Abstract
Opioids create a neuroinflammatory response within the CNS, compromising opioid-induced analgesia and contributing to various unwanted actions. How this occurs is unknown but has been assumed to be via classic opioid receptors. Herein, we provide direct evidence that morphine creates neuroinflammation via the activation of an innate immune receptor and not via classic opioid receptors. We demonstrate that morphine binds to an accessory protein of Toll-like receptor 4 (TLR4), myeloid differentiation protein 2 (MD-2), thereby inducing TLR4 oligomerization and triggering proinflammation. Small-molecule inhibitors, RNA interference, and genetic knockout validate the TLR4/MD-2 complex as a feasible target for beneficially modifying morphine actions. Disrupting TLR4/MD-2 protein–protein association potentiated morphine analgesia in vivo and abolished morphine-induced proinflammation in vitro, the latter demonstrating that morphine-induced proinflammation only depends on TLR4, despite the presence of opioid receptors. These results provide an exciting, nonconventional avenue to improving the clinical efficacy of opioids.
Keywords: protein–protein interaction, pain management therapy, drug discovery
Opioids, such as morphine, have been used to treat pain for millennia. However, it is now well-documented that morphine can create neuroinflammation in the CNS (1), which has been linked to suppression of morphine analgesia as well as enhancement of morphine-induced tolerance, dependence, and reward associated with drug abuse (1–7). Although morphine-induced neuroinflammation leads to the release of neuroexcitatory, proinflammatory substances such as proinflammatory cytokines, how morphine creates such neuroinflammation has remained a mystery. Various studies have alternatively suggested that such effects of morphine may either be via classic opioid receptors or via an interaction somewhere along the intracellular cascade activated by Toll-like receptor 4 (TLR4) signaling. Here we provide direct evidence that morphine creates its neuroinflammatory effects (i) by binding to the TLR4 accessory protein, myeloid differentiation protein 2 (MD-2), and inducing TLR4/MD-2 oligomerization and subsequent TLR4 signaling activation in a similar fashion to the classic TLR4 ligand, LPS (endotoxin); (ii) not via μ-opioid receptors, but through activation of TLR4 signaling; (iii) not via an interaction along the intracellular signaling pathway of TLR4, but solely through binding in a specific LPS-binding pocket of MD-2; and (iv) not just via microglia but also via CNS endothelial cells, which are exposed to the highest concentrations of blood-borne morphine as it transits to the CNS. Genetic knockout of TLR4/MD-2 suppressed morphine-induced neuroinflammatory responses and potentiated acute morphine analgesia in vivo, indicating that blocking the TLR4/MD-2 association is sufficient to suppress morphine-induced neuroinflammation. Finally, we validated this strategy of disrupting the TLR4/MD-2 complex using drug-like small-molecule inhibitors that potentiate acute morphine analgesia, thereby laying the groundwork for the development of therapeutic TLR4-targeting drugs for improving the clinical efficacy of opioids.
Results
Morphine Binds to MD-2.
X-ray crystal structure of the TLR4/MD-2/LPS complex shows that LPS binds to a hydrophobic pocket on MD-2 and induces oligomerization of the TLR4/MD-2 complex (8), forming a (TLR4/MD-2)/(TLR4/MD-2) heterotetramer and facilitating signal transduction (9). Human MD-2 was expressed and purified after the methods previously reported (8). The prepared protein was shown to fold properly by circular dichroism spectroscopy (SI Appendix, Fig. S1 A and B) with a melting temperature (Tm) of ≈80 °C, which is consistent with previous literature reports (10). We previously demonstrated that MD-2 produced in this fashion effectively binds LPS (11). To further demonstrate that the prepared MD-2 protein retains its ligand-binding activity, we tested its binding with a well-documented MD-2 ligand, curcumin (12, 13). A Kd of 0.55 ± 0.36 μM was obtained (SI Appendix, Fig. S1C), which is comparable to the literature value (13).
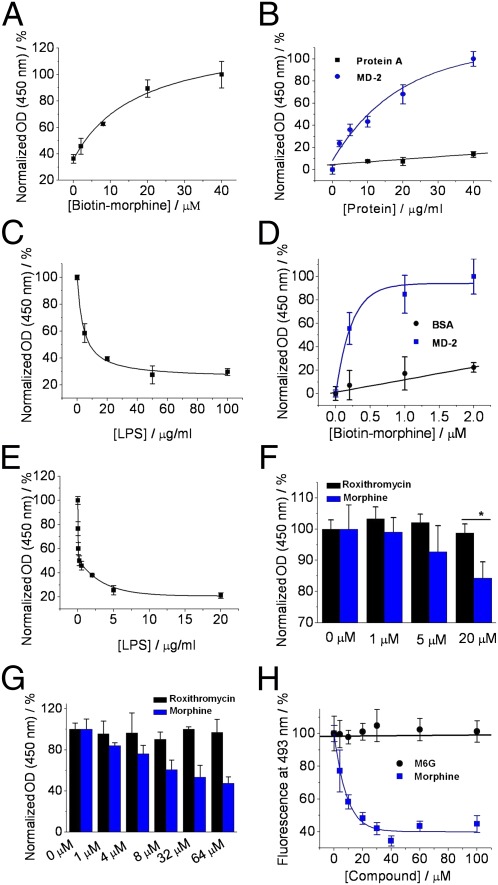
Prior observations have suggested that morphine can induce neuroinflammation in vivo (14). To test whether morphine may functionally mimic LPS, we first tested whether morphine binds to human MD-2. Biotin-labeled morphine was synthesized using our previously developed method (SI Appendix, Fig. S2) (15). The biotin tag was attached via a flexible-PEG linker to the 3′ hydroxyl group that has been shown not to interfere with the morphine-induced proinflammatory activation (15). Various concentrations of biotinylated morphine then were immobilized on a streptavidin-coated plate as the capturing probe in an ELISA. As shown in Fig. 1A, morphine bound to human MD-2 in a concentration-dependent manner. We titrated MD-2 into streptavidin-coated plates in the presence of 10 μM morphine. As shown in Fig. 1B, human MD-2 was captured in a concentration-dependent manner. By contrast, the binding to a negative control (protein A) was negligible, indicating that the morphine–MD-2 binding is specific. Additionally, LPS was found to compete with immobilized morphine for binding to MD-2 (Fig. 1C). Fig. 1D shows that, when MD-2 was immobilized on plates, biotinylated morphine bound to MD-2 in a concentration-dependent manner, whereas morphine showed little binding to the control, BSA. Further, LPS antagonized biotinylated morphine binding to human MD-2 immobilized on the ELISA plate (Fig. 1E), which is consistent with the result observed in Fig. 1C. Morphine and MD-2 binding was also directly determined by using a monoclonal antibody that specifically recognizes apoMD-2 but not MD-2 with bound LPS (16). This assay detected decreased antibody binding to MD-2 in the presence of morphine (Fig. 1F). As a comparison, roxithromycin, a natural product used as a negative control in previous reports (SI Appendix, Fig. S3) (17), showed no MD-2 binding activity. As shown in Fig. 1G, morphine was found to compete with biotin-LPS immobilized on the plate for binding to MD-2, suggesting that morphine binds to the LPS binding pocket of MD-2, whereas roxithromycin showed no inhibition. However, roxithromycin (SI Appendix, Fig. S3) shares little structural similarity with morphine (SI Appendix, Fig. S4A). We therefore used the morphine metabolite morphine-6-glucuronide (M6G; SI Appendix, Fig. S4B) as a negative control in the binding assays, because it has previously been shown to not activate the TLR4 pathway (6, 18). Fluorescence probe curcumin binds to MD-2, and its fluorescence intensity enhances when binding to MD-2 (13). As shown in Fig. 1H, morphine caused a concentration-dependent decrease of curcumin fluorescence from the curcumin–MD-2 complex, suggesting that morphine replaces curcumin binding to MD-2. Compared with morphine, M6G induced negligible decrease in curcumin fluorescence, again supporting that morphine rather than M6G interacts with MD-2. By fitting the binding curve to a one-site competitive model, we calculated the Ki value of 4.3 ± 3.3 μM for morphine competing with curcumin for MD-2 binding.
Fig. 1.
Biophysical characterizations of morphine binding to human MD-2. (A) Different concentrations of biotin-morphine were coated onto streptavidin-precoated plate as the probe. Human MD-2 (10 μg/mL, protein A tagged) was added, and the morphine-bound MD-2 was detected by IgG-HRP conjugate. Absorbance with 40 μM biotin-morphine was set as 100%. (B) Biotin-morphine (10 μM) was coated onto streptavidin precoated plate. Different concentrations of human MD-2 were added, and the morphine-bound MD-2 was detected by IgG-HRP conjugate. Protein A was used as the negative control. Absorbance with 40 μg/mL MD-2 was set as 100%. (C), 10 μM of biotin-morphine was coated onto streptavidin-precoated plate. Human MD-2 (40 μg/mL) and different concentrations of LPS were added. The morphine-bound MD-2 was detected by IgG-HRP conjugate. Absorbance with 0 μg/mL LPS was set as 100%. (D) Human MD-2 (10 μg/mL) or BSA (used as the control) was coated onto the plate. Different concentrations of biotin-morphine were added, and the bound morphine was detected by streptavidin conjugated with HRP. Absorbance with MD-2 in the presence of 2 μM biotin-morphine was set as 100%. (E) Human MD-2 (10 μg/mL) was coated onto the plate. Biotin-morphine (4.0 μM) and different concentrations of LPS were added, and the bound morphine was detected by streptavidin conjugated with HPR. Absorbance with 0 μg/mL LPS was set as 100%. (F) MD-2 capturing antibody (2 μg/mL) was coated onto the plate. MD-2 (5 μg/mL; protein A tag was removed) and different concentrations of morphine or roxithromycin (used as the control) were added. Anti-MD-2 antibody 9B4 (0.1 μg/mL), which specifically recognizes apoMD-2, was added. MD-2 without morphine bound was detected by HRP-coupled secondary antibody. Absorbance with 0 μg/mL morphine or roxithromycin was set as 100%. *P < 0.05 vs. roxithromycin control group. (G) Biotin-LPS (0.1 μg/mL) was coated onto streptavidin-precoated plate as the probe. Human MD-2 (10 μg/mL) and different concentrations of morphine were added. The LPS-bound MD-2 was detected by IgG-HRP conjugate. Absorbance with 0 μg/mL morphine or roxithromycin was set as 100%. (H) Morphine replaced curcumin binding to MD-2. Different concentrations of morphine or M6G were titrated into MD-2 (0.5 μM). Curcumin (0.5 μM) was added as the extrinsic fluorescence probe. The excitation wavelength was set at 430 nm, and the fluorescence signal at 493 nm was plotted against the titrated morphine concentration. Fluorescence intensity of curcumin–MD-2 complex in the absence of morphine was set as 100%. Data fitting to a one-site competitive model gives a Ki of 4.3 ± 3.3 μM.
Morphine Induces TLR4 Oligomerization.
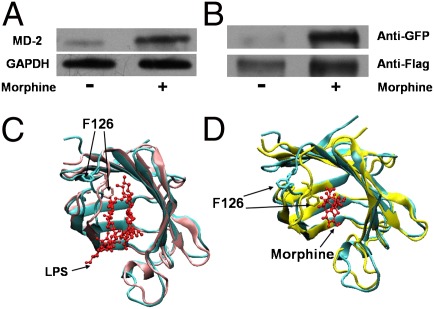
Next, we tested whether morphine binds to MD-2 on the cell surface and induces TLR4 oligomerization. HEK Blue hTLR4 cells, which overexpress the human TLR4 and MD-2, were stimulated with morphine. Cell lysates were subjected to immunoprecipitation with morphine-specific antibody and immuno-detected by MD-2 antibody. As shown in the Fig. 2A, MD-2 was coimmunoprecipitated with morphine, showing that morphine interacts with MD-2 in a whole-cell environment.
Fig. 2.
Morphine induces TLR4 oligomerization and activates TLR4 signaling. (A) Morphine bound to MD-2 in a cellular environment. HEK Blue hTLR4 cells, which overexpress the human TLR4 and MD-2, were treated with morphine (300 μM) for 12 h, and cell lysates were immunoprecipitated by morphine antibody and then detected by Western blotting by MD-2 antibody. GAPDH served as the cell lysates input control. (B) Morphine-induced TLR4 receptor oligomerization. Ba/F3 cells simultaneously overexpressing human TLR4-Flag, human TLR4-GFP, human CD14, and human MD-2 were stimulated with morphine (300 μM) for 72 h. Cells were then subjected to immunoprecipitation with anti-Flag antibody and immunoprobing with anti-GFP antibody (Upper) and anti-Flag antibody (Lower). (C and D) Structural comparison of apo- and ligand-bound MD-2. The ligands are shown in a ball-and-stick presentation. X-ray crystal structure of apoMD-2 (cyan; PDB ID 2E56) superimposed with (C) X-ray crystal structure of the LPS-bound MD-2 (pink; PDB ID 3FXI) or (D) morphine-bound MD-2 (yellow) derived from atomic molecular dynamics simulation.
The binding of LPS to MD-2 induced the oligomerization of TLR4 (SI Appendix, Fig. S5), facilitating TLR4 signaling (9). To test whether morphine induced TLR4 receptor oligomerization, Ba/F3 cells, which overexpress human TLR4-Flag and human TLR4-GFP, as well as human CD14 and human MD-2 (19), were stimulated with morphine. Cell lysates were subjected to immunoprecipitation with anti-Flag antibody and immuno-detected by anti-Flag and anti-GFP antibodies. As shown in Fig. 2B, upon stimulation with morphine, the coprecipitation of TLR4-GFP and TLR4-Flag significantly increased compared with the barely detectable basal level observed in the control sample. The data imply that morphine, similar to LPS, induced TLR4 oligomerization, possibly in a dimeric form as previously documented (8).
X-ray crystallographic analyses of apoMD-2 and LPS-bound MD-2 indicated that the binding of LPS induces conformational changes of MD-2, primarily in the F126 loop region (8, 20). Residue F126 is located in the core of the (TLR4/MD-2)/(TLR4/MD-2) oligomerization interface and initiates structural changes in MD-2 upon LPS binding. These conformational changes facilitated hydrogen bonds and electrostatic interactions between the F126 loop residues of MD-2 and a second copy of TLR4, stabilizing the dimeric (TLR4/MD-2)/(TLR4/MD-2) heterotetramer complex (21). Mutation of F126 and the surrounding residues in MD-2 blocked LPS-induced oligomerization (8). Comparison of the dihedral angles of the residues in the F126 loop of the apoMD-2 [Protein Data Bank (PDB) ID 2E56] and LPS–MD-2 (PDB ID 3FXI) structures showed variation of at least 100° for the backbone dihedral angles of residues K122, G123, K125, and F126. Further inspection of side-chain dihedral angles showed variation of at least 100° for K125 and K128, indicating that these residues were affected most by the LPS binding. To gain insight into how morphine possibly affects MD-2 structurally, we conducted molecular dynamics simulation using fully atomistic models of morphine and MD-2 in an implicit solvent. A total of 3 ns of simulation time were performed, and the structural changes in the F126 loop were examined (Fig. 2 C and D). These ligand-induced conformational changes were also monitored by the φ-ψ backbone dihedral angles (SI Appendix, Fig. S6) and the χ1-χ2 side-chain dihedral angles (SI Appendix, Fig. S7) extracted from the simulation trajectory. It was observed that residues I124, K125, F126, and S127 underwent large transitions of their backbone dihedral angles, whereas residues K122, I124, K125, and S127 showed prominent shifts in the orientation of their side chains. Of these, K122, K125, and F126 were the residues that also showed large differences in the apoMD-2 and LPS-bound MD-2 structures. These observations suggested that morphine, similar to LPS, induced MD-2 conformational change in the F126 loop, resulting in oligomerization of the TLR4/MD-2 complex.
Morphine Triggers Proinflammation Responses in Immunocompetent Cells.
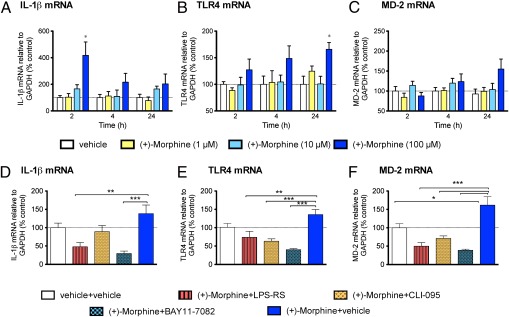
To demonstrate that morphine induces proinflammatory responses in TLR4-expressing nonneuronal cells, the effect of morphine on TLR4-expressing CNS endothelial cells (22) was investigated. To eliminate confounding effects of μ-opioid receptor binding of (-)-morphine in these primary CNS endothelial cells, (+)-morphine, which activates TLR4 signaling (5) but has no μ-opioid receptor activity (23), was used. Using biophysical methods, we have confirmed that (+)-morphine indeed showed binding affinity comparable to MD-2 with (-)-morphine (SI Appendix, Fig. S8). Further, (+)-morphine similarly induces TLR4 oligomerization (SI Appendix, Fig. S9). In-cell Western results showed that LPS and (+)-morphine both caused dose-dependent increases in p38 phosphorylation during 30 min treatment, as well as dose-dependent increases in ERK phosphorylation (SI Appendix, Fig. S10). The data showed that morphine behaved like LPS in activating the TLR4 pathway and induced rapid phosphorylation of the MAP kinases p38 and ERK in primary CNS endothelial cells documented to express TLR4 (22). Quantitative RT-PCR (qRT-PCR) was also performed to investigate the effect of (+)-morphine and LPS on expression of mRNA for TLR4, MD-2, and downstream proinflammatory cytokine IL-1β (Fig. 3 and SI Appendix, Fig. S11). In the presence of complex FBS medium, 100 μM (+)-morphine, similar to LPS, potently increased IL-1β mRNA in endothelial cells at 2 h, with a gradual decline in IL-1β mRNA expression by 24 h. Morphine and LPS also each increased TLR4 and MD-2 mRNA expression over time. LPS-RS, a structural analog of LPS and a potent LPS antagonist that targets MD-2 (8, 24, 25) and inhibits TLR4 signaling (9, 24), attenuated morphine and LPS-induced IL-1β and TLR4 mRNA elevations. CLI-095, an intracellular TLR4 antagonist acting on Cys747 of TLR4 (26), significantly attenuated morphine and LPS-induced TLR4 and MD-2 mRNA overexpression. IκB-α is an inhibitor of NF-κB activation and an important downstream regulator of TLR4 signaling pathway (9). IκB-α inhibitor BAY11-7082 also significantly attenuated morphine- and LPS-induced IL-1β, TLR4, and MD-2 mRNA overexpression.
Fig. 3.
Isolated primary CNS endothelial cells incubated with (+)-morphine induce proinflammatory mediator (IL-1β) and TLR (TLR4 and the coreceptor MD-2) mRNA up-regulation after 2-h, 4-h, and 24-h incubations. (A) (+)-Morphine (100 μM) increased IL-1β mRNA at 2 h with a gradual decline in IL-1β mRNA expression by 24 h. (B and C) Gradual increase in TLR4 and MD-2 mRNA expression over time with the 100 μM (+)-morphine compared with vehicle control. (+)-Morphine (100 μM) was coincubated for 24 h with 10 ng/mL LPS-RS, a competitive TLR-4 antagonist, 1 μM CLI-095, an intracellular TLR4 antagonist, and 1 μM BAY11-7082, an IκB-α inhibitor. LPS-RS significantly attenuated (+)-morphine-induced IL-1β (D) and TLR4 (E) mRNA. CLI-095 significantly attenuated TLR4 (E) and MD-2 (F) mRNA. BAY11-7082 significantly attenuated IL-1β (D), TLR4 (E), and MD-2 (F) mRNA. Experimental procedures are described in SI Appendix, SI Materials and Methods. n = 6 per group per time interval. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. One-way ANOVA compared with vehicle control, with Bonferroni post hoc comparisons.
TLR4/MD-2 Complex Mediates Morphine-Induced Proinflammation.
The association of TLR4 and MD-2 is essential for downstream activation of NF-κB and production of proinflammatory cytokines (9). NF-κB activation and up-regulation of proinflammatory cytokines were used to monitor TLR4 activation. As shown in SI Appendix, Fig. S12A, morphine induced NF-κB activation in a dose-dependent manner in BV-2 microglia, macrophage-like immunocompetent cells in the CNS that express TLR4 (1, 14). The competitive inhibitor curcumin (SI Appendix, Fig. S12B) and LPS-RS (SI Appendix, Fig. S12C), which by themselves showed no influence on NF-κB activity (SI Appendix, Fig. S13), suppressed morphine-induced NF-κB activation in a dose-dependent manner. Furthermore, the MD-2 binding agent curcumin inhibited morphine-induced IκB-α degradation (SI Appendix, Fig. S14A). Morphine stimulation also increased the NF-κB p65 protein expression level, an effect that was blocked by the MD-2 competitive inhibitor curcumin (SI Appendix, Fig. S14B). These data confirmed that morphine did not activate NF-κB beyond the TLR4/MD-2 complex, suggesting a unique strategy to suppress morphine-induced inflammation by targeting the TLR4/MD-2 protein complex. Importantly, in addition, because these microglia also express opioid receptors (27), these data demonstrate that the morphine-induced proinflammatory responses are not mediated by opioid receptors because the response was abolished by blocking only MD-2.
Next, RNAi was performed to demonstrate that an intact TLR4/MD-2 complex is essential for CNS immunocompetent cell activation. We optimized siRNA transfection procedures so as to attain ≈60% specific knockdown efficiency in BV-2 microglia for both TLR4 and MD-2, as confirmed by Western blot analysis (SI Appendix, Fig. S15). Proinflammatory factors such as nitric oxide (NO), IL-1β, and TNF-α were monitored as indicators of TLR4 activation (1). As shown in SI Appendix, Fig. S12D, knockdown of MD-2 or TLR4 by RNAi suppressed morphine-induced NO production to a significant extent. Further, stimulation with morphine increased production of the proinflammatory cytokines IL-1β and TNF-α, which was abolished by knocking down either TLR4 or MD-2 (SI Appendix, Fig. S12 E and F). In the absence of morphine, RNAi suppression of MD-2 or TLR4 slightly reduced the background level of proinflammatory factors, including IL-1β and TNF-α (SI Appendix, Fig. S16). It should be noted that no cell proliferation inhibition was observed at the concentrations of morphine used in these experiments (SI Appendix, Fig. S17). Taken together, RNAi knockdown of either TLR4 or MD-2 suppresses morphine-induced proinflammatory responses, suggesting that TLR4/MD-2 mediated TLR4 activation and provides a target for small-molecule regulation. Also importantly, these results provided further support that morphine neither activates TLR4 signaling pathway beyond MD-2 nor creates neuroinflammatory responses via the classic opioid receptors coexpressed on BV-2 microglial cells.
MyD88-Dependent TLR4 Signaling Is Involved in Morphine Analgesia.
Knockout mice for TLR4 and MyD88, a critical downstream effector of TLR4 (9), were used separately to assess the involvement of TLR4 and the MyD88-dependent signaling cascade in acute morphine analgesia over a range of concentrations. Prior studies have demonstrated that morphine-induced neuroinflammation suppressed acute morphine analgesia (4, 14, 28, 29). Hence, we predicted that knocking out TLR4 or MyD88 should potentiate acute morphine analgesia if morphine-induced neuroinflammatory responses are via this pathway. Pain responsivity was compared for the knockout strains and their respective wild-type controls dosed by i.p. injection of morphine, ranging from no analgesia to maximal analgesia. As expected, increasing doses of morphine resulted in significant increases in hotplate latencies in all strains. Further analysis of the morphine dose–response curves revealed a main TLR4 and MyD88 effect whereby both TLR4- and MyD88-knockout mice achieved significantly longer hotplate latencies compared with their respective wild-type controls. In both cases there was a 2.2-fold leftward shift in the knockout morphine analgesic dose–response curve (ED50 wild-type 2.8 mg/kg vs. TLR4-knockout 1.2 mg/kg; ED50 wild-type 11.9 mg/kg vs. MyD88-knockout 5.4 mg/kg) compared with wild-type mice (SI Appendix, Fig. S18). In addition, both knockout strains had a significantly altered dose–response Hill slope compared with wild-types.
After behavioral testing, wild-type and TLR4-knockout mice spinal cord samples were collected after a range of morphine doses to enable quantification of in vivo signaling via the TLR4 cascade. Samples were analyzed for downstream TLR4 MAP kinase phosphorylation. After morphine administration, there was a main strain (P < 0.01) and dose effect (P < 0.001) and interaction (P < 0.01) as assessed using p38. Post hoc analysis revealed that morphine significantly increased p38 phosphorylation at doses of 0.1 and 50 mg/kg (P < 0.05; SI Appendix, Fig. S19A) compared with TLR4-knockout mice. Analysis also revealed a main strain and dose effect (P < 0.05) for JNK, whereas ERK displayed a main strain effect (P < 0.05) and interaction (P < 0.001) (SI Appendix, Fig. S19 B and C). Together, the data demonstrated that the MyD88-dependent TLR4 signaling is involved in morphine analgesia.
Small-Molecule Inhibitors of the TLR4/MD-2 Association Potentiate the Efficacy of Morphine.
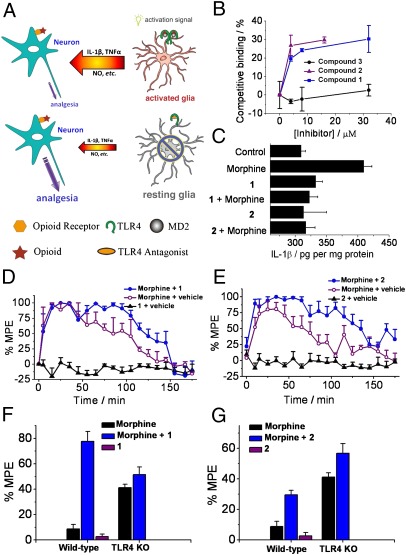
Having shown that disruption of TLR4/MD-2 was sufficient to regulate the morphine-induced proinflammatory responses, we continued the investigation using drug-like, small-molecule agents. These small-molecule agents may validate the TLR4/MD-2 complex as a potential target for drug development, laying the groundwork for a new strategy to potentiate morphine analgesia (Fig. 4A). Two previously identified small-molecule probes that selectively inhibited the TLR4/MD-2 interactions (30, 31) were used to study their effects in morphine-induced proinflammatory response both in vitro and in vivo. Compound 1 (SI Appendix, Fig. S20) has been identified to target TLR4, and compound 2 (SI Appendix, Fig. S21) was identified as the MD-2 antagonist (30, 31). To biophysically characterize compounds 1 and 2 and their targets interactions, competitive ELISAs were performed. As shown in the Fig. 4B, compound 1 competed against MD-2-I, a 17-residue peptide derived from the TLR4-binding region of MD-2, which tightly binds to TLR4 (11, 32). By contrast, negative control compound 3 (SI Appendix, Fig. S20), a structural analog to 1, show no inhibitory effects to MD-2-I binding to TLR4 (Fig. 4B). Similarly, compound 2 competed against morphine binding to MD-2 (Fig. 4B). It should be noted that racemic form of 1 was used in these studies because previous reports showed that the stereocenter in 1 did not affect its TLR4 inhibition activities (31).
Fig. 4.
TLR4/MD-2 antagonists abolish morphine-induced proinflammatory activation in vitro and in vivo. (A) A strategy of potentiating opioid analgesia by disrupting the nonneuronal TLR4/MD-2 complex. Upper: Opioids activate CNS immunocompetent cells by triggering a signal transduction cascade mediated by TLR4 (dimeric form in complex with MD-2). This results in the release of the cytokine intercellular mediator IL-1β and other proinflammatory factors, which suppresses the desired opioid-induced neuronal analgesic effect. Lower: In the presence of TLR4-signaling antagonists such as inhibitors of the critical TLR4/MD-2 interaction, CNS immunocompetent cells remain in the resting state. Opioids (red star) then show higher analgesic efficacy by binding solely to opioid receptors (orange hexagon) on neurons. (B) Competitive ELISA binding. Compound 1 competes against MD-2-I peptide binding to TLR4, and compound 2 competes against morphine binding to MD-2. Negative control compound 3, which shares similar structure with compound 1, showed no apparent binding to TLR4. (C) TLR4/MD-2 antagonists inhibited morphine-induced IL-1β overproduction in microglial BV-2 cells. BV-2 cells were incubated in the presence of morphine (200 μM), morphine (200 μM), and 10 μM of compound 1 or 2. Cell lysates from BV-2 cells were assayed for IL-1β protein by ELISA. (D and E), TLR4/MD-2 antagonists 1 (D) and 2 (E) potentiated the acute intrathecal morphine analgesia. After predrug (baseline) assessment of responsivity to radiant heat (Hargreaves test), rats received intrathecal morphine (1 μL, 15 mg/mL), 1/2 (1 μL, 30 mM), or the combination of morphine and 1/2 at same doses. Data are expressed as percent maximum potential effect (MPE). Data are means from six animals. (F and G) Selectivity of potentiation of morphine analgesia by TLR4/MD-2 antagonists 1 (F) and 2 (G) in wild-type vs. TLR4 knockout mice. It should be noted here that we chose a morphine dose that produced low analgesia in wild-type mice (has greater analgesia in TLR4 KO mice). Therefore, behavioral tests were not hampered by the ceiling cutoff. After predrug (baseline) assessment of responsivity to radiant heat (Hotplate test), mice received i.p. 1 (82 mg/kg) or 2 (153 mg/kg), followed 10 min later by i.p. morphine (2.5 mg/kg). n = 6 animals for morphine groups and n = 3 for small molecules alone. It should be note here that morphine used here is (-)-morphine.
The in vitro inhibitory effects of compounds 1 and 2 on morphine-induced microglial cell activation were investigated. Treatment of BV-2 cells with morphine elevated the IL-1β expression level, indicative of activation of TLR4 signaling. Coincubation of morphine with compound 1 or 2 abolished morphine-induced IL-1β production to the background level (Fig. 4C). In contrast, negative control compound 3, an inactive analog to 1, did not show any significant reduction of IL-1β expression (SI Appendix, Fig. S22). These results demonstrated that small-molecule inhibitors of TLR4 signaling reduce morphine-induced proinflammatory responses.
An established rat model (3) was used to test whether compounds 1 and 2 were able to potentiate the analgesic effect of morphine in vivo. The Hargreaves test (33) was used to measure pain response via the time taken to observe radiant heat-induced withdrawal responses by tails of unrestrained rats. Before drug administration, two readings were recorded for controls. After these baseline measurements, drugs were injected intrathecally, and the rats’ responses to radiant heat were assessed across a 3-h time course. Whereas compounds 1 (Fig. 4D) and 2 (Fig. 4E) had no effect on pain responsivity in the absence of coadministered morphine, they robustly potentiated the acute analgesic effects of morphine such that the rats exhibited the maximal analgesia recordable on the test, presumably by inhibiting morphine-induced proinflammatory response in the CNS. These results indicated that TLR4/MD-2 was a valid target for the regulation of opioid-induced TLR4 activation in vivo.
Using a knockout mouse model, we further demonstrated that the TLR4 signaling pathway is the target for potentiating morphine analgesia. Pain responsivity was compared for wild-type vs. TLR4-knockout mice dosed via i.p. injection of morphine at a dose causing low analgesia in the absence of TLR4 inhibitors (34). Compounds 1 and 2 were highly effective in potentiating analgesia in wild-type mice, as expected if TLR4 mediated suppression of morphine analgesia via morphine-induced neuroinflammation. In contrast, TLR4-knockout mice were unaffected by treatment of morphine coadministered with compounds 1 or 2 (Fig. 4 F and G), again, as expected if morphine was acting only via TLR4. These data provided firm evidence that 1 and 2 exerted their analgesic effect by targeting TLR4/MD-2. Last, compounds 1 and 2 were administered in these experiments with negligible cytotoxicity (SI Appendix, Fig. S23).
Discussion
It is now well accepted that opioids such as morphine induce neuroinflammatory responses within the brain and spinal cord, which potently dysregulate the actions of opioids. Although it was long assumed that opioid-induced neuroinflammation must be mediated via activation of classic opioid receptors, recent data from Hutchinson et al. contested this assumption. Their data demonstrated that morphine-induced CNS neuroinflammation was created by nonclassic (nonstereoselective) opioid actions that cannot be explained as being mediated by classic opioid receptors (1, 4–6, 14, 35). Although nonstereoselective effects demonstrate that some receptor, enzyme, or second messenger other than classic, stereoselective opioid receptors must underlie opioid-induced neuroinflammatory effects, how this occurs was unknown. Although follow-on studies suggested that opioids were likely interacting somewhere along (at least) the TLR4 signaling pathway, how and where this interaction was occurring remained a mystery. Had opioids interacted directly with various intracellular signaling elements of the TLR4 pathway that are also downstream of receptors other than TLR4, this would have indicated that morphine impacts multiple signaling cascades, as do direct modulators of p38, NF-κB, etc. In contrast, the present series of multidisciplinary studies provided converging lines of evidence that morphine activates TLR4 signaling, not via interactions along the intracellular signaling cascade, but rather explicitly and only via LPS-like interactions with TLR4's coreceptor MD-2. Thus, opioid-induced neuroinflammation is via a highly selective extracellular interaction affecting solely MD-2. Further, these studies provided converging lines of evidence that opioid receptors do not mediate opioid-induced neuroinflammatory responses because blocking only MD-2 blocks morphine-induced neuroinflammation despite the simultaneous expression of opioid receptors in the same cells.
Developing highly specific small-molecule inhibitors of TLRs is a dynamic research area in drug discovery owing to the great disease relevance of these innate immune receptors. A previously used strategy involved developing LPS-mimicking antagonists of TLR4 (e.g., LPS-RS). Nonetheless, agents derived from this approach suffer from the significant shortcoming of poor pharmacological properties (e.g., poor brain–blood barrier permeability). A recent example is eritoran, an LPS mimetic, failed in phase III clinical trials (36). An alternative strategy is to design small-molecule inhibitors to target downstream receptors of TLR4. Using this strategy, several TLR4 antagonists have been developed although, to the best of our knowledge, none has successfully advanced through late-stage clinical trials. TAK-242 (resatorvid), the most successful TLR4-binding small-molecule antagonist, reached phase III clinical trials as an antisepsis agent, but studies were recently discontinued owing to a failure to suppress cytokine levels in patients despite showing promising preclinical efficacy in animal models (37). These results might have implied that the current approaches to targeting the TLR4 receptor are flawed. Novel strategies to regulate the innate immune response are urgently needed.
In this present study, we attempted a unique approach for optimization of opioid therapies by selectively targeting the TLR4/MD-2 complex. TLR4/MD-2 is required by morphine-induced TLR4 activation, and its disruption abolished the downstream neuroinflammation. Among all members of the TLR protein family, TLR4 is the only one that has been confirmed to form an active heterodimer with MD-2 [although there are still ongoing debates whether TLR2 also binds to MD-2 (38)]. Specific inhibition of the TLR4/MD-2 association might eventually lead to selective drug candidates to target the TLR4 signaling pathway without affecting the normal functions of other homologous TLR protein family members.
In summary, we have demonstrated that morphine bound to MD-2, induced TLR4 oligomerization, and activated TLR4 signaling, in a fashion similar to that of LPS. RNAi, in vivo knockout, and small-molecule inhibitors of the TLR4/MD-2 complex suppressed morphine-induced immunocompetent cells activation in vitro and in vivo, validating TLR4/MD-2 complex as a target for therapeutic development to improve the current opioid-based pain management therapies. Optimized small-molecule inhibitors of TLR4-mediated immunocompetent cells activation may provide novel drug candidates to enhance opioid efficacy and possibly improve safety.
Materials and Methods
SI Appendix, SI Materials and Methods provides complete experimental methods. It includes reagents and details of MD-2 and TLR4 expression and purification, chemical synthesis, ELISAs, fluorescence titration, morphine/MD-2 molecular dynamics simulation, qRT-PCR and Western blot, coimmunoprecipitation of MD-2 with morphine, TLR4 receptor oligomerization assay, dual luciferase reporter assay, secreted alkaline phosphatase assay, RNAi, cell proliferation assay, CNS endothelial cell isolation and culture, in-cell Western blots, behavioral assessment of responsivity to radiant heat in rats, and behavioral assessment of responsivity to radiant heat in TLR4-knockout and wild-type mice. Methods for statistical analysis are also included.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants DA026950, DA025740, NS067425, RR025780, DA029119, DA024044, and DA023132. M.R.H. is an Australian National Health and Medical Research Council C. J. Martin Fellow (ID 465423; 2007-2010) and an Australian Research Council Research Fellow (DP110100297; 2011).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200130109/-/DCSupplemental.
References
- 1.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narita M, Suzuki M, Kuzumaki N, Miyatake M, Suzuki T. Implication of activated astrocytes in the development of drug dependence: Differences between methamphetamine and morphine. Ann N Y Acad Sci. 2008;1141:96–104. doi: 10.1196/annals.1441.032. [DOI] [PubMed] [Google Scholar]
- 3.Hutchinson MR, et al. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun. 2008;22:1178–1189. doi: 10.1016/j.bbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watkins LR, Hutchinson MR, Rice KC, Maier SF. The “toll” of opioid-induced glial activation: Improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci. 2009;30:581–591. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutchinson MR, et al. Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences. Neuroscience. 2010;167:880–893. doi: 10.1016/j.neuroscience.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutchinson MR, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L, Coller JK, Watkins LR, Somogyi AA, Hutchinson MR. Naloxone-precipitated morphine withdrawal behavior and brain IL-1β expression: Comparison of different mouse strains. Brain Behav Immun. 2011;25:1223–1232. doi: 10.1016/j.bbi.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park BS, et al. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Keber MM, Gradisar H, Jerala R. MD-2 and Der p 2—a tale of two cousins or distant relatives? J Endotoxin Res. 2005;11:186–192. doi: 10.1179/096805105X35206. [DOI] [PubMed] [Google Scholar]
- 11.Liu LP, et al. An MD2 hot-spot-mimicking peptide that suppresses TLR4-mediated inflammatory response in vitro and in vivo. ChemBioChem. 2011;12:1827–1831. doi: 10.1002/cbic.201100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Youn HS, Saitoh SI, Miyake K, Hwang DH. Inhibition of homodimerization of Toll-like receptor 4 by curcumin. Biochem Pharmacol. 2006;72:62–69. doi: 10.1016/j.bcp.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Gradisar H, Keber MM, Pristovsek P, Jerala R. MD-2 as the target of curcumin in the inhibition of response to LPS. J Leukoc Biol. 2007;82:968–974. doi: 10.1189/jlb.1206727. [DOI] [PubMed] [Google Scholar]
- 14.Hutchinson MR, et al. Exploring the neuroimmunopharmacology of opioids: An integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev. 2011;63:772–810. doi: 10.1124/pr.110.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, et al. Design, synthesis, and evaluation of biotinylated opioid derivatives as novel probes to study opioid pharmacology. Bioconjug Chem. 2008;19:2585–2589. doi: 10.1021/bc8003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viriyakosol S, et al. Characterization of monoclonal antibodies to human soluble MD-2 protein. Hybridoma (Larchmt) 2006;25:349–357. doi: 10.1089/hyb.2006.25.349. [DOI] [PubMed] [Google Scholar]
- 17.Resman N, et al. Taxanes inhibit human TLR4 signaling by binding to MD-2. FEBS Lett. 2008;582:3929–3934. doi: 10.1016/j.febslet.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 18.Lewis SS, et al. Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta. Neuroscience. 2010;165:569–583. doi: 10.1016/j.neuroscience.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saitoh S, et al. Lipid A antagonist, lipid IVa, is distinct from lipid A in interaction with Toll-like receptor 4 (TLR4)-MD-2 and ligand-induced TLR4 oligomerization. Int Immunol. 2004;16:961–969. doi: 10.1093/intimm/dxh097. [DOI] [PubMed] [Google Scholar]
- 20.Ohto U, Fukase K, Miyake K, Satow Y. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science. 2007;316:1632–1634. doi: 10.1126/science.1139111. [DOI] [PubMed] [Google Scholar]
- 21.Manavalan B, Basith S, Choi S. Similar structures but different roles—an updated perspective on TLR structures. Front Physiol. 2011;2:41. doi: 10.3389/fphys.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagyoszi P, et al. Expression and regulation of toll-like receptors in cerebral endothelial cells. Neurochem Int. 2010;57:556–564. doi: 10.1016/j.neuint.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Jacquet YF, Klee WA, Rice KC, Iijima I, Minamikawa J. Stereospecific and nonstereospecific effects of (+)- and (-)-morphine: Evidence for a new class of receptors? Science. 1977;198:842–845. doi: 10.1126/science.199942. [DOI] [PubMed] [Google Scholar]
- 24.Kirikae T, et al. Diphosphoryl lipid A derived from the lipopolysaccharide (LPS) of Rhodobacter sphaeroides ATCC 17023 is a potent competitive LPS inhibitor in murine macrophage-like J774.1 cells. FEMS Immunol Med Microbiol. 1994;9:237–243. doi: 10.1111/j.1574-695X.1994.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 25.Smith A, et al. Targeting the TLR4/MD-2 complex for imaging inflammation by SPECT/CT. J Nucl Med. 2011;52(Suppl 1):1515. [Google Scholar]
- 26.Matsunaga N, Tsuchimori N, Matsumoto T, Ii M. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol Pharmacol. 2011;79:34–41. doi: 10.1124/mol.110.068064. [DOI] [PubMed] [Google Scholar]
- 27.Bokhari SM, et al. Morphine enhances Tat-induced activation in murine microglia. J Neurovirol. 2009;15:219–228. doi: 10.1080/13550280902913628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchinson MR, et al. Minocycline suppresses morphine-induced respiratory depression, suppresses morphine-induced reward, and enhances systemic morphine-induced analgesia. Brain Behav Immun. 2008;22:1248–1256. doi: 10.1016/j.bbi.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutchinson MR, et al. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast) Brain Behav Immun. 2009;23:240–250. doi: 10.1016/j.bbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joce CM, et al. Application of a novel in silico high-throughput screen to identify selective inhibitors for protein-protein interactions. Bioorg Med Chem Lett. 2010;20:5411–5413. doi: 10.1016/j.bmcl.2010.07.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bevan DE, et al. Selection, preparation, and evaluation of small-molecule inhibitors of toll-like receptor 4. ACS Med Chem Lett. 2010;1:194–198. doi: 10.1021/ml100041f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slivka PF, et al. A peptide antagonist of the TLR4-MD2 interaction. ChemBioChem. 2009;10:645–649. doi: 10.1002/cbic.200800769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 34.Hoshino K, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 35.Hutchinson MR, et al. Opioid-induced glial activation: Mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barochia A, Solomon S, Cui X, Natanson C, Eichacker PQ. Eritoran tetrasodium (E5564) treatment for sepsis: Review of preclinical and clinical studies. Expert Opin Drug Metab Toxicol. 2011;7:479–494. doi: 10.1517/17425255.2011.558190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rice TW, et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit Care Med. 2010;38:1685–1694. doi: 10.1097/CCM.0b013e3181e7c5c9. [DOI] [PubMed] [Google Scholar]
- 38.Dziarski R, Wang Q, Miyake K, Kirschning CJ, Gupta D. MD-2 enables Toll-like receptor 2 (TLR2)-mediated responses to lipopolysaccharide and enhances TLR2-mediated responses to Gram-positive and Gram-negative bacteria and their cell wall components. J Immunol. 2001;166:1938–1944. doi: 10.4049/jimmunol.166.3.1938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.