Abstract
Dendritic cells (DC) are antigen-presenting cells found in both lymphoid and nonlymphoid organs, including the brain (bDC) of Cd11c/eyfp transgenic C57BL/6 mice. Using an intranasal vesicular stomatitis virus infection, we demonstrated that EYFP+ cells amass in areas associated with viral antigens, take on an activated morphology, and project their processes into infected neuronal tissue within the olfactory bulb. These bDC separated into three EYFP+ CD45+ CD11b+ populations, all but one being able to functionally promote both T lymphocyte proliferation and TH1 cytokine production. One population was shown to emanate from the brain and a second population was peripherally derived. The third population was of indeterminate origin, being both radiosensitive and not replenished by donor bone marrow. Finally, each EYFP+ population contained CD11b+ CD103+ subpopulations and could be distinguished in terms of CD115, Gr-1, and Ly-6C expression, highlighting mucosal and monocyte-derived DC lineages.
Keywords: antigen presentation, neuroinflammation
Dendritic cells (DC) are immune cells specialized in antigen capture, processing, and presentation to T lymphocytes for the induction of adaptive immunity (1) or tolerance (2, 3). Since their initial identification (4) and characterization (5, 6), DC are now recognized to represent a heterogeneous group consisting of many distinct subpopulations identifiable by a diverse array of cell-surface markers and functions (7, 8). DC subpopulations are found in both lymphoid and nonlymphoid tissue, such as the dermis, intestine, liver, lungs, kidneys, and the heart (9–12). Some examples of this phenotypic heterogeneity include: plasmacytoid DC, the primary function of which is the production of type I interferons (13); the CD8+ DEC205+ DC in the spleen, which are involved in cross-presentation of antigen to cytotoxic T lymphocytes; and CD103+ CD11b+ DC in the intestine, which migrate to draining lymph nodes in a CCR7-dependent manner and promote the proliferation of regulatory T cells (Treg) (14–16).
DC are also found within the brain (bDC) and meninges of the Itgax (Cd11c)/eyfp transgenic (Tg) mouse (17, 18). In the steady state, bDC are found within discrete regions of the CNS (17). Furthermore, bDC have been shown to respond to physical trauma and intracranial treatment with the proinflammatory cytokine IFN-γ (19, 20). Given the ability of bDC to present antigen ex vivo after stimulus with IFN-γ, and their association with blood brain barrier (BBB) compromised or deficient anatomical regions of the CNS [e.g., the olfactory bulb (OB) and circumventricular organs], bDC appear to possess the CNS antigen presenting cell function first attributed to bone marrow-derived perivascular microglia by Hickey and Kimura (21). To further elucidate in vivo bDC function, we capitalized on a naturally occurring infectious route to the brain, namely CNS access via olfactory nerves within the nasal epithelia.
Using vesicular stomatitis virus (VSV) to elicit an encephalitic state after intranasal administration (22), we sought to better understand the bDC response to an acute infection via phenotypic and functional characterization. VSV is a member of the Rhabdoviridae viral family, most notably characterized by its enveloped bullet-shaped virion, negative sense single-stranded RNA genome, and high sensitivity to IFN-elicited immune responses (23). Many VSV attributes, including host range, natural reservoirs, endemic regions, and infectious cycle are well known. One characteristic in particular, the polarized nature of VSV virion budding, is the basis for the intranasal VSV infection model used here—and by many others—to study virally induced encephalitis in the mouse (24, 25). VSV is capable of accessing the CNS via olfactory nerve cells that span the cribriform plate; this, and its neuro-invasive pathology, make it amenable to studying proinflammatory responses within the brain (26, 27).
In this work we found that VSV infection of Cd11c/eyfp Tg mice results in anatomical changes in CD11c+ cell distribution and morphology relative to regions associated with viral antigen expression. Flow cytometric analysis reveals the presence of unique CD45+, CD11b+, and CD11c+ cell populations within the VSV-infected OB, each with its own distinct phenotype. Functional analysis of these populations demonstrates the ability of some to process and present antigen to T lymphocytes on par with splenic conventional DC (cDC). Finally, we explore the origin and lineage of these unique bDC populations within the context of viral infection using radiation chimeras, EdU-labeling, and phenotyping. These data revealed the presence of CD103+ CD11b+ double-positive cells similar to those found in mucosal DC subsets of the intestinal lamina propria and the basal lamina of the bronchial epithelium (10, 14), as well as a population of radio-sensitive, bone marrow-independent cells resembling monocyte-derived DC.
Results
Cd11c/eyfp-Expressing Cells Respond to Early Stages of Acute VSV Infection Within the OB.
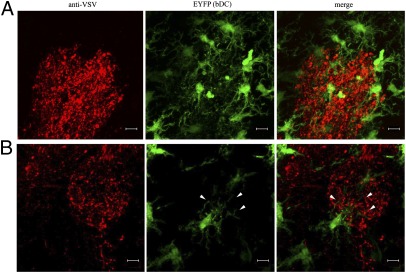
To investigate how CD11c-expressing cells respond to an infection caused by a pathogen's ability to circumvent the BBB, we intranasally infected Cd11c/eyfp Tg mice with VSV. Three cohorts of 12 mice received a LD50 of VSV, UV-inactivated VSV, or vehicle control. Mice were killed following 24, 48, 72, and 96 h postinfection (hpi), and 60-μm OB sections were processed for immunofluorescence staining with an anti-VSV antiserum. Over the first 24 hpi, we observed VSV antigen within the olfactory nerve layer with no apparent change in the EYFP+ distribution pattern, relative to naive mice (Fig. S1A). By 48 hpi, the VSV infection had progressed through the olfactory nerve and penetrated the glomerular layer, again with little change in EYFP+ cell distribution. However, by 72 hpi, EYFP+ cells were found in abundance throughout VSV-infected areas and continued to increase after 96 hpi. This accumulation of EYFP+ cells was only observed with viable VSV and was not reproduced with either UV-inactivated virus or vehicle controls at any time point postinfection (Fig. S1B). These data indicate that a putative bDC response was solely attributable to an active VSV infection rather than from irritation caused by inhalation of liquid or innate immune responses within infected neurons triggered by pathogen-associated molecular patterns. Furthermore, CD11c+ cells that accumulated within these VSV-infected regions exhibited an activated morphology signified by stout cell bodies with stunted processes (28), unlike the thin, delicately branched processes associated with bDC in unchallenged mice (Fig. S2). Closer examination revealed that CD11c+ cells clustered around VSV-infected neurons within glomeruli (Fig. 1A), and projected their processes into neuropil-expressing VSV antigens (Fig. 1B). This phenomenon was reminiscent of the hallmark probing behavior attributed to both brain resident microglia (MG) and peripheral DC subsets (4, 29, 30).
Fig. 1.
bDC surround and probe VSV-infected glomeruli. Representative confocal z-stack analysis of an OB 72 hpi after intranasal VSV infection. (A) A glomerulus labeled with anti-VSV antiserum (red) showed clustering bDC (green). (B) Confocal section visualizing a bDC extending cellular projections (arrowheads) into VSV infected glomerular neuropil. Representative images from three experiments with an n = 3. (Scale bars, 10 μm.)
To correlate the putative bDC response to MG, changes in MG distribution were analyzed in response to the VSV infection. In previously published work, MG were discriminated from putative bDC in the Cd11c/eyfp Tg mouse, primarily based on EYFP expression in conjunction with either an Iba1+ or F4/80+ phenotype (17). Using this definition, the vast majority of EYFPneg Iba1+ cells showed no distributive differences between areas proximal and distal to the infection relative to vehicle control groups (Fig. S3), even though EYFPneg Iba1+ displayed an activated morphology near the site of infection. Given the MG's developmental plasticity (31), we subsequently sought to phenotypically and functionally characterize the CD11c+ cell response to verify their role as bDC.
bDC Responding to Acute VSV Infection Separate into Three Phenotypically and Functionally Distinct Populations.
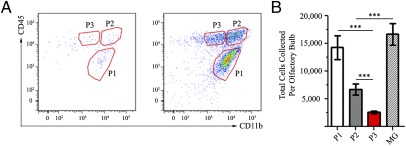
Given that the BBB remains uncompromised during an intranasal VSV infection until 6–8 d postinfection (22), we chose to analyze bDC accumulation at 96 hpi to maximize cell numbers while minimizing peripheral immune contributions. As such, bDC were isolated and phenotypically characterized by flow cytometry at 96 hpi in the VSV-infected OB. After gating for live, singlet, TCRβneg, and CD19neg cells (Fig. S4), EYFP+ cells resolved into three distinct CD45+ and CD11b+ populations only prevalent in infected OB (Fig. 2A). The EYFP+ CD45int CD11bhi (P1) phenotypic population had a similar CD45 and CD11b phenotype as previously described resident MG populations found in the steady state and inflamed brains of wild-type animals (32–35). The two other populations, only found at appreciable levels in the VSV-infected OB, were defined by EYFP+ CD45hi CD11bhi (P2) and EYFP+ CD45hi CD11bint (P3) phenotypes. P1 had the greatest number of EYFP+ cells compared with P2 (P valueP1vsP2 < 0.001) (Fig. 2B) and P3 (P valueP1vsP3 < 0.0001), and P3 had the least number of EYFP+ cells (P valueANOVA < 0.0001). In summary, most EYFP+ cells within the VSV-infected OB at 96 hpi fall within the P1 population.
Fig. 2.
EYFP+ bDC within the VSV-infected OB exhibit three phenotypically distinct populations at 96 hpi. (A) Three distinct EYFP+, CD45+, and CD11b+ populations. (B) Analysis of the differences in each CD45+ and CD11b+ population shows that the P1 population represents the majority of EYFP+ cells; the P2 and P3 populations were less abundant. The EYFP+ cell numbers found within the P1 population are nearly double those in the P2 and P3 populations combined. Data representative of three separate experiments with n = 3; error bars represent SEM. ***P < 0.001
Next, the ability of the three EYFP+ populations to function as antigen-presenting cells was examined using a recombinant version of VSV (rVSV-OVA) able to transduce ovalbumin (OVA) expression in the cells it infects (36). rVSV-OVA was used to induce the robust increases in EYFP+ cells observed in wild-type VSV infections, while simultaneously priming those cells in vivo with OVA. This rVSV-OVA infection model was substantiated by (i) confocal micrographs demonstrating colocalization of OVA and VSV antigens within infected glomeruli (Fig. 3A), and (ii) flow cytometry analysis that revealed an increase in EYFP+ bDC levels in the OB, which resolved into the three populations identified during wild-type VSV infections (Fig. 3B).
Fig. 3.
Recombinant VSV-OVA induced bDC process and present antigen to OVA-specific T lymphocytes. (A) Representative confocal images of anti-OVA (red) and EYFP (green) from OB sections from mice intranasally infected with rVSV-OVA at 96 hpi, resulting in OVA and VSV antigen (blue) colocalization within glomeruli. (Scale bars, 50 μm.) (B) rVSV-OVA infection elicited EYFP+ populations analogous to wild-type VSV. (C) P2 and P3 processed in vivo antigens during a rVSV-OVA intranasal infection and drove ex vivo proliferation of OVA-restricted CD8α+, but not CD4+ T cells. Bar graphs represent the average number of proliferating T cells, as determined by a shift in 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester intensity. (D) All three bDC populations facilitated OVA-restricted CD8α+ T-cell proliferation after ex vivo supplementation with OT-I peptide; however P1 was ineffective at presenting OVA. Representative histograms from OT-I peptide treated DC populations plated 1:30 with T cells. (E) P2 and P3 bDC effectively presented OVA antigens to promote OVA-restricted CD4+ T-cell proliferation, but P1 was ineffective. Histograms from OT-II peptide-treated DC populations plated 1:30, and OVA treated plated 1:5. Error bars represent SEM; 6–12 replicates per treatment group. *P valuevs. T Cell Only < 0.05, **P valuevs. T Cell Only < 0.01, ***P valuevs. T Cell Only < 0.001.
Using CD45, CD11b, and EYFP as markers, cells from P1, P2, and P3 were then isolated by FACS at 96 hpi from rVSV-OVA intranasally infected OB, along with MG (identified as CD45int CD11bhi EYFPneg). As controls, splenic cDC (B220neg EYFPhi) were also isolated from both steady state and rVSV-OVA intraperitoneally infected Cd11c/eyfp Tg mice. Sorted P2 and P3 bDC, as well as splenic cDC from infected mice, were capable of directly stimulating CD8α OT-I, but not CD4 OT-II, T-cell proliferation (Fig. 3C). The magnitude of the observed proliferation was consistent with both the small percentage of OT-I and OT-II epitopes present within the OVA primary sequence (SIINFEKL ∼2% and ISQAVHAAHAEINEAGR ∼4% of total OVA amino acids, respectively) and the low levels of OVA mRNA produced by the VSV RNA-dependent RNA polymerase, given the genes position in the recombinant viral genome (37). P1 and MG from rVSV-OVA infected OB, as well as splenic cDC from uninfected mice, were unable to stimulate OT T-cell proliferation without further antigen supplementation. In summary, these data illustrate ongoing in vivo processing of OVA antigens by P2, P3, and cDC controls, but not P1 and MG, as illustrated by their ability to facilitate OVA specific T-cell proliferation.
When the EYFP+ populations were supplemented with excess OVA or the appropriate OT peptide significant proliferation of OT T cells was observed, in some cases out-performing splenic cDC populations. However, in all antigen-presentation assays, MG were unable to present OVA, OT-I or OT-II peptide antigens to their respective OT T cells (Fig. 3 D and E). Similarly, P1 cells were unable to process and present OVA or OT-II peptide; however, they were able to facilitate CD8α OT-I T-cell proliferation with the SIINFEKL peptide, albeit at very low levels.
In stark contrast, both P2 and P3 populations were effectively able to present supplemented peptide antigens and OVA ex vivo to OT T cells. In most cases, the P3 population induced a more robust proliferation than that observed with steady state or rVSV-OVA cDC controls (Fig. 3 D and E). This higher functional ability most likely arose from greater levels of phenotypically mature DC found within the intranasally infected OB than spleens from intraperitoneally infected mice, because the spleen primarily monitors the blood and is not likely to be the draining lymphoid organ for infections of the peritoneal cavity.
Finally, cytokine profiles were analyzed from proliferating CD4+ OT-II T cells induced during antigen presentation assays (Fig. S5). Both P2 and P3 populations promoted a proinflammatory helper T-cell (TH1) biased CD4 T lymphocyte response characterized by elevated levels of IL-2, IFN-γ, and TNF, similar to both splenic cDC populations. MG and the P1 population, however, had undetectable levels of both pro- and anti-inflammatory cytokines, which was consistent with the lack of CD4+ T-cell proliferation elicited by those cells. In summary, P2 and P3, much like their cDC counterparts, were effectively able to orchestrate both OVA-specific cytotoxic and TH1-mediated responses after in vitro supplementation with OVA or its peptides. Conversely, P1 and MG did not participate in establishing such antiviral related responses, although P1 did show a mild ability to induce cytotoxic effector cells.
Origin of VSV-Induced bDC Populations.
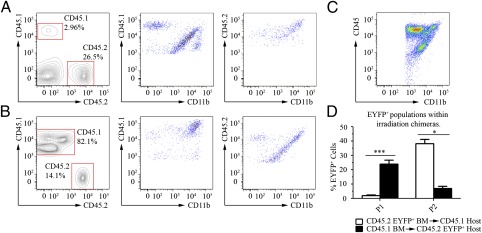
Radiation chimeras were used to ascertain the origin of EYFP+ cell populations that respond to acute VSV infection. After verifying chimerism by analyzing CD45.1+ and CD45.2+ leukocytes in the blood, CD11b+ cells within the OB were assessed for surface CD45.1+ or CD45.2+ expression. In lethally irradiated Cd45.1 genetically engineered host mice replenished with Cd11c/eyfp (CD45.2) Tg bone marrow, radio-resistant cells were identified at 96 hpi that resembled the P1 population in that they were CD45.1int CD11bhi (Fig. 4A). Furthermore, a second population was identified that expressed the CD45.2hi CD11bhi donor phenotype and resembled P2. These observations were verified by EYFP fluorescence because the population resembling P1 was found to be EYFPneg, but the population similar to P2 was EYFP+ (Fig. 4D). These data were also anatomically corroborated in the VSV-infected chimeric OB, because EYFP+ cells were present in low numbers only at the site of acute viral infection (Fig. S6A). These data were recapitulated in reciprocally chimeric mice: that is, lethally irradiated Cd11c/eyfp (CD45.2) Tg host mice replenished with Cd45.1 genetically engineered bone marrow. In short, the P1 population was CD45.2int CD11bhi, radio-resistant and EYFP+, and a second population resembling P2 originated from CD45.1hi CD11bhi donor bone marrow and was EYFPneg (Fig. 4 B and D). These observations were anatomically represented by the high accumulation of EYFP+ cells typically seen virally infected, nonirradiated Cd11c/eyfp (CD45.2) Tg mice (Fig. S6B). Taken together, these data suggest that P1 are radio-resistant and represent a resident population within the brain, and P2 are derived from bone marrow progenitors.
Fig. 4.
The P1 population is associated with the brain parenchyma, but P2 EYFP+ cells are part of the infiltrating peripheral response. (A) Lethally irradiated Cd45.1 mice, successfully rescued with Cd11c/eyfp (CD45.2) Tg bone marrow and their (B) reciprocal chimeras were intranasally infected with VSV at the LD50. At 96 hpi, blood and OB were analyzed by flow cytometry using antibodies able to discriminate between CD45.1 and CD45.2 variants. In both scenarios a CD45int CD11bhi population (containing P1) was identified as brain resident and radio-resistant, and a CD45hi CD11bhi population (containing P2) was consistently derived from donor progenitor cells from the periphery. The P3 (CD45hi CD11bint) population was conspicuously absent from each chimera set, however, as control (C) CD11c/eyfp mice infected with the same batch of VSV possessed all three populations. These findings were corroborated by (D) EYFP+ cell populations. Data are representative of three experiments with n = 5–6 per experiment; error bars represent SEM. *P valuevs. T Cell Only < 0.05, ***P valuevs. T Cell Only < 0.001.
Unexpectedly, no population resembling P3 was associated with CD45.1+ or CD45.2+ leukocytes in either chimeric model, even though all three populations were observed in a control infection of Cd11c/eyfp Tg mice (Fig. 4C). Consequently, the source for P3 is ambiguous at this point and its absence could be indicative of (i) a radiosensitive population that is not replenished by bone marrow progenitor cells within the time constraints of our viral infection model (i.e., mice are optimally susceptible to VSV infection between 5 and 7 wk of age, allowing only 4 wk for chimerism), or (ii) a confound of the irradiation model.
Given that the chimera data supports P1 cells as part of the brain parenchyma, the question still remains as to how these EYFP+ cells go from very low numbers in the steady-state OB to such high levels between 72 and 96 hpi of acute VSV infection. To address this question, EdU-labeled proliferation was assessed in Cd11c/eyfp Tg mice intranasally infected with VSV (LD50) or vehicle alone. After 96 hpi, there was an 71.7% increase in EdU+ cells found within the VSV-infected OB, relative to HBSS controls (Fig. S7A). However, none of the three identified EYFP+ CD45+ CD11b+ populations had incorporated EdU (Fig. S7B); this was interesting considering P1 comprised the largest proportion of EYFP+ cells responding to the VSV infection.
Lineage of VSV-Induced bDC Populations.
In an effort to better understand the lineage associated with all three EYFP+ populations, these cells were separately phenotyped in nonirradiated mice by flow cytometry using cellular markers commonly associated with DC subsets, as well as other immunological cells. As controls, MG isolated from VSV-infected OB were characterized along with cDC (TCRβneg, CD19neg, NK1.1neg, and EYFPhi) from the spleen of VSV-infected mice (Fig. 5A and summarized in Table S1). As expected, all three EYFP+ populations were positive for CD11c and MHC I, although P1 cells expressed the lowest levels of CD11c relative to all other EYFP+ populations. All three EYFP+ populations also contained CD103+ cells, with P3 possessing the least; MG were mostly CD103low cells. Additionally, all populations were found mostly negative for CD8α and DEC-205, with the exception being a very small P3 subpopulation, and all populations were found negative for CD4 and Siglec H. In summary, the three EYFP+ populations were distinguishable from MG based on CD11c and CD103 expression, and from cDC subsets in the spleen associated with cross-presentation (i.e., CD8α+ and DEC-205+).
Fig. 5.
bDC populations contain a diverse array of CD103+ CD11b+ and CD103neg CD11b+ cells. (A) Phenotypic analysis of TCRβneg, CD19neg, CD45+, and CD11b+ cells represented by the three EYFP+ populations using a variety of markers characteristic of DC and other immunological subsets. MG (CD45int, CD11b+ and EYFPneg) and common DC (Linneg EYFPhi) from the spleen of infected mice were used as a control. bDC populations were further discriminated by (B) CD103+ and (C) CD103neg subpopulations possessing MHC II, Gr-1, and Ly-6c+ cells. P1 is composed of a more-or-less equal number of CD103+ and CD103neg cells, but all are MHC II, Gr-1, and Ly-6c–negative. P2 contains several populations of cells with phenotypes analogous to mucosal DCs. Finally, P3 is predominantly composed of CD103neg cells with phenotypes similar to monocytes-derived DC. These histograms and dot plots are representative of three separate experiments, with two mice OB pooled per antibody per experiment.
Further differences between EYFP+ populations were evident when examining MHC II and the expression patterns of the costimulatory molecules. First, P1 cells were found to be MHC IIneg, and a majority of the P2 and P3 populations contained MHC II+ cells. Second, P1 and MG expressed low levels of the costimulatory molecules CD40 and CD80, and moderate levels of CD86, but these markers were found at relatively higher levels within P2 and P3. These results were consistent with P2 and P3 representing mature DC subsets capable of antigen presentation.
In addition to differences in the expression of DC maturation markers, there were noted differences in the markers used to classify DC lineage. Both P1 and P2 populations contained macrophage colony-stimulating factor receptor (CD115)-positive and F4/80+ cells, but P3 cells were both CD115neg and F4/80low. In terms of Ly-6C surface expression, unlike the P1 population, which was Ly-6Cneg, all of P2 and a majority of P3 cells were identified as Ly-6C+. Interestingly, the P3 population also contained cells positive for NK1.1, CD49b, CD122, and CD45R, phenotypes reminiscent of NK cells and a unique IFN-producing killer DC found within both lymph nodes and spleen (38, 39). Finally, using a clone capable of discriminating Ly-6G (Gr-1) from Ly-6C, P2 was the only population found to be Gr-1+.
Subsequent phenotypic analysis using CD103, MHC II, Gr-1, and Ly-6C, as depicted in Fig. 5 B and C, revealed the three EYFP+ cell populations could be further divided into CD103+ and CD103neg subpopulations. P1 was composed of 45.3% ± 7.5% CD103+ cells that were MHC II, Gr-1, and Ly-6C–negative. However, unlike P1 EYFP+ cells, a majority of P2 was composed of CD103+ cells (62.8% ± 2.0%) that were also MHC II+ and Ly-6C+ (45.0% ± 9.5%) with Gr-1+ cells associated with 31.7% ± 4.5% of the CD103+ MHC II+ Ly-6C+ population. Finally, P3 EYFP+ cells were predominantly CD103neg (85.0% ± 0.1%) cells that were also MHC II+ and Ly-6C+ (45.5% ± 4.5%).
Discussion
Using intranasal VSV infection we show that bDC, normally positioned near sites associated with olfactory nerve entry into the steady-state OB, amass in great numbers and surround infected neuronal tissue. Furthermore, we were able to discriminate three phenotypically and functionally distinct EYFP+ cell populations from the phenotype commonly associated with MG, two of which with potentially different lineage associations. Because the pathology associated with intranasal VSV, in terms of viral progression and leukocyte recruitment, is well established (25–27, 40), we chose to examine a time point (96 hpi) where the greatest accumulation of bDC coincided with the ability to analyze their early contributions toward afferent immune responses before breakdown of the BBB. Our data not only depicts an increase of bDC within VSV-infected areas, but closer examination revealed these bDC often projected cellular processes directly into infected tissue. Conversely, EYFPneg Iba1+ MG, although morphologically activated, did not redistribute in response to the acute viral infection. These findings substantiate our hypothesis that EYFP+ bDC occupy vulnerable CNS access points (in this case they exist within tissue that have olfactory nerve projections from the nasal epithelium) to act as first responders capable of orchestrating immunological responses. The nature of those immune responses was the focus of our further investigation.
Phenotypic characterization demonstrated that VSV-induced EYFP+ cells resolved into three distinct populations based on CD45 and CD11b expression not observed in the steady state. One of these populations, described as a radio-resistant resident CD45int CD11bhi (P1) population, was similar in phenotype to MG (34, 35), except that it contains a CD103+ subpopulation, a phenotype resembling migratory DC (10, 11). P1, however, differed from migratory DC cell types based on surface MHC II and costimulatory molecule expression. Furthermore, this population failed to incorporate EdU during an acute VSV infection, all of which strongly suggests P1 may differentiate from the CD103neg CD11clow MG population found within the VSV-infected OB.
P1 and MG populations were also functionally distinguishable from one another. Unlike MG, P1 cells were able to weakly facilitate CD8α+ OT-I T-cell receptor-restricted T lymphocyte proliferation. Given these observations, it would be interesting to assess the phenotypic and functional characteristics of P1 during later stages of the infection (e.g., between 6 and 8 d postinfection). Such an analysis might reveal either a reversion to an inactivated microglial phenotype, or provide evidence for this population's further differentiation. Given that we have not identified an immunogenic role for P1 and that it represents the largest population responding early on during the acute VSV infection, we are currently exploring other functional possibilities for these cells.
The second EYFP+ population of radio-sensitive CD45hi CD11bhi (P2) cells was reminiscent of peripherally infiltrating leukocytes identified during Theiler's murine encephalitis virus infection of rats, and experimental autoimmune encephalomyelitis mouse models (34, 35). P2 cells differed from the P1 and MG populations in terms of MHC II and costimulatory molecule expression. P2 cells were also found to contain CD103+ CD11c+ F4/80+ Gr-1+ and Ly-6Chi subpopulations, a phenotype similar to mucosal DC (10), and were found to be functionally active in most T-cell proliferation assays. The P2 bDC were also able to promote a TH1 bias in proliferating CD4+ T lymphocytes. Taken as a whole, these observations have led us to formulate the hypothesis that P2 derive from a population of mucosal DC (10), which infiltrates the OB of VSV intranasally infected mice, perhaps from the nasal epithelium.
The third EYFP+ population observed was classified by a CD45hi CD11bint (P3) phenotype. We believe P3 cells represent a unique population of DC yet to be described in CNS viral infection models. This population was the least abundant of the EYFP+ cells, but had costimulatory molecule expression on par with splenic cDC and P2 bDC, was able to process antigen and promote T-cell proliferation (often more robustly than splenic cDC controls), and induced a TH1 bias in proliferating CD4+ T lymphocytes. The P3 population, however, was different from the other two EYFP+ populations in that, although it possessed both MHC II+ and Ly-6Chi populations, it was also mostly negative for CD103, CD115, F4/80, and Gr-1. These phenotypes draw similarities to inflammatory monocytes (41, 42), which have the ability to form monocyte derived DC populations. Taken as a whole, the exact DC-subset and source of P3 remains uncertain, as our efforts to identify the origin and source of the P3 population were confounded by those cells being completely absent from VSV-infected irradiation chimeras. We suspect this might be because of P3 representing a radiosensitive population, the source of which is some other organ (e.g., spleen or liver) that is not immediately replenished by bone marrow. However, an alternate hypothesis is that the absence of P3 is because of a lingering proinflammatory condition caused by the lethal irradiation (43).
In conclusion, we identified immune cells acting as sentinels at vulnerable points of the CNS. These cells have the capacity to orchestrate adaptive immune responses in the face of a viral pathogen and demonstrate characteristics of DC. Furthermore, our data provide insight by building upon our previous observations of CD11c+ cells with a dendritic morphology to show a heterogeneous group of immune cells with complex immunological functions. Our hope is that further dissection of the interaction between bDC and disease, resulting from viral stimuli or in other present day models of neurodegenerative diseases, may ultimately lead to the identification of therapeutic targets for ameliorating neuro-inflammatory diseases.
Materials and Methods
Viral Infection Model.
VSV, Indiana Serotype (ATCC VR-158) and rVSV-OVA (36) were isolated from infected BHK (ATCC CCL-10) supernatants and purified on a 5–40% sucrose gradient. Endotoxin levels were determined using a ToxinSensor Chromogenic LAL Endotoxin Assay Kit (GenScript). See SI Materials and Methods for intranasal administration procedure.
Flow Cytometry and FACS.
After 96 hpi, mice were rapidly decapitated, brains extracted, and placed in ice-cold HBSS. OB were subsequently removed and single cell suspensions obtained by digesting with collagenase D (Roche), dispase II (Roche), and DNase I (Invitrogen) in conjunction with repeated trituration using a GentleMACS Dissociator (Miltenyi Biotec) and serological pipetting. See SI Materials and Methods for antibodies, flow cytometry staining procedure, and spleen DC isolation.
Antigen Presentation Assays.
Because of the limited number of viable bDC collected per OB, DC to T-cell ratios were titered, alongside antigen concentrations and T-cell plating number, to maximize both proliferation sensitivity and sample replicates within each experiment. See SI Materials and Methods for details about the antigen presentation assay.
See SI Materials and Methods for additional details on the mouse models, antibodies, immunofluorescence, radiation chimeras, EdU incorporation assays, and the statistics used in this article.
Supplementary Material
Acknowledgments
We thank Drs. Carol Shoshkes Reiss, Margaret MacDonald, Charles Rice, and Leo Lefrançois for providing useful reagents; Drs. Juliana Idoyaga, Andres Gottfried-Blackmore, Elizabeth Waters, Ulrike W. Kaunzner, Cheolho Cheong, and Jae-Hoon Choi for their valuable recommendations; and The Rockefeller University's Comparative Bioscience Center, Flow Cytometry Resource Center, and the Bio-Imaging Resource Center for their expert technical assistance. This research is supported by a gift from the Peter Deane Trust (to K.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203941109/-/DCSupplemental.
References
- 1.Steinman RM, Idoyaga J. Features of the dendritic cell lineage. Immunol Rev. 2010;234:5–17. doi: 10.1111/j.0105-2896.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, et al. Dendritic cell function in vivo during the steady state: A role in peripheral tolerance. Ann N Y Acad Sci. 2003;987:15–25. doi: 10.1111/j.1749-6632.2003.tb06029.x. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 4.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinman RM, Witmer MD. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci USA. 1978;75:5132–5136. doi: 10.1073/pnas.75.10.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nussenzweig MC, Steinman RM. Contribution of dendritic cells to stimulation of the murine syngeneic mixed leukocyte reaction. J Exp Med. 1980;151:1196–1212. doi: 10.1084/jem.151.5.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 8.Dudziak D, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 9.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 11.Ginhoux F, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi JH, et al. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med. 2009;206:497–505. doi: 10.1084/jem.20082129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu YJ. IPC: Professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 14.Sung SS, et al. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006;176:2161–2172. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- 15.Johansson-Lindbom B, et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaensson E, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bulloch K, et al. CD11c/EYFP transgene illuminates a discrete network of dendritic cells within the embryonic, neonatal, adult, and injured mouse brain. J Comp Neurol. 2008;508:687–710. doi: 10.1002/cne.21668. [DOI] [PubMed] [Google Scholar]
- 18.Anandasabapathy N, et al. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J Exp Med. 2011;208:1695–1705. doi: 10.1084/jem.20102657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottfried-Blackmore A, et al. Acute in vivo exposure to interferon-gamma enables resident brain dendritic cells to become effective antigen presenting cells. Proc Natl Acad Sci USA. 2009;106:20918–20923. doi: 10.1073/pnas.0911509106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felger JC, et al. Brain dendritic cells in ischemic stroke: Time course, activation state, and origin. Brain Behav Immun. 2010;24:724–737. doi: 10.1016/j.bbi.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 22.Bi Z, Barna M, Komatsu T, Reiss CS. Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J Virol. 1995;69:6466–6472. doi: 10.1128/jvi.69.10.6466-6472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogel SN, Fertsch D. Macrophages from endotoxin-hyporesponsive (Lpsd) C3H/HeJ mice are permissive for vesicular stomatitis virus because of reduced levels of endogenous interferon: possible mechanism for natural resistance to virus infection. J Virol. 1987;61:812–818. doi: 10.1128/jvi.61.3.812-818.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez Boulan E, Sabatini DD. Asymmetric budding of viruses in epithelial monlayers: A model system for study of epithelial polarity. Proc Natl Acad Sci USA. 1978;75:5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grodums EI. Ultrastructure of mouse periventricular and choroid plexus tissues in experimental vesicular stomatitis virus infection. Arch Virol. 1976;51:75–85. doi: 10.1007/BF01317836. [DOI] [PubMed] [Google Scholar]
- 26.Lundh B, Kristensson K, Norrby E. Selective infections of olfactory and respiratory epithelium by vesicular stomatitis and Sendai viruses. Neuropathol Appl Neurobiol. 1987;13:111–122. doi: 10.1111/j.1365-2990.1987.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 27.Huneycutt BS, et al. Distribution of vesicular stomatitis virus proteins in the brains of BALB/c mice following intranasal inoculation: An immunohistochemical analysis. Brain Res. 1994;635:81–95. doi: 10.1016/0006-8993(94)91426-5. [DOI] [PubMed] [Google Scholar]
- 28.Raivich G, et al. Neuroglial activation repertoire in the injured brain: Graded response, molecular mechanisms and cues to physiological function. Brain Res Brain Res Rev. 1999;30:77–105. doi: 10.1016/s0165-0173(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 29.Lindquist RL, et al. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5:1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- 30.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 31.Santambrogio L, et al. Developmental plasticity of CNS microglia. Proc Natl Acad Sci USA. 2001;98:6295–6300. doi: 10.1073/pnas.111152498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mildner A, et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 33.Getts DR, et al. Ly6c+ “inflammatory monocytes” are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J Exp Med. 2008;205:2319–2337. doi: 10.1084/jem.20080421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greter M, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 35.Sedgwick JD, et al. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci USA. 1991;88:7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim SK, et al. Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals. Proc Natl Acad Sci USA. 1998;95:10814–10819. doi: 10.1073/pnas.95.18.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barr JN, Whelan SP, Wertz GW. Transcriptional control of the RNA-dependent RNA polymerase of vesicular stomatitis virus. Biochim Biophys Acta. 2002;1577:337–353. doi: 10.1016/s0167-4781(02)00462-1. [DOI] [PubMed] [Google Scholar]
- 38.Chan CW, et al. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med. 2006;12:207–213. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- 39.Taieb J, et al. A novel dendritic cell subset involved in tumor immunosurveillance. Nat Med. 2006;12:214–219. doi: 10.1038/nm1356. [DOI] [PubMed] [Google Scholar]
- 40.McGavern DB, Kang SS. Illuminating viral infections in the nervous system. Nat Rev Immunol. 2011;11:318–329. doi: 10.1038/nri2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geissmann F, et al. Blood monocytes: Distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86:398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 42.Geissmann F, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linard C, et al. Acute induction of inflammatory cytokine expression after gamma-irradiation in the rat: effect of an NF-kappaB inhibitor. Int J Radiat Oncol Biol Phys. 2004;58:427–434. doi: 10.1016/j.ijrobp.2003.09.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.