx.corporate-ir.net/phoenix.zhtml?c=120919&p=irol-newsArticle&ID=1148660&highlight=
- 22. Adolor_Corporation Entereg, Prescribing Information. Available at: http://www.entereg.com/
- 23. Delaney CP, Weese JL, Hyman NH, et al. Phase III trial of alvimopan, a novel, peripherally acting, mu opioid antagonist, for postoperative ileus after major abdominal surgery. Dis Colon Rectum. June 2005;48(6):1114–1125; discussion 1125-1116; author reply 1127–1119 [DOI] [PubMed] [Google Scholar]
- 24. Ludwig K, Enker WE, Delaney CP, et al. Gastrointestinal tract recovery in patients undergoing bowel resection: results of a randomized trial of alvimopan and placebo with a standardized accelerated postoperative care pathway. Arch Surg. November 143(11):1098–1105, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Viscusi ER, Goldstein S, Witkowski T, et al. Alvimopan, a peripherally acting mu-opioid receptor antagonist, compared with placebo in postoperative ileus after major abdominal surgery: results of a randomized, double-blind, controlled study. Surg Endosc. January 20(1):64–70, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Wolff BG, Michelassi F, Gerkin TM, et al. Alvimopan, a novel, peripherally acting mu opioid antagonist: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial of major abdominal surgery and postoperative ileus. Ann Surg. October 240(4):728–734, 2004; discussion 734–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buchler MW, Seiler CM, Monson JR, et al. Clinical trial: alvimopan for the management of post-operative ileus after abdominal surgery: results of an international randomized, double-blind, multicentre, placebo-controlled clinical study. Aliment Pharmacol Ther. August 1 28(3):312–325, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Delaney CP, Yasothan U, Kirkpatrick P. Alvimopan Nat Rev Drug Discov. September 2008;7(9):727–728 [DOI] [PubMed] [Google Scholar]
- 29. Delaney CP, Wolff BG, Viscusi ER, et al. Alvimopan, for postoperative ileus following bowel resection: a pooled analysis of phase III studies. Ann Surg. March 245(3):355–363, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kraft M, Maclaren R, Du W, Owens G. Alvimopan (Entereg) for the management of postoperative ileus in patients undergoing bowel resection. P T. January 35(1):44–49, 2010 [PMC free article] [PubMed] [Google Scholar]
- 31. Wolff BG, Weese JL, Ludwig KA, et al. Postoperative ileus-related morbidity profile in patients treated with alvimopan after bowel resection. J Am Coll Surg. April 204(4):609–616, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Delaney CP, Marcello PW, Sonoda T, Wise P, Bauer J, Techner L. Gastrointestinal recovery after laparoscopic colectomy: results of a prospective, observational, multicenter study. Surg Endosc. March 24(3):653–661, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Whelpley R, Pierce M, Collins R, Timmerman W. An evaluation of alvimopan use as part of perioperative management of patients undergoing laparoscopic small and large bowel resections. Hospital Pharmacy. 2011;46(1):26–32 [Google Scholar]
- 34. Absher RK, Gerkin TM, Banares LW. Alvimopan use in laparoscopic and open bowel resections: clinical results in a large community hospital system. Ann Pharmacol. 2010;44:1701–1708 [DOI] [PubMed] [Google Scholar]
- 35. Augestad KM, Delaney CP. Postoperative ileus: impact of pharmacological treatment, laparoscopic surgery and enhanced recovery pathways. World J Gastroenterol. May 7 16(17):2067–2074, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kehlet H. Multimodal approach to postoperative recovery. Curr Opin Crit Care. August 15(4):355–358, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Zargar-Shoshtari K, Connolly AB, Israel LH, Hill AG. Fast-track surgery may reduce complications following major colonic surgery. Dis Colon Rectum. November 51(11):1633–1640, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Scheer A, Martel G, Moloo H, et al. Laparoscopic colon surgery: does operative time matter? Dis Colon Rectum. October 52(10):1746–1752, 2009 [DOI] [PubMed] [Google Scholar]