Abstract
In native tissues, microscale variations in the extracellular matrix (ECM) structure can drive different cellular behaviors. Although control over ECM structure could prove useful in tissue engineering and in studies of cellular behavior, isotropic 3D matrices poorly replicate variations in local microenvironments. In this paper, we demonstrate a method to engineer local variations in the density and size of collagen fibers throughout 3D tissues. The results showed that, in engineered multiphase tissues, the structures of collagen fibers in both the bulk ECM phases (as measured by mesh size and width of fibers) as well as at tissue interfaces (as measured by density of fibers and thickness of tissue interfaces) could be modulated by varying the collagen concentrations and gelling temperatures. As the method makes use of a previously published technique for tissue bonding, we also confirmed that significant adhesion strength at tissue interfaces was achieved under all conditions tested. Hence, this study demonstrates how collagen fiber structures can be engineered within all regions of a tightly integrated multiphase tissue scaffold by exploiting knowledge of collagen assembly.
Keywords: Adhesion, Alginate, Collagen, Hydrogel, Interface, Micropatterning
1. Introduction
Native tissues are structurally inhomogeneous and anisotropic, consisting of numerous types of cells and extracellular matrices (ECMs) that are integrated together in defined spatial hierarchies. Fibrillar proteins, such as type I collagen, which impart structural, mechanical, and signaling functions to tissues are major components of the ECM. The size, density, and orientation of collagen and other ECM fibers vary among different tissues and at tissue interfaces [1, 2] in a manner that is intimately related to the function of the tissue. For example, different zones in cartilage exhibit varying types, densities, and orientations of collagen fibers; these variations are important for the proper dissipation of loads throughout the tissue [3]. In tendon interfaces (such as the transition from tendon to fibrocartilage to bone), the structure of ECM fibers also change markedly [4]. Cells can also change their basic migration mechanisms when encountering matrices of different pore size [5–7]. Hence, it would be useful for tissue engineers and cell biologists to construct 3D ECM scaffolds with local control over the size, density and strength of collagen fibers.
In bulk gels made of type I collagen [8], the structure of collagen fibers is sensitive to a number of experimental variables during the gelling step: concentration of collagen, pH, ionic strength, gelling temperature, presence of ECM macromolecules (such as glycosaminoglycans) and physical forces such as shear [6, 9–14]. Although these methods enable different collagen fiber structures to be fabricated, isotropic matrices are poorly suited to mimic microscale variations in ECM structure and composition.
Inhomogeneous tissues can be constructed by assembling multiple layers of tissues [3, 4, 15, 16] or by using microfabrication [17–19]. It remains challenging, however, to assemble multiple phases of tissues together at the microscale in a manner that can withstand cellular contractile forces over time. We have previously developed a technique to tightly integrate different blocks of a multiphase tissue by using type I collagen fibers [20]. The method works as follows: first, a cold suspension of multiple ECM precursor components, including type I collagen precursors, is warmed to induce self-assembly of collagen into a fibrous matrix (Fig. 1, left panel). Other soluble ECM materials are then gelled. This network of collagen fibers, which we call “pECM” (for “pre-formed ECM”), provides a template for nucleation and growth of new collagen fibers at the surface of this hybrid ECM (Fig. 1, right panel). When a second cold hydrogel suspension containing collagen precursors, which we call “iECM” (for “integrated ECM”) is placed adjacent to the pECM and the solution warmed, new collagen fibers assemble from the preformed collagen network. We refer to the fibers that span both ECM phases (pECM and iECM) as integrating fibers. Whereas in bulk solutions the collagen fibers form from only nuclei within the solution, in the case of gelling beside a preformed ECM, collagen fibers form from the surface nuclei in the pECM in addition to the nuclei in the bulk solution.
Fig. 1.
Schematic diagram of the collagen fiber-mediated tissue integration method.
In this paper, we hypothesize that varying the assembly conditions in each ECM phase could change the structure of collagen fibers throughout the multiphase construct. Further, we performed shear adhesion tests to verify that creation of continuous collagen networks across the interface under the tested assembly conditions would result in significant bonding strength.
2. Materials and Methods
2.1 Collagen fiber integration in microfluidic hydrogels
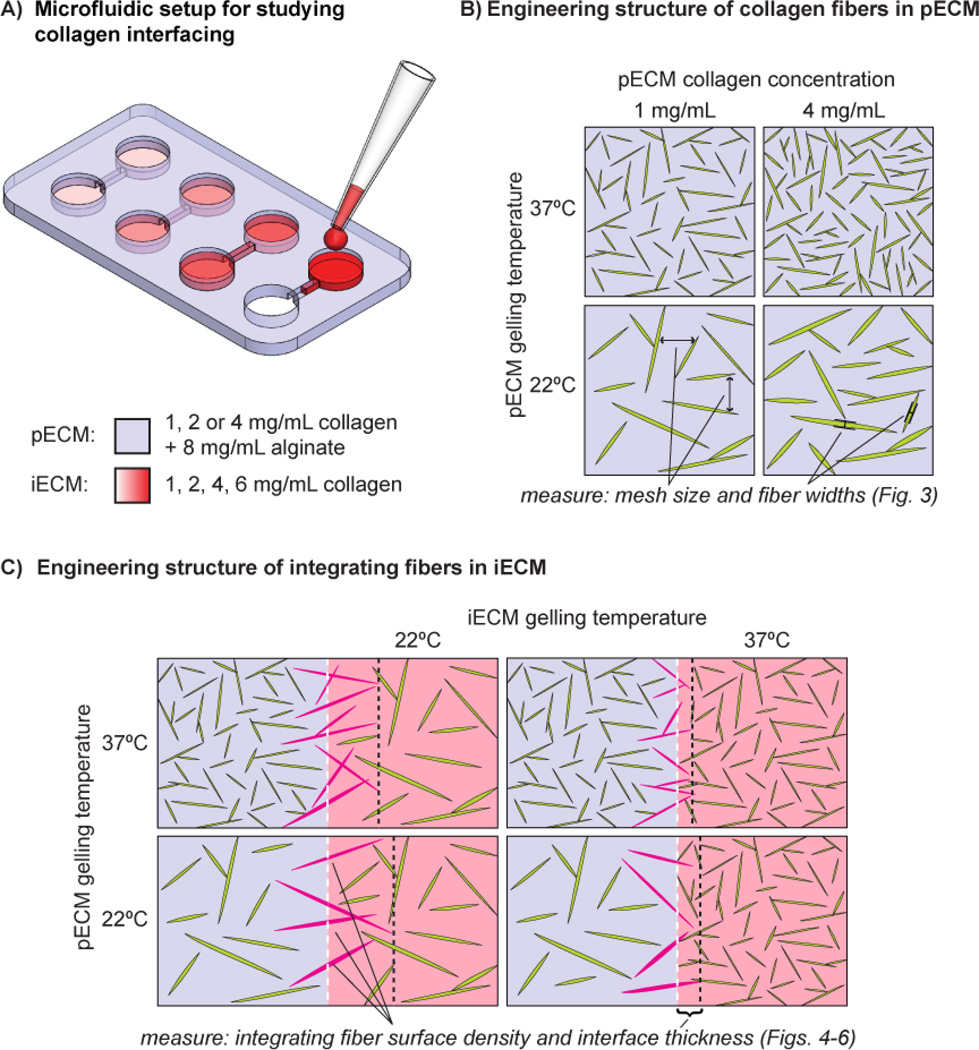
We developed an experimental setup to investigate multiple interfaces in parallel featuring the collagen interfacing method which we previously reported [20]. A schematic of the fabrication of this microfluidic hydrogel setup is shown in Supplementary Fig. 1. Each microchannel was 2 mm in length by 0.2 mm wide by 0.12 mm height. Our method to mold microfluidic hydrogels [20] was adapted from a previous work [21]. We fabricated master molds for the microfluidic hydrogels in SU-8 (SU-8 3050, Microchem, Newton, MA) on 3-inch Si wafers (Silicon Sense, Nashua, NH) by photolithography [20]. We cast polydimethylsiloxane (PDMS, Sylgard 184, Essex Brownell, Fort Wayne, IN) replicas from the masters, made them hydrophilic by plasma treatment, and submerged them in distilled water to retain their hydrophilicity. We then autoclaved the molds and cooled them in a refrigerator prior to casting collagen. Next, we prepared solutions of ice-cold type I collagen (rat tail, BD biosciences, Franklin Lakes, NJ) at 2X final concentration by mixing the acid solubilized stock solution with 10XPBS, 1N NaOH, and distilled water according to the manufacturer protocol. We then mixed the cold collagen solutions with cold 1.6% w/v alginate (Pronova UltraPure MVG, NovaMatrix, Drammen, Norway) in a 1:1 ratio, and held the resulting collagen-alginate solution on ice before quickly pipetting the solution into the PDMS molds. Thus, the hydrogels contained a constant amount of alginate (0.8% w/v), which we found to be a minimum amount to support molding and handling of microfluidic hydrogels at low collagen density. We used positive-displacement pipettes for accurate pipetting of viscous collagen-alginate solutions and to avoid bubbles. We closed the top of the molds with cellulose dialysis membranes (6000 kD MWCO), and flattened the membranes using the edge of a sterile glass slide. We allowed the collagen component to gel for 50 minutes at either room temperature (22 ± 2°C) or 37°C before pipetting a 60 mM CaCl2 HEPES buffer solution on top of the membrane for at least 45 minutes to crosslink the alginate at room temperature. We removed the hydrogels from the molds using sterilized tools, and placed the hydrogels in HEPES saline buffer solution (Ultrasaline A, Lonza, Basel, Switzerland) supplemented with 1.8 mM CaCl2 (to prevent leaching of the calcium ions from the hydrogels). We then transferred the hydrogels into Lab-Tek chambered coverglasses (NUNC, Rochester NY) to permit high resolution confocal reflectance imaging of collagen fibers at the interfaces. We removed the buffer solution and dried the inlets using sterile PVA surgical spears (Ultracell, Braintree Scientific, Braintree, MA), and then manually pipetted collagen solutions into the inlets to drive flow through the channels by hydrostatic pressure (Fig. 2A). We held the coverglasses on a cold plate during flow, and then allowed them to settle for several minutes before heating so that flow stops prior to collagen assembly. This process allowed the formation of relatively isotropic collagen matrices within the channels, since gelling collagen solutions while the solution is flowing in microfluidic channels can produce matrices with aligned collagen fibers [10, 11]. Collagen solutions were prepared immediately before use for all experiments, as some degree of nucleation has been shown to occur even when held on ice [11].
Fig. 2.
(A) Schematic diagram of experimental setup. To permit high throughput study of interface structure under varying conditions, we used microfluidic hydrogels with 4 independent channels for flowing different collagen concentrations within a single hydrogel composition. The microchannels were sealed against glass coverslips to permit high resolution confocal imaging of collagen fibers along the microchannel sidewalls using immersion objectives. Collagen solutions were flowed into the channels by manual pipetting into the inlets. (See Supplementary Fig. 1 for a complete schematic of the hydrogel microfabrication process.) (B) Schematic diagram depicting the influence of collagen concentration and gelling temperature on fiber structure in a bulk hydrogel (actual images of these conditions are shown in Fig. 3). Similarly to previous studies, we characterize the structure of bulk collagen fibers by quantifying the mesh size (i.e. the spacing between fibers, indicated by arrows in lower left panel), and fiber widths (manually measured across the approximate midpoint of fibers, indicated by calipers in lower right panel). (C) Schematic diagram depicting the hypothesized influence of collagen gelling temperature in each phase on the structure of integrating fibers (shown in purple) for a given collagen concentration in each phase (actual images of these conditions are shown in Fig. 4 and Supplementary Figs 3–6). The tissue interface is highlighted by white dotted lines. We measure two parameters to characterize the structure of integrating fibers: the integrating fiber surface density (number of fibers per unit interface area) and the interface thickness (distance from the interface to the tips of integrating fibers, indicated by the black dotted lines).
2.2 Imaging and image analysis to measure structural parameters
We took stacks of confocal images (1024×1024 pixels, 41 images with a z-spacing of 0.25 microns) using a Leica SP5 confocal microscope, with a 100× 1.43 N.A. oil immersion objective (Leica Microsystems) at a resolution of 0.132 microns/pixel (image stacks were thus 135 µm * 135 µm * 10 µm). Stacks were started at 10 µm beyond the coverslip to avoid noise from reflection of the glass. We simultaneously collected collagen reflectance [22] and differential interference contrast (DIC) images using the 488-nm laser line.
Within the pECM, we determined two structural parameters: “mesh size” and “fiber widths”. To determine the collagen-fiber mesh size [23], we thresholded confocal reflectance images and used Image Pro software (Media Cybernetics, Bethesda, MD) to make them binary, so that fiber pixels had a value of one whereas background pixels had a value of zero. We then lowered the noise using a ‘despeckle’ filter. After loading the stacks of images into MATLAB software (The MathWorks, Natick, MA), we measured the distances between fibers by counting the number of background pixels between fiber pixels across the rows and columns of the images. This distance represents the mesh size of the collagen fibers. Previously, distributions of inter-fiber distances in collagen gels have been shown to fit well to an exponential decay, which is expected for a random array in which fiber locations are independent of each other [23]. Thus, we fit the distributions of fiber spacings to the equation A*exp(−βx), where×is the inter-fiber distance and 1/β is the characteristic mesh size. We measured fiber widths in bulk microfluidic hydrogels in Image Pro software by tracing across the approximate midpoint of representative fibers (45 fibers were measured for each condition).
For integrating fibers, we measured another two structural parameters: “surface density” and “interface thickness”. To visualize integrating fibers, we captured image stacks centered at the microchannel walls. To determine the surface density of integrating fibers, we overlaid the collagen reflectance images with the DIC images, the latter of which clearly highlighted the boundary of the alginate channel. As we moved through the stack of images, we marked the fibers that crossed the interface, manually counted the numbers of fibers using Image J software (National Institutes of Health, Bethesda, MD) (Supplementary Fig. 2), and then divided the number of fibers by the total interface area. We measured the interface thickness by following integrating fibers from the interface to the fiber tips while advancing through the image stack, then measuring the distance from the fiber tip to the interface boundary (at least 15 integrating fibers were counted per condition).
2.3 Shear adhesion testing
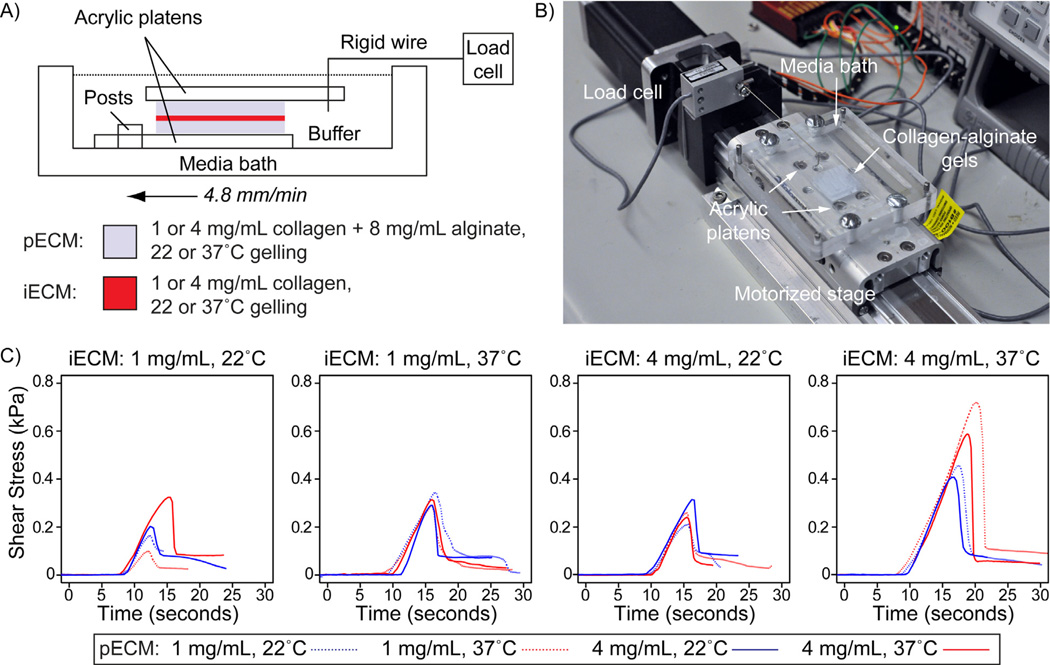
Based on previous setups to study collagen gel adhesion [24, 25], we built a custom mechanical testing apparatus. This device consisted of a media bath container (machined from acrylic) attached to a motorized stage, with a load cell fixed at one end (see Fig. 7A). The bath contained a set of posts that were used for fixing the sample in place. The sample consisted of two slabs of collagen-alginate gel (2 cm × 2 cm × 0.5mm), each glued to an acrylic platten with cyanoacrylate. We fabricated collagen-alginate slabs using the same molding approach used to fabricate the microfluidic hydrogels. We used a laser cutter (VersaLASER, Scottsdale, AZ) to fabricate disposable acrylic platens; the top platen had a triangular cutout for aligning and attaching a rigid stainless steel wire between the sample and the load cell, and the bottom platen had cutouts for attachment to posts in the media bath. We glued the collagen-alginate gels to the platens and cooled the collagen samples in a refrigerator. We then bonded the two collagen-alginate samples to each other by pipetting 20 µL of collagen onto one gel, then placing the second gel on top while ensuring the gels were aligned to each other. Similar to the microfluidic gels, the samples were held on a cold plate while gluing until they were ready to be placed at 22 or 37°C for gelling. We expected this volume of collagen distributed across the 4 cm2 surface would result in a 50 µm-thick iECM, so that there would be adequate space between the layers for the growth of integrating fibers from the pECM surfaces. We then attached the integrated gels to the posts in the media bath, and used the HEPES buffer solution to maintain sample hydration. We then positioned the wire attached to the load cell in the cutout in the top platen. We used a microcontroller to drive the motorized stage at a constant rate of 4.8 mm/min relative to the load cell. The output of the load cell was transmitted by a multimeter to a PC and recorded using LabView software (National Instruments, Austin, TX). We converted forces to stresses by diving by the interface area (4 cm2). We removed noise from the stress vs. time curves and determined the shear adhesion strength of the interface as the maximum value of stress that the sample could endure before breaking.
Fig. 7.
Characterization of interface adhesion strength by single lap shear adhesion testing. (A) Schematic of mechanical testing apparatus. Two collagen-alginate gels are glued to acrylic platens using cyanoacrylate, and then glued to each other with collagen. The gels on platens are then transferred to the media bath. Posts in the bath constrain the lower platen. A rigid steel wire connected to the load cell is inserted into a triangular cut in the top platen. A microcontroller controlled motorized stage is used to translate the specimen at 4.8 mm/min relative to the load cell. (B) Image of testing platform. (C) Representative shear adhesion test curves for each assembly condition. Each plot shows representative curves for each pECM assembly condition for a given iECM assembly condition.
2.4 Statistical analysis
In performing ANOVA (followed by Tukey-Kramer posthoc test), we judged the differences among data sets to be statistically significant if p < 0.05.
3. Results
3.1 Experimental design and hypotheses of how assembly conditions would influence collagen structure
We developed an experimental setup in which the collagen-interfacing method for multiple ECM compositions could be investigated in parallel within a single gel (Fig. 2A). Using previously published methods [20], we fabricated four open microchannels inside hydrogels made of a collagen-alginate composite gel; this bulk gel served as the pECM. Inside the four open microchannels, we flowed in suspensions of different concentrations of collagen; these liquid suspensions served as the iECM. To further tune the structure of collagen fibers at the interfaces and within each ECM, we systematically varied the gelling temperatures for both pECM and iECM (Fig. 2B–C). We refer to the set of collagen concentrations and gelling temperatures as assembly conditions. We set out to examine how different assembly conditions would affect the collagen structures both in the bulk phase and at interfaces.
To examine the structure of collagen fibers in the bulk phase, we quantified two structural parameters for the preformed collagen fibers within the pECM: mesh size and fiber widths (Fig. 2B). Recent experimental evidence suggests that collagen self-assembles in vitro by a nucleation and growth mechanism in which the structure of collagen fibers is determined by the size and density of nuclei formed during a temperature-dependent initial phase of gelling [6]. Therefore, a low temperature during the nucleation phase results in few large nuclei, which would be expected to produce long and thick collagen fibers, and an open network (Fig. 2B). Also, varying the collagen concentration would be expected to alter the density of nuclei and thus the density of fibers in the pECM (Fig. 2B).
To examine the structure of collagen fibers at the interface, we quantified two other structural parameters for the integrating fibers in the iECM: surface density (number of integrating fibers per unit surface area of the interface) and interface thickness (the distance from the tips of integrating fibers to the interface) (Fig. 2C). We expected that we could control the structure of integrating fibers at the interface by varying the structure of preformed collagen fibers in pECM, as well as by modulating the assembly conditions for the nucleating collagen fibers in iECM. In particular, we hypothesized that a higher density of preformed fibers would result in a higher surface density of integrating fibers, and that larger preformed fibers (as measured by fiber width) or a lower temperature for gelling the iECM would result in an increased interface thickness (Fig. 2C).
3.2 Characterization of collagen structure in pECM under different assembly conditions
First, we characterized the structure of bulk collagen fibers in pECM by measuring the mesh size and fiber widths. In agreement with previous studies of bulk collagen gels [6, 23], collagen fibers gelled at 37°C exhibited a higher fiber density and thinner fibers compared to those gelled at 22°C (Fig. 3). Our results showed that, at each gelling temperature, each tested collagen concentration resulted in a statistically unique mesh size (Fig. 3B). 37°C assembly conditions also produced significantly thinner fibers than 22°C for each collagen concentration (Fig. 3C). At 37°C, where most fibers remained as single fibers (as was observed previously [26]), the collagen concentration had no statistically significant effect on average fiber width. At 22°C, although we observed a statistically significant increase in fiber width for the 2 mg/mL collagen concentration, the fiber widths were more difficult to interpret due to significant fiber bundling under that gelling condition (see top images from Fig. 3A). Thus, each assembly condition for pECM provided a unique combination of collagen mesh size and fiber width at the surface of the gel for subsequent growth of integrating fibers.
Fig. 3.
Modulation of preformed collagen fiber structure in collagen-alginate microfluidic hydrogels. (A) Representative confocal reflectance microscopy images of collagen fibers formed under varying collagen concentration and gelling temperature. Scale bar is 10 microns. B–C) Plots of (B) mesh size (n = 12 image stacks per condition, taken from 6 independent experiments) and (C) fiber width (n = 45 representative fibers per condition) measured from confocal reflectance images for each condition (see Methods for details). Different numbers of asterisks indicates a significant difference between groups.
3.3 Characterization of collagen structure in iECM under different assembly conditions
Next, we sought to determine how both the unique preformed fiber structures of the pECM and the subsequent assembly conditions of the iECM modulated the structure of integrating fibers at the interface. We performed collagen fiber integration under 48 assembly conditions (3 collagen concentrations for pECM, 4 collagen concentrations for iECM, and two gelling temperatures for each ECM phase), and took images of the resulting interfaces using confocal reflectance microscopy. Representative images of selected assembly conditions are shown in Fig. 4 (representative images of all 48 conditions are shown in Supplementary Figs. 3–6).
Fig. 4.
Representative images of interface structure for: A) 1 mg/mL collagen in both pECM and iECM, B) 2 mg/mL collagen in both pECM and iECM, C) 4 mg/mL collagen in both pECM and iECM, and D) 1 mg/mL collagen in pECM and 6 mg/mL collagen in iECM for all combinations of gelling temperature for each phase. Scalebar is 10 microns. Representative images for all 48 combinations of concentration and gelling temperature are shown in Supplementary Figs. 3–6. Note that each image is one slice (0.25 µm thick) out of 41 images in the z-stack. A single fiber typically crosses multiple z-slices.
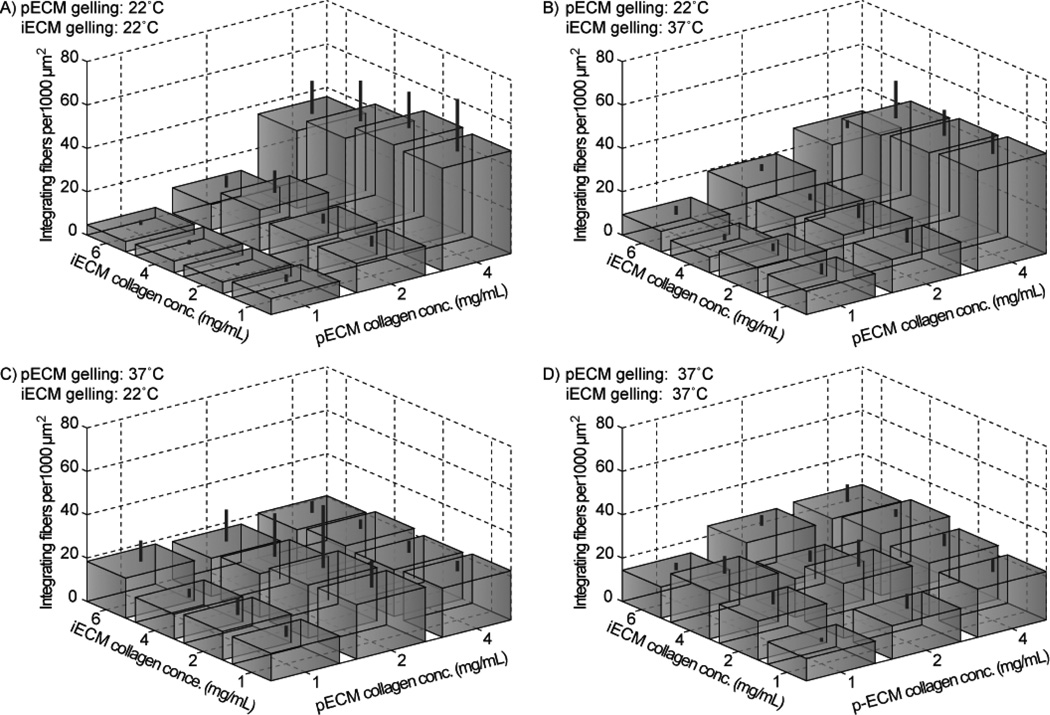
We quantified the surface density of integrating fibers (Fig. 5) and the interface thickness (Fig. 6). Under 22°C pECM assembly conditions which produced a low density of surface nuclei, the surface density of integrating fibers significantly increased with increasing pECM collagen concentration (Fig. 5A–B and Supplementary Table 1). However, under 37°C pECM assembly conditions which already produced a large density of surface nuclei, no significant differences in integrating fiber density were seen across pECM collagen concentrations (Fig. 5C–D and Supplementary Table 1). The iECM collagen concentration and gelling temperature generally had little effect on the surface density of integrating fibers, indicating that the surface density of integrating fibers is determined primarily by the structure of the preformed fibers in pECM.
Fig. 5.
Integrating fiber surface density under varying assembly conditions. Standard deviation is indicated by lines above the boxes. Significant differences between groups (determined by ANOVA followed by Tukey-Kramer post-hoc test) are shown is Supplementary Table 1.
Fig. 6.
Interface thickness under varying assembly conditions. Standard deviation is indicated by lines above the boxes. Significant differences between groups (determined by ANOVA followed by Tukey-Kramer post-hoc test) are shown is Supplementary Table 2.
By contrast, the interface thickness depended strongly on the gelling temperature of iECM (compare Fig. 6 panels A, C to panels, B, D). Specifically, the interface thickness was significantly larger for the 22°C iECM assembly conditions, which is an expected trend since collagen that gels at lower temperatures exhibits longer fibers. Similar to the results for surface density of integrating fibers, changing the iECM collagen concentration had no significant effect on the interface thickness. Interestingly, for iECM gelled at 37°C, there also appeared to be a slight increase in the interface thickness when pre-existing fibers were thick in the pECM (compare Fig. 6 panel B to panel D). Hence, for this iECM gelling temperature, wide fibers in the pECM may result in long fibers in the iECM and hence increased interface thickness, similar to the case for gelling in bulk ECM in which larger nuclei resulted in longer fibers [6].
From the representative interface images (Fig. 4, Supplementary Figs. 3–6), it also appears that the iECM assembly conditions strongly influence the fiber structure within the bulk of the iECM. Although we attempted to minimize flow-based alignment of collagen fibers in iECM by allowing flow to stop for several minutes before raising the temperature, we occasionally observed matrices with fibers appearing to be partially aligned along the microchannels. This alignment is likely due to the relatively imprecise flow control inherent in the hydrostatic delivery method.
3.4 Adhesion strength of collagen fiber-mediated bonding
In order to confirm that an assembly condition resulted in strong integration of the ECM phases, as well as to examine whether varying the structure of integrating fibers would have an impact on the bond strength, we subjected interfaced ECMs to a shear adhesion test. We performed a single lap shear test in which two preformed collagen-alginate hydrogel slabs (pECM) were bonded with a layer of collagen (iECM) (Fig. 7). The slabs were glued on acrylic platens, and then “bonded” to each other using a small volume of collagen gelled (equivalent to ~50 µm-thick layer of iECM) at two different concentrations and two gelling temperatures. Similar setups have been used previously to measure the bond strength of tissue adhesives and cell-mediated interfacing of collagen-based ECMs [24, 25, 27, 28]. Because we expected the crosslinked alginate in the composite pECM would prevent failure within the pECM slabs (since these composite gels are more robust than pure collagen matrices), we expected failure to always occur in the iECM phase. Visual inspection of the samples after separation confirmed that failure occurred in the iECM phase in all samples. Moreover, as expected, adhesion curves exhibited a linear increase in force upon generation of tension between the sample and load cell, followed by a rapid drop in force upon breakage of the interface (Fig. 7C).
We performed two negative control experiments. In one control, we attempted to place two pECM gels together without using the iECM glue; this control tested whether placing the pECM surfaces together without creating integrated collagen fibers resulted in significant adhesion strength. For the second control, we made pECM gels of pure alginate with no collagen, and attempted to glue the pECM slabs together using the iECM glue under the same conditions; this control tested whether the presence of preformed collagen fibers was necessary for forming a significant bond strength between pECM layers. For both of these controls, adhesions of the pECM layers were too weak to be tested, as the layers floated apart in the media bath or fell apart when we attempted to place them in the bath. These observations confirmed our previous result that the collagen-integration method helps to bond tissue structures [20]. Moreover, the failures of these negative controls confirmed that the shapes of the curves in Fig. 7C reflected that the adhesion was due to the integrated fibers.
From the adhesion test curves, we determined the “adhesion strength” from the maximum value of shear stress. The range of mean adhesion strengths was 0.1 to 0.75 kPa, which was significantly lower than other natural ECM-based tissue adhesives such as fibrin glues (1 to 10 kPa) [29, 30] (note that commercial fibrin glues have a much higher fibrillar protein concentration, such as 10 to 100 mg/mL [31]). (Despite the popularity of collagen as a biomaterial, to our knowledge there are no previous studies that investigated the lap shear adhesion strength of tissues bound together using a pure collagen glue.) The adhesion strengths of collagen-mediated bonding in this study were several times stronger than as for cell-mediated interfacing of multiple collagen-based tissue layers [25], suggesting that assembly of soluble collagen at ECM interfaces may more strongly integrate adjacent ECMs than the collagen fibers deposited in short term culture by cells. These adhesion strengths are also about two orders of magnitude weaker than cyanoacrylate based tissue adhesives [27] (which was how the pECM was glued to the acrylic platen).
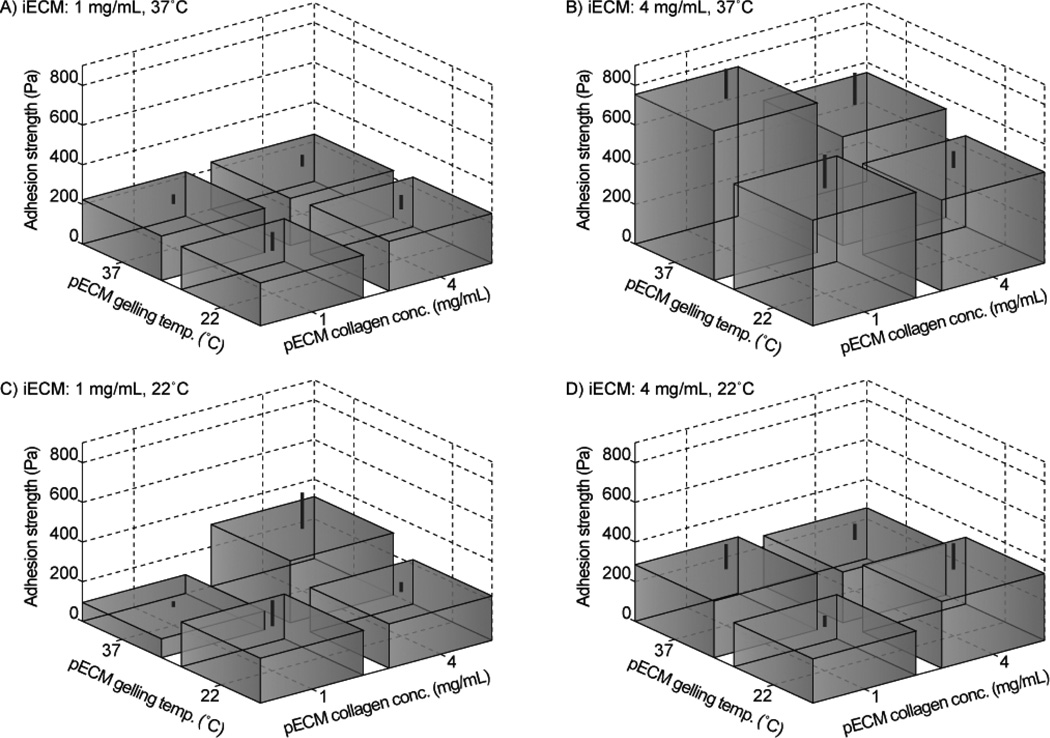
In order to compare the influences of preformed fiber structure on the adhesion strength, we grouped the adhesion results for different pECM gelling conditions under each of the four iECM gelling conditions (Fig. 8). The results showed that for certain conditions (panels A and D), there was little impact of the preformed fiber structure on the adhesion strength. Such a result could reflect that the weakest point of the samples were in the bulk iECM fibers away from the interface, such that the structure of integrating fibers did not impact the adhesion strength. By contrast, for the 1 mg/mL, 22°C iECM gluing condition, the 4 mg/mL, 37°C pECM condition exhibited significantly stronger adhesion than the 1 mg/mL, 37°C pECM condition (Fig. 8C). This result could be rationalized due to a high collagen concentration in the pECM, which would result in a greater density of nucleation sites at the surface for bonding the adjacent ECM. However, for the 4 mg/mL, 37°C iECM gluing condition, the 1 mg/mL, 37°C pECM condition was significantly stronger than the three other pECM conditions (Fig. 8B). Taken together, these results point to the complex interactions among nucleation sites in the pECM, integrating fibers, and bulk fibers in the iECM in determining the adhesion strength.
Fig. 8.
Maximum adhesion strength under varying assembly conditions for pECM and iECM. Standard deviation is indicated by lines above the boxes.
4. Discussion
4.1 Influence of assembly conditions on collagen structure
Our results provided data on how microscale control of collagen structures can be attained in a multiphase tissue for both bulk fibers and fibers at interfaces. The data confirmed that the collagen in the bulk pECM exhibited a higher fiber density and thinner fibers when gelled at 37°C compared to those gelled at 22°C. These trends are similar to previous studies of bulk matrices made of pure collagen and collagen-glycosaminoglycan (GAG) [6, 23]. We believe that the fiber densities in this study were somewhat lower than those for pure collagen matrices because alginate increases the collagen mesh size in a similar fashion as GAGs [32, 33].
Given our ability to produce pECM with statistically unique mesh sizes (Fig. 3B), we could analyze how the structure of preformed collagen fibers in the pECM would influence the structure of the integrating fibers in the iECM. Surprisingly, although we expected that decreasing pECM mesh size would consistently increase the surface density of integrating fibers, we found that there was an intermediate pECM mesh size (~6 µm, under 4 mg/mL, 22°C pECM assembly conditions) that produced the highest surface density of integrating fibers (~40 integrating fibers per 1000 µm2). A possible explanation is that very close spacing of preformed fibers causes the growing integrating fibers to sterically crowd each other, inhibiting further elongation, at least for fibers that were large enough to be visualized.
Based on previous studies of in vitro collagen assembly that showed large collagen fibers assembled from large nuclei during the temperature-dependent nucleation phase [6], we also hypothesized that a low temperature during gelling of the iECM would result in a greater interface thickness. The results showed that iECM gelled at 22°C compared to 37°C resulted in significantly larger interface thickness (Fig. 5). By contrast, the gelling temperature of pECM had only a slight influence on the interface thickness when iECM was gelled at 37°C. Interestingly, changing the concentration of collagen in the iECM resulted in no significant effect on the surface density of integrating fibers or interface thickness.
4.2 Utility of collagen-integration method for tissue engineering
The collagen-interfacing method can be used to integrate microfabricated tissue blocks or multiple layers of scaffolds [20, 34], even in the face of cellular contractile forces. This study shows that by using this same collagen-interfacing method, it is possible to exploit the manipulation of collagen assembly in order to systematically engineer collagen fiber structures. Although collagen matrices have been extensively utilized for in vitro biological studies and tissue engineering, the influence of collagen fiber structure on cell behaviors is only beginning to be explored. This study shows how the structures of collagen fibers, in both the bulk phases and across tissue interfaces, can be effectively controlled and engineered throughout multiphase 3D tissues.
Control of collagen fiber structure throughout multiphase tissues is potentially useful for a number of applications. Recent tissue engineering efforts have focused on recreating the fibrillar architecture of native ECMs, in effort to impart proper mechanical and cell-signaling functions to engineered tissues [14, 16, 35]. Collagen fiber-mediated integration has been used to integrate multilayer electrospun scaffolds for example [34], which could be potentially applied to strengthening the integration of lamellar structures such as the annulus fibrosus of the intervertebral disk [36]. Furthermore, since collagen fiber microstructure and tissue mechanical properties are tightly correlated [14, 26], cell behavior in engineered tissues can strongly depend on how global loads are transmitted to cells by their local ECM [14]. For example, in the future, control over collagen fiber microstructure in distinct regions of a multiphase tissue could aid in recapitulating mechanically dependent cellular processes that exist in different zones of native tissues (such as in cartilage), thereby improving the overall response of the tissue to globally applied mechanical forces.
Moreover, the ability to exert further control over collagen fiber structure may aid in understanding cellular responses to local changes in ECM structure for numerous physiological situations, including angiogenesis and tumor metastasis. For example, the structure of capillaries forming in in vitro collagen gels is dramatically altered through changing structural and mechanical properties of the collagen matrix [37]. In tumor microenvironments, metastasizing cells have been observed to align and compact collagen fibers locally, creating regions of varied fiber alignment and density [38]. Some tumor cells have shown the ability to use different migration mechanisms when encountering matrices of different pore size [5]. Creation of interfaces having transitions in fiber structure could be used to investigate dynamic cell migration behavior through changes in matrix architecture. Although the presence of alginate in the pECM in this study would prevent migration of cells between phases (due to the small pore size of alginate), uncrosslinking of the alginate using a calcium chelator can allow for dynamic removal of this spatial migration constraint [20, 32]. Overall, the approach of exploiting collagen assembly conditions as described in this study complements existing approaches to control local collagen structures such as flow alignment or electrospinning [6, 10–13].
5. Conclusion
This paper demonstrates a method for engineering microscale variations in collagen structure in multiphase tissues. Using this collagen-interfacing strategy, the results indicate that it is important to control the structure of preformed fibers in the pECM, which determines the interfacial fiber structure and adhesion strength. Because collagen structures vary throughout tissues and cell behavior is driven in part by ECM structure, control over the bulk fiber structure and interfacial properties of multiphase collagen-based ECMs should prove highly useful in tissue engineering and studies of cells in 3D environments.
Supplementary Material
Acknowledgements
We thank the American Heart Association (Scientist Development Grant), the National Science Foundation (CAREER), and the National Institutes of Health (Grant R01HL095477) for financial support of this work. We thank Prof. Helen Lu, Nora Khanarian, and Sid Subramony for advice in the mechanical testing experiments, and Keith Yeager and Mario Modena for assisting with building the testing rig.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lu HH, Subramony SD, Boushell MK, Zhang X. Tissue engineering strategies for the regeneration of orthopedic interfaces. Ann Biomed Eng. 2010;38:2142–2154. doi: 10.1007/s10439-010-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mikos AG, Herring SW, Ochareon P, Elisseeff J, Lu HH, Kandel R, et al. Engineering complex tissues. Tissue Eng. 2006;12:3307–3339. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma B, Williams CG, Kim TK, Sun D, Malik A, Khan M, et al. Designing zonal organization into tissue-engineered cartilage. Tissue Eng. 2007;13:405–414. doi: 10.1089/ten.2006.0068. [DOI] [PubMed] [Google Scholar]
- 4.Spalazzi JP, Dagher E, Doty SB, Guo XE, Rodeo SA, Lu HH. In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. J Biomed Mater Res A. 2008;86:1–12. doi: 10.1002/jbm.a.32073. [DOI] [PubMed] [Google Scholar]
- 5.Beadle C, Assanah MC, Monzo P, Vallee R, Rosenfeld SS, Canoll P. The role of myosin II in glioma invasion of the brain. Mol Biol Cell. 2008;19:3357–3368. doi: 10.1091/mbc.E08-03-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang YL, Motte S, Kaufman LJ. Pore size variable type I collagen gels and their interaction with glioma cells. Biomaterials. 2010;31:5678–5688. doi: 10.1016/j.biomaterials.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 7.Miron-Mendoza M, Seemann J, Grinnell F. The differential regulation of cell motile activity through matrix stiffness and porosity in three dimensional collagen matrices. Biomaterials. 2010;31:6425–6435. doi: 10.1016/j.biomaterials.2010.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillette BM, Perez N, Varghese P, Sia SK. Collagen: A natural nanobiomaterial for high-resolution studies in tissue engineering. In: Sitharaman B, editor. Nanobiomaterials Handbook. New York: CRC Press; 2011. (in press) [Google Scholar]
- 9.Chrobak KM, Potter DR, Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res. 2006;71:185–196. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Lee P, Lin R, Moon J, Lee LP. Microfluidic alignment of collagen fibers for in vitro cell culture. Biomed Microdevices. 2006;8:35–41. doi: 10.1007/s10544-006-6380-z. [DOI] [PubMed] [Google Scholar]
- 11.Sung KE, Su G, Pehlke C, Trier SM, Eliceiri KW, Keely PJ, et al. Control of 3-dimensional collagen matrix polymerization for reproducible human mammary fibroblast cell culture in microfluidic devices. Biomaterials. 2009;30:4833–4841. doi: 10.1016/j.biomaterials.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo C, Kaufman LJ. Flow and magnetic field induced collagen alignment. Biomaterials. 2007;28:1105–1114. doi: 10.1016/j.biomaterials.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Dong B, Arnoult O, Smith ME, Wnek GE. Electrospinning of collagen nanofiber scaffolds from benign solvents. Macromol Rapid Commun. 2009;30:539–542. doi: 10.1002/marc.200800634. [DOI] [PubMed] [Google Scholar]
- 14.Roeder BA, Kokini K, Voytik-Harbin SL. Fibril microstructure affects strain transmission within collagen extracellular matrices. J Biomech Eng. 2009;131:031004. doi: 10.1115/1.3005331. [DOI] [PubMed] [Google Scholar]
- 15.Tan W, Desai TA. Microscale multilayer cocultures for biomimetic blood vessels. J Biomed Mater Res A. 2005;72:146–160. doi: 10.1002/jbm.a.30182. [DOI] [PubMed] [Google Scholar]
- 16.Baker BM, Nathan AS, Gee AO, Mauck RL. The influence of an aligned nanofibrous topography on human mesenchymal stem cell fibrochondrogenesis. Biomaterials. 2010;31:6190–6200. doi: 10.1016/j.biomaterials.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du Y, Lo E, Ali S, Khademhosseini A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc Natl Acad Sci U S A. 2008;105:9522–9527. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albrecht DR, Underhill GH, Mendelson A, Bhatia SN. Multiphase electropatterning of cells and biomaterials. Lab Chip. 2007;7:702–709. doi: 10.1039/b701306j. [DOI] [PubMed] [Google Scholar]
- 19.Cheung YK, Azeloglu EU, Shiovitz DA, Costa KD, Seliktar D, Sia SK. Microscale control of stiffness in a cell-adhesive substrate using microfluidics-based lithography. Angew Chem Int Edit. 2009;48:7188–7192. doi: 10.1002/anie.200900807. [DOI] [PubMed] [Google Scholar]
- 20.Gillette BM, Jensen JA, Tang B, Yang GJ, Bazargan-Lari A, Zhong M, et al. In situ collagen assembly for integrating microfabricated three-dimensional cell-seeded matrices. Nat Mater. 2008;7:636–640. doi: 10.1038/nmat2203. [DOI] [PubMed] [Google Scholar]
- 21.Cabodi M, Choi NW, Gleghorn JP, Lee CS, Bonassar LJ, Stroock AD. A microfluidic biomaterial. J Am Chem Soc. 2005;127:13788–13789. doi: 10.1021/ja054820t. [DOI] [PubMed] [Google Scholar]
- 22.Brightman AO, Rajwa BP, Sturgis JE, McCallister ME, Robinson JP, Voytik-Harbin SL. Time-lapse confocal reflection microscopy of collagen fibrillogenesis and extracellular matrix assembly in vitro. Biopolymers. 2000;54:222–234. doi: 10.1002/1097-0282(200009)54:3<222::AID-BIP80>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y-l, Kaufman LJ. Rheology and confocal reflectance microscopy as probes of mechanical properties and structure during collagen and collagen/hyaluronan self-assembly. Biophys J. 2009;96:1566–1585. doi: 10.1016/j.bpj.2008.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marenzana M, Kelly DJ, Prendergast PJ, Brown RA. A collagen-based interface construct for the assessment of cell-dependent mechanical integration of tissue surfaces. Cell Tissue Res. 2007;327:293–300. doi: 10.1007/s00441-006-0316-z. [DOI] [PubMed] [Google Scholar]
- 25.Hadjipanayi E, Brown RA, Mudera V. Interface integration of layered collagen scaffolds with defined matrix stiffness: implications for sheet-based tissue engineering. J Tissue Eng Regen Med. 2009;3:230–241. doi: 10.1002/term.157. [DOI] [PubMed] [Google Scholar]
- 26.Yang YL, Leone LM, Kaufman LJ. Elastic moduli of collagen gels can be predicted from two-dimensional confocal microscopy. Biophys J. 2009;97:2051–2060. doi: 10.1016/j.bpj.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kull S, Martinelli I, Briganti E, Losi P, Spiller D, Tonlorenzi S, et al. Glubran2 surgical glue: in vitro evaluation of adhesive and mechanical properties. J Surg Res. 2009;157:e15–e21. doi: 10.1016/j.jss.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 28.Mahdavi A, Ferreira L, Sundback C, Nichol JW, Chan EP, Carter DJ, et al. A biodegradable and biocompatible gecko-inspired tissue adhesive. Proc Natl Acad Sci U S A. 2008;105:2307–2312. doi: 10.1073/pnas.0712117105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silver FH, Wang MC, Pins GD. Preparation of fibrin glue: a study of chemical and physical methods. J Appl Biomater. 1995;6:175–183. doi: 10.1002/jab.770060306. [DOI] [PubMed] [Google Scholar]
- 30.Burke SA, Ritter-Jones M, Lee BP, Messersmith PB. Thermal gelation and tissue adhesion of biomimetic hydrogels. Biomed Mater. 2007;2:203–210. doi: 10.1088/1748-6041/2/4/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buchta C, Hedrich HC, Macher M, Höcker P, Redl H. Biochemical characterization of autologous fibrin sealants produced by CryoSeal® and Vivostat® in comparison to the homologous fibrin sealant product Tissucol/Tisseel®. Biomaterials. 2005;26:6233–6241. doi: 10.1016/j.biomaterials.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Gillette BM, Jensen JA, Wang M, Tchao J, Sia SK. Dynamic hydrogels: switching of 3D microenvironments using two-component naturally derived extracellular matrices. Adv Mater. 2009;22:686–691. doi: 10.1002/adma.200902265. [DOI] [PubMed] [Google Scholar]
- 33.Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCullen SD, Miller PR, Gittard SD, Gorga RE, Pourdeyhimi B, Narayan RJ, et al. In situ collagen polymerization of layered cell-seeded electrospun scaffolds for bone tissue engineering applications. Tissue Eng Part C Methods. 2010;16:1095–1105. doi: 10.1089/ten.tec.2009.0753. [DOI] [PubMed] [Google Scholar]
- 35.Mauck RL, Baker BM, Nerurkar NL, Burdick JA, Li WJ, Tuan RS, et al. Engineering on the straight and narrow: the mechanics of nanofibrous assemblies for fiber-reinforced tissue regeneration. Tissue Eng Part B Rev. 2009;15:171–193. doi: 10.1089/ten.teb.2008.0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nerurkar NL, Han W, Mauck RL, Elliott DM. Homologous structure-function relationships between native fibrocartilage and tissue engineered from MSC-seeded nanofibrous scaffolds. Biomaterials. 2011;32:461–468. doi: 10.1016/j.biomaterials.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sieminski AL, Hebbel RP, Gooch KJ. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp Cell Res. 2004;297:574–584. doi: 10.1016/j.yexcr.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 38.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.