Abstract
Neurospora crassa colonizes burnt grasslands in the wild and metabolizes both cellulose and hemicellulose from plant cell walls. When switched from a favored carbon source such as sucrose to cellulose, N. crassa dramatically upregulates expression and secretion of a wide variety of genes encoding lignocellulolytic enzymes. However, the means by which N. crassa and other filamentous fungi sense the presence of cellulose in the environment remains unclear. Here, we show that an N. crassa mutant carrying deletions of two genes encoding extracellular β-glucosidase enzymes and one intracellular β-glucosidase lacks β-glucosidase activity, but efficiently induces cellulase gene expression in the presence of cellobiose, cellotriose, or cellotetraose as a sole carbon source. These data indicate that cellobiose, or a modified version of cellobiose, functions as an inducer of lignocellulolytic gene expression in N. crassa. Furthermore, the inclusion of a deletion of the catabolite repressor gene, cre-1, in the triple β-glucosidase mutant resulted in a strain that produces higher concentrations of secreted active cellulases on cellobiose. Thus, the ability to induce cellulase gene expression using a common and soluble carbon source simplifies enzyme production and characterization, which could be applied to other cellulolytic filamentous fungi.
Liquid biofuels produced from lignocellulosic biomass are an environmentally clean and renewable source of energy that could displace a significant fraction of the current demand for petroleum (1). However, the costs associated with conversion of insoluble plant cell wall polysaccharides to fermentable sugars represent a significant barrier to the production of cost-competitive biofuels (2). Filamentous fungi have the capacity to secrete large amounts of lignocellulosic enzymes that release fermentable sugars from plant cell walls, and this ability has been exploited by industry to produce cellulases in quantities exceeding 100 g/L of culture (3).
Regulating enzyme synthesis is crucial for the economics of biofuel production from lignocellulose. The best inducers of plant cell wall-degrading enzyme expression by filamentous fungi are insoluble substrates that include cellulose, hemicellulose, or mixtures of plant polymers. However, the use of insoluble substrates to induce enzyme secretion is not ideal for industrial processes. Because these naturally inducing substances cannot enter fungal cells, it is believed that oligosaccharides released from polymers and their derivatives function as the actual molecules that trigger enzyme induction (4). Cellobiose, the major soluble end product of cellulases, moderately induces cellulase gene expression and activity in Hypocrea jecorina (Trichoderma reesei) (5–7) and Aspergillus species (8), which are commonly used fungi for high-level enzyme production (9). However, cellobiose is unable to induce cellulase gene expression in the more distantly related fungus Phanerochaete chrysosporium, which instead responds to cellotriose or cellotetraose (10). Notably, the oligosaccharide sophorose, which can be generated by the transglycosylation of cellobiose by an extracellular β-glucosidase, acts as a potent inducer of cellulases in T. reesei (11–13), although differences in both gene expression and protein production are apparent in cellulose- versus sophorose-induced cultures (14). Sophorose does not induce cellulase gene expression or activity in Aspergillus niger (15) or P. chrysosporium (16). A complicating factor in understanding the regulation of cellulase gene expression is inhibition of cellulase production due to carbon catabolite repression (CCR) (4) by the end product of cellulose hydrolysis, glucose. Previous studies in T. reesei showed that nojirimycin inhibition of β-glucosidase, the enzyme that converts cellobiose into glucose in the final step of cellulose hydrolysis, allows a moderate induction of cellulases by cellobiose (17–19).
In this study, we use the model cellulolytic fungus Neurospora crassa (20) to show that deletion of key genes encoding predicted extracellular and intracellular β-glucosidase enzymes allows cellobiose to induce cellulase gene expression to the same level as insoluble cellulose. Further deletion of cre-1, which encodes a carbon catabolite repressor transcription factor (21), enables N. crassa to produce a higher level of secreted active cellulases when induced with cellobiose, as compared to enzyme levels observed during growth on crystalline cellulose (Avicel). An analysis of the transcriptome and secretome of these deletion strains lays the foundation for understanding the molecular mechanism underlying the induction of lignocellulose-degrading enzymes in filamentous fungi. These results also provide insights that can be applied to industrial fungi that produce high levels of cellulases.
Results
Induction of Cellulase Transcripts in Cellodextrin-Induced Cultures of N. crassa Lacking Three β-Glucosidase Genes.
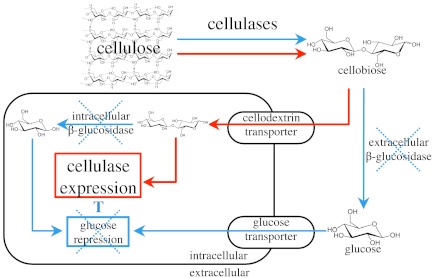
Lignocellulolytic genes are not induced nor is cellulolytic enzyme activity detected when WT N. crassa is grown on sucrose, cellobiose, cellotriose, or cellotetraose as the sole carbon source (Figs. S1, S2A). We hypothesized that when N. crassa is grown on cellodextrins, glucose produced by β-glucosidase enzymes masks the inducing capacity of oligosaccharides (Fig. 1). Whereas the genome of N. crassa encodes at least seven genes encoding predicted β-glucosidase enzymes, a previous systems-level study indicated that only three (NCU00130, NCU04952, and NCU08755) showed a significant increase in transcription during growth on Avicel or Miscanthus (20). NCU00130 encodes an intracellular member of the glycosyl hydrolase family one (GH1-1) (22). Glycosyl hydrolase family three member NCU04952 (GH3-4) was identified by mass spectrometry in the supernatant of an N. crassa culture grown on Avicel and Miscanthus (20) and NCU08755 (GH3-3) was identified in the cell wall fraction of conidia (23) and its enzymatic activity was recently verified (24). All three β-glucosidases showed significant homology to both predicted and experimentally verified β-glucosidase enzymes in other filamentous fungi (Fig. S3). Based on the expression data, we predicted that GH1-1, GH3-3, and GH3-4 would be the most relevant enzymes in converting cellobiose to glucose when N. crassa was grown on either cellulose or cellodextrins as sole carbon sources.
Fig. 1.
Model for transcriptional regulation of cellulases in β-glucosidase deletion strains of N. crassa. Both transcriptional derepression and specific induction are required to achieve maximal transcriptional activation of cellulase gene expression. Arrows indicate possible pathways for cellulose metabolites. Blue lines and red lines indicate pathways hypothesized to be minimized and to be most active, respectively, in the Δ3βG and Δ3βGΔcre deletion strains.
To examine the hypothesis that cellobiose induces cellulase gene expression in N. crassa, we tested whether the expression of three major cellulase genes (cbh-1, NCU07340; gh6-2, NCU09680; gh5-1, NCU00762) were induced in strains carrying deletions in the β-glucosidase genes gh1-1, gh3-3, or gh3-4 via a transfer experiment (Materials and Methods). To eliminate the possibility of redundancy between the β-glucosidase enzymes, double and triple mutant strains carrying different combinations of β‐glucosidase gene deletion sets were also constructed and tested. Following a 4-h induction with 0.2% cellobiose, the individual β-glucosidase deletion strains (Δgh1-1, Δgh3-3, or Δgh3-4) did not show a significant induction of cbh-1, gh6-2, or gh5-1 expression, whereas a Δgh1-1Δgh3-3 double mutant showed some cellulase gene induction (Fig. S4). However, a strain carrying deletions for all three β-glucosidase genes (Δgh1-1, Δgh3-3, and Δgh3-4; Δ3βG) showed similar relative expression levels of cbh-1, gh5-1, and gh6-2 when shifted to 0.2% cellobiose as did a WT culture shifted to Avicel (Fig. 2A). In addition, the Δ3βG strain showed similar relative expression levels of cbh-1, gh5-1, and gh6-2 when shifted to cellobiose, cellotriose, or cellotetraose (Fig. S2B). The transcriptional response in the Δ3βG mutant was specific for cellobiose and was not due to starvation as the expression of cbh-1 and gh5-1 in WT and the Δ3βG strain when transferred to media lacking any carbon source showed only small increases in transcription levels (less than 50-fold induction). These values are negligible when compared to the approximately 20,000-fold (minimum) induction of cbh-1 and gh5-1 by Avicel in WT N. crassa and in the Δ3βG strain shifted to cellobiose (Fig. S5).
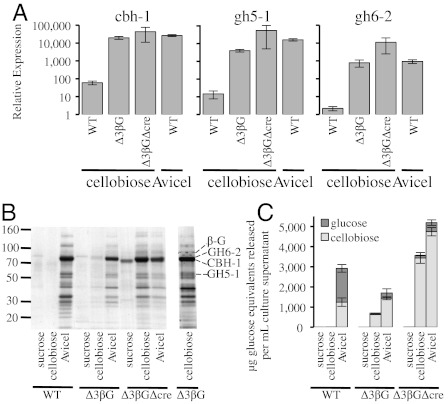
Fig. 2.
Cellulase expression levels in WT and β-glucosidase deletion strains after induction with cellobiose or Avicel. (A) Gene expression of select cellulases after 4 h induction with 0.2% cellobiose or 1% Avicel in WT, Δ3βG, and Δ3βGΔcre strains. Gene expression levels of cbh-1, gh6-2, and gh5-1 were normalized to 1 when induced with 1% sucrose. Expression levels of actin (NCU04173) were used as an endogenous control in all samples. Each strain was grown in triplicate and error bars indicate 1 SD. (B) SDS-PAGE of secreted proteins in culture filtrates from WT, Δ3βG, and Δ3βGΔcre strains. Growth and induction conditions are described in Materials and Methods. Protein bands representing CBH-1, GH6-2, and GH5-1 are marked. In addition, the absence of the extracellular β-glucosidase (NCU04952) is marked in the triple knockout. The presence of glucoamylase I (NCU01517) correlates with the deletion of the cre-1 gene (21). (C) Activity of supernatant from B toward Avicel. Glucose (dark gray) and cellobiose (light gray) were measured after 24 h of incubation with 1% Avicel at 50 °C. Error bars are 1 SD.
The most widely used soluble inducers of cellulases in the industrial species T. reesei are sophorose and lactose (25). We therefore examined whether exposure to sophorose or lactose induced cellulase gene expression in N. crassa using both the WT and the Δ3βG deletion strain. As observed for other filamentous fungal species (15), transfer of either WT or the Δ3βG mutant to media containing sophorose, lactose, or d-(+)-galactose (an expected degradation product of lactose), did not significantly induce cellulase gene expression (Fig. S6).
CCR acts in filamentous fungi to repress cellulase and hemicellulase gene expression in the presence of preferred carbon sources, such as glucose or sucrose, even when lignocellulose is present (4). The C2H2 zinc finger transcription factor CreA/CRE1/CRE-1 (26) plays a key role in CCR as strains lacking CreA/CRE1/CRE-1 in Aspergillus sp., T. reesei, and N. crassa, respectively, produce increased amounts of both cellulases and hemicellulases when grown on cellulose or hemicellulose (21, 27, 28). Consistent with previous data (21), quantitative RT-PCR analysis of RNA isolated from an N. crassa cre-1 deletion strain (ΔNCU08807) showed that the basal expression of cbh-1 and gh5-1 increased about 10-fold relative to a WT strain (Fig. S5). When shifted from sucrose to 0.2% cellobiose for 4 h, the Δcre-1 strain showed increased induction of cbh-1, gh5-1, and gh6-2 (3,000-, 500-, and 85-fold, respectively) (Fig. S4). However, the level of induction in the Δcre-1 mutant was significantly lower than induction levels obtained for WT exposed to Avicel or the Δ3βG mutant exposed to cellobiose (Fig. S2A). Notably, a Δ3βG strain that also carried the Δcre-1 deletion (Δ3βGΔcre) exhibited stronger induction of cbh-1, gh5-1, and gh6-2 than either the WT strain shifted to Avicel or the Δ3βG strain shifted to cellobiose (Fig. 2A). These data indicate that the induction of cellulase gene expression in the Δ3βG mutant when exposed to cellobiose is comparable to induction by cellulose and is not a consequence of relief from CCR.
Recapitulation of Wild-Type N. crassa Cellulolytic Response in the Triple β-Glucosidase Mutant on Cellobiose.
High throughput sequencing (RNA-Seq) was used to assess whether the full genomic response in the Δ3βG strains to cellobiose was similar to or different from a WT strain exposed to Avicel. The full genomic pattern of gene expression changes showed that the response of the Δ3βG mutant to cellobiose closely matched that of WT induced by Avicel, but was significantly different from the response of WT on cellobiose or when subjected to starvation (Fig. S7 A–C). To identify which genes were significantly and specifically induced in WT N. crassa in response to Avicel, a pairwise analysis was performed between expression profiles of WT transferred to Avicel versus WT transferred to no added carbon source. These analyses identified 321 genes (including the three deleted β-glucosidase genes) that were significantly and specifically induced in WT cultures in response to Avicel (cellulose regulon) (Materials and Methods, Dataset S1). This gene set included 16 predicted cellulase and 12 predicted hemicellulase genes. Additional genes in the cellulose regulon included 41 genes encoding proteins predicted to be active on carbohydrates by CAZy (29) and 111 genes encoding secreted proteins (SignalP) (30). Of the 321 genes in the cellulose regulon, 156 encode proteins that are characterized as unclassified proteins (MIPS FunCat database) (31). Of specific interest, the ortholog for xlnR/xyr1 (NCU06971), which has been shown to play a major role in the regulation of cellulases in Aspergillus sp. (32) and T. reesei (33), falls into the cellulose regulon (Dataset S1). Although NCU06971 was previously identified as an xlnR/xlr1 homologue in N. crassa (34), its role in plant cell wall degradation is unknown.
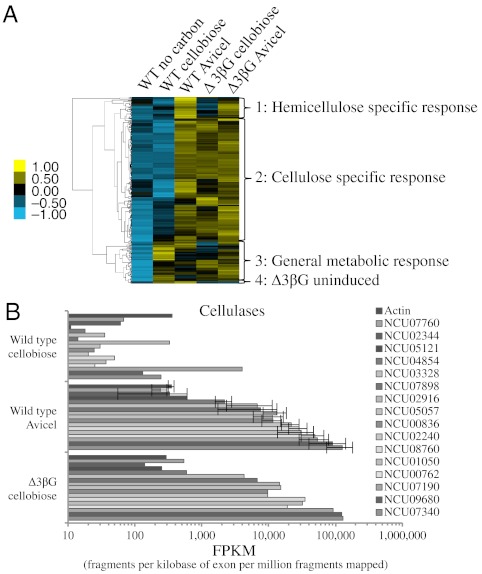
Hierarchical clustering of genes within the cellulose regulon from expression data of WT transferred to media containing no carbon source, cellobiose or Avicel and the Δ3βG strain transferred to media containing cellobiose or Avicel resulted in the identification of four distinct expression clusters (Fig. 3A, Dataset S1). The largest cluster (cluster 2) contained 210 genes that showed high expression in the WT strain on Avicel, as well as in the Δ3βG strain under either cellobiose- or Avicel-induced conditions. This group of 210 genes contained all 16 predicted cellulases (NCU00762, gh5-1; NCU00836, gh61-7; NCU01050, gh61-4; NCU02240, gh61-1; NCU02344, gh61-12; NCU02916, gh61-3; NCU03328, gh61-6; NCU04854, gh7-2; NCU05057, gh7-1; NCU05121, gh45-1; NCU07190, gh6-3; NCU07340, cbh-1; NCU07760, gh61-2; NCU07898, gh61-13; NCU08760, gh61-5; NCU09680, gh6-2) as well as three genes identified in previous analyses (20, 35) to be accessory proteins for cellulose degradation (NCU00206, cdh-1; NCU07143, lac-2; NCU09764, CBM1-containing protein). This cluster also contained nine hemicellulase genes (NCU02343, gh51-1; NCU02855, gh11-1; NCU04997, gh10-3; NCU05924, gh10-1; NCU05955, gh74-1; NCU07225, gh11-2; NCU07326, gh43-6; NCU08189, gh10-2; NCU09775, gh54-1). Of the 182 proteins remaining in this cluster, 29 are predicted to be active on carbohydrates by CAZy (29) and 76 are predicted to be secreted by signalP, with 25 genes falling into both categories. The remaining 102 genes were grouped into their predicted functional categories (31) resulting in 10 genes expected to be involved in C-compound and carbohydrate metabolism, 8 genes involved in protein folding, modification or transport, and 62 genes encoding unclassified proteins.
Fig. 3.
RNA sequencing of the WT and Δ3βG strains. (A) Hierarchical clustering analysis of 318 genes differentially induced in WT N. crassa by Avicel, compared to induction by cellobiose. Yellow indicates higher relative expression and blue indicates lower relative expression. (B) Cellulase expression in FPKMs for the WT induced with cellobiose or Avicel compared to Δ3βG induced with cellobiose. All strains were grown for 16 h on 2% sucrose, followed by a transfer to no carbon source (Vogel’s salt solution only), 0.2% cellobiose, or 1% Avicel for 4 h. Expression levels for each condition are shown in the order of genes listed in the legend.
A small cluster of 36 genes (cluster 1) showed high expression levels in either the WT or Δ3βG deletion strain when exposed to Avicel (Fig. 3A), but had lower expression levels in the Δ3βG deletion strain on cellobiose. This group contained a predicted β-xylosidase gene (NCU09652, gh43-5) and several other genes encoding proteins active on hemicellulose (NCU00710, acetyl xylan esterase; NCU01900, xylosidase/arabinosidase; NCU00891, xylitol dehydrogenase; NCU08384, xylose reductase). These results suggest that these genes were induced by the 0.5–1.0% hemicellulose found in Avicel (20) and are not part of the regulon induced by cellobiose.
When comparing the induction of the Δ3βG strain on cellobiose versus WT on Avicel, a striking pattern appears (Fig. 3B). Genes induced in the WT by Avicel are very close to the value seen in the Δ3βG mutant. For example, the fragments per kilobase of exon per million fragments mapped (FPKM) for cbh-1 in the WT on Avicel is 126,816 ± 53,016 whereas the FPKM in Δ3βG on cellobiose is 130,865. This pattern extends even to the lesser-induced cellulases like NCU07760 (gh61-2), which has an FPKM of 239 ± 62 for WT on Avicel and 538 for Δ3βG mutant on cellobiose. In contrast, some hemicellulase genes in the Δ3βG mutant were induced in response to cellobiose (Fig. S7D), but had lower expression levels than in Avicel-induced WT or Δ3βG cultures. For example, whereas NCU05924 (endoxylanase, gh10-1) has 20,023 ± 9,888 FPKMs in WT induced with Avicel, an expression level of 10,000 FPKMs was observed in the Δ3βG mutant induced with cellobiose. These results indicate that although all of the cellulase genes are in the same regulon, the hemicellulase genes are divided into those that are coordinately regulated with cellulases and those that require additional signals for full induction.
The third cluster contained 67 genes that showed no response to conditions of starvation, but were most highly induced by cellobiose in WT (Dataset S1). This cluster can be further subdivided into two groups: 31 genes that were most highly induced in the WT strain by cellobiose, but were not induced by the Δ3βG strain and 36 genes that are moderately induced by cellobiose in the WT and Δ3βG strains, but were most highly induced in the Δ3βG strain in response to Avicel. The majority of these genes fall into one of two categories: unclassified proteins or proteins involved in the metabolism of amino acids, nitrogen, phosphate, or carbohydrates, with only three that are predicted to have activity toward carbohydrates by CAZy (29). The final cluster contains four genes that are induced by cellobiose or Avicel in WT cultures, but are not induced by the Δ3βG mutant when challenged with either cellobiose or Avicel. Three of the four were predicted to be active on carbohydrates by CAZy (29), and one, NCU08087 (gh26-1), is predicted to be a hemicellulase (20).
Transcription of Plant Cell Wall Degrading Enzymes in the Δ3βG Mutant Correlates with Cellulase Secretion and Activity.
Historically, studies examining cellulases in filamentous fungi have used transcription as a readout for enzyme induction (10, 36). To determine whether the transcriptional response of the Δ3βG and Δ3βGΔcre strains in response to cellobiose corresponds to an increase in functional protein, we assessed secreted proteins and cellulase activity of the Δ3βG and Δ3βGΔcre strains in response to induction with either cellobiose or Avicel (SI Materials and Methods), as compared to WT cultures. As expected, supernatants from all sucrose-grown cultures (Δ3βG, Δ3βGΔcre, and WT) were unable to produce glucose or cellobiose from crystalline cellulose in an Avicel hydrolysis assay (Materials and Methods), whereas supernatants from all three Avicel-induced cultures (Δ3βG, Δ3βGΔcre, and WT) were able to degrade crystalline cellulose to cellobiose and glucose (Fig. 2C). When grown on cellobiose, the Δ3βG and Δ3βGΔcre strains displayed a secreted protein pattern similar to WT Avicel-grown cultures (Fig. 2B) (20). Importantly, supernatants from both the Δ3βG and Δ3βGΔcre deletion strains induced by cellobiose hydrolyzed crystalline cellulose, whereas supernatants from WT cellobiose-grown cultures did not (Fig. 2C). The Δ3βG and Δ3βGΔcre strains, which lack three β-glucosidases, produced mostly cellobiose. These data are consistent with the hypothesis that the three β-glucosidase enzymes provide the bulk of the glucose-generating activity in WT cultures (37).
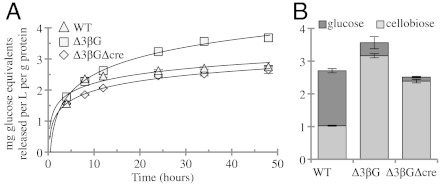
Industrial filamentous fungi are grown in submerged cultures for high-level production of a variety of products (38). We therefore examined the induction of cellulases in the Δ3βG and Δ3βGΔcre deletion strains in a controlled bioreactor process (Fig. S8 A–D). After 24 h growth on sucrose, WT, Δ3βG, and Δ3βGΔcre produce a similar amount of biomass (approximately 3.5 g/L) (Fig. S8 A–C). After induction with 0.2% cellobiose, WT did not secrete a significant amount of protein (0.05 mg/mL; Fig. S8C). In contrast, the Δ3βG and Δ3βGΔcre cultures produced 0.12 mg/mL and 0.24 mg/mL protein, respectively, in the supernatant (Fig. S8 A–B). In addition, the cellobiose-induced Δ3βG and Δ3βGΔcre cultures showed a significant increase in endoglucanase activity over this same period of induction (Fig. S8F). Examining the aggregate Avicel hydrolysis from the 24-h time point indicated that the Δ3βGΔcre strain produced 60% more glucose equivalents (0.424 mg/mL) as compared to the Δ3βG strain (0.296 mg/mL) (Fig. S8E). However, when the total concentration of protein was normalized, the Δ3βGΔcre strain had less specific activity than either the WT or Δ3βG culture supernatants (Fig. 4). These data indicate that although the addition of the cre-1 deletion to Δ3βG strain allowed for greater protein secretion, this secretion was not specific for cellulases.
Fig. 4.
Enzyme activity in WT, Δ3βG, and Δ3βGΔcre strains after induction with cellobiose or Avicel. (A) The 24 h induced supernatant activity (from Fig. S8 A, B, and D) toward Avicel. Cellulase activity of culture supernatant from Δ3βG (□) and Δ3βGΔcre (◊) strains when induced with cellobiose for 24 h compared to culture supernatants from WT grown on Avicel for 5 d (▵). (B) Breakdown of cellobiose (light gray) and glucose (dark gray) produced in the Avicel hydrolysis assay (from A) after 36 h. Error bars are 1 SD.
Proteomic Analysis of Secreted Proteins.
To compare the identity of proteins secreted by WT N. crassa grown on Avicel versus the Δ3βG strains when induced with cellobiose, we analyzed the secretome using a shotgun proteomics approach (Table 1) (SI Materials and Methods). There were 39 proteins identified in the WT Avicel-grown culture supernatant. In cellobiose-grown cultures, 38 proteins were identified in the Δ3βG broth and 24 were identified in the Δ3βGΔcre broth (Dataset S1). Using quantitative mass spectrometry Phillips, et al. concluded that 76% of the WT N. crassa secretome on Avicel is composed of six individual proteins (35). All of these proteins were identified in the WT, Δ3βG, and Δ3βGΔcre culture broths (except for the deleted β-glucosidase, gh3-4) (Table 1). In addition to the cellulases, we identified a number of lower abundance accessory proteins, which make up a total of 6.5% of the secretome (35): a cellobiose dehydrogenase (CDH-1), a type 2 lactonase (LAC-2), and two hypothetical proteins, which include NCU09764, a CBM1-containing protein of unknown function and NCU05137, a gene, which when deleted leads to an increase in cellulase activity (20). These data indicate that, similar to the transcriptional response of the Δ3βG mutant to cellobiose, the identity of proteins secreted and the amount of protein secreted in the Δ3βG strain on cellobiose mimicked the WT N. crassa response to Avicel.
Table 1.
Mass spectrometry of the most abundant secreted proteins in N. crassa WT (Avicel), Δ3βG (cellobiose), and Δ3βGΔcre (cellobiose) strains
| Gene | Annotation | WT | Δ3βG | Δ3βGΔcre | Secretome (%)* |
| Cellulases | |||||
| NCU07340 | CBH-1 | + | + | + | 39.5 |
| NCU09680 | GH6-2 | + | + | + | 13.4 |
| NCU07898 | GH61-2 | + | — | + | 6.6 |
| NCU00762 | GH5-1 | + | + | + | 5.9 |
| NCU08760 | GH61-5 | + | + | + | 4.6 |
| NCU05057 | GH7-1 | + | + | + | 4.0 |
| NCU02240 | GH61-1 | + | — | + | 3.4 |
| NCU07190 | GH6-3 | + | + | + | 3.2 |
| Accessory proteins | |||||
| NCU04952 | GH3-4 | + | ND | ND | 3.8 |
| NCU00206 | CDH-1 | + | + | + | 2.4 |
| NCU09764 | N/A | + | + | + | 1.6 |
| NCU05137 | NCW-1 | + | + | + | 1.5 |
| NCU07143 | LAC-2 | + | + | + | 1.0 |
GH, glycoside hydrolase; ND, not detected, gene knockout
*Avicel-induced secretome identified by AQUA Mass Spectrometry, Phillips, et al. (35) (13 proteins represent 91% of the total secretome with all other proteins representing less than 1% of the secretome each).
Discussion
In this study, we examined the hypothesis that cellobiose functions as an inducer of cellulase gene expression and secretion when filamentous fungi, such as N. crassa, are exposed to cellulose, but that the action of extracellular and intracellular β-glucosidases and CCR mask this inducing activity. Our results revealed that a strain of N. crassa carrying deletions for the three major β-glucosidase genes induces cellulases when exposed to cellobiose and this induction recapitulates, on both a transcriptional and protein level, the WT response to Avicel.
Although many industrially focused studies have attempted to identify the best inducers of lignocellulose-degrading enzymes, little is known about the molecular mechanism of this induction. Early studies used the β-glucosidase inhibitor nojirimycin to show that cellobiose induces cellulases in the lignocellulose-degrading filamentous fungus, T. reesei (17, 18). However, this induction was not as robust as with other soluble inducers including lactose (25) or sophorose (12). Although both molecules have been used extensively to induce cellulases in T. reesei, neither can exactly reproduce the response to insoluble cellulose (39, 40). In contrast to these results, exposure of the N. crassa Δ3βG mutant to cellobiose recapitulated the response of a WT strain to Avicel.
Our results indicate that deletion of the predicted major β-glucosidase genes enabled N. crassa to induce both transcription and secretion of the complete repertoire of cellulases upon exposure to cellobiose. In P. chrysosporium, cellobiose does not induce cellulase gene expression (10), although cellotriose or cellotetraose were able to do so. We hypothesize that in P. chrysosporium, as in N. crassa, any inducing affect of cellobiose is masked by its degradation to glucose by endogenous β-glucosidases, leading to catabolite repression. Our results also indicate that sophorose does not act as an inducer in N. crassa. In T. reesei, sophorose is likely produced by transglycosylation of cellobiose by an extracellular β-glucosidase (11) and readily transported into the cell by a β-linked disaccharide permease (41). However, in the N. crassa Δ3βG mutant all of the predicted extracellular β-glucosidase enzymes were deleted, suggesting that transglycosylation of cellobiose to sophorose is not relevant to cellulase induction. Given the high conservation of the lignocellulose-degrading machinery in filamentous fungi, we predict that deletion of the bulk of β-glucosidase activity in other fungi would enable the use of cellobiose for full induction of the cellulose regulon in these species.
An advantage for the industrial production of cellulolytic enzymes is the ability to use a soluble inducer. Because the action of sophorose is not generally applicable to other filamentous fungi, we predict that the production of cellulases in filamentous fungi will be possible upon exposure to cellobiose, providing that either mutational inactivation or chemical inhibition of their major β-glucosidases associated with plant cell wall utilization can be achieved. Because the number of predicted cellulases and hemicellulases in the genomes of filamentous fungi varies considerably, this approach may provide a tool for analysis of such proteins in a soluble environment, avoiding the complication of separating enzymes from insoluble plant cell wall material. Although utilization of cellodextrins as inducers currently might not be economically feasible in an industrial setting, understanding the molecular mechanism of cellulase induction in filamentous fungi could allow researchers to manipulate other (e.g., thermophilic) cellulolytic organisms to enable the production, purification, and examination of new lignocellulolytic enzymes. Furthermore, a considerable number of unclassified and hypothetical proteins are induced as part of the N. crassa cellulose regulon, many of which are secreted or are predicted to be secreted. A comparative analysis of the cellulose regulon in a variety of filamentous fungi should reveal which of these genes/proteins are conserved and thus worthy of further characterization. By understanding the mechanism of cellulase induction and utilization in a model organism using reverse genetics, we expect that this knowledge can be translated into currently used industrial filamentous fungi to further improve their ability to produce lignocellulose-degrading enzymes and allow for the production of a renewable source of cost-competitive biofuels.
Finally, we note that current ethanologenic yeast used for the production of biofuels require that cellulose be completely degraded to glucose. Cellulolytic fungi engineered as described here to enable cellodextrin induction of cellulases would lack the β-glucosidase enzymes required to produce glucose. However, recent studies using N. crassa cellodextrin transporters indicate organisms that ferment xylose and cellobiose simultaneously may perform better than those that ferment glucose and xylose sequentially (22, 42). The combination of cellodextrin induction of cellulases in filamentous fungi with yeasts that can ferment cellodextrins may prove beneficial in developing an economical process of biofuel production from plant biomass.
Materials and Methods
Strains.
All strains were obtained from the Fungal Genetics Stock Center (FGSC) (SI Materials and Methods). Multiple deletion strains were made by performing sequential crosses and confirmed by PCR (SI Materials and Methods).
Transcriptional Studies.
RNA was prepared as previously described (20) and quantitative RT-PCR was performed using the EXPRESS One-Step SYBR GreenER Kit (Invitrogen) and the StepOnePlus Real-Time PCR System (Applied Biosystems) (SI Materials and Methods).
RNA-Seq.
mRNA sequencing was performed using an Illumina kit (RS-100-0801). The cDNA library was sequenced using an Illumina Genome Analyzer-II (Vincent J. Coates Genomic Sequencing Laboratory, University of California, Berkeley) using standard Illumina operating procedures. Libraries were mapped against the N. crassa OR74A genome (version 10) with Tophat (version 1.1.4) (43) and abundance was estimated with Cufflinks (version 0.9.2) (44). Genes exhibiting statistically significant expression changes by Cuffdiff were hierarchically clustered by their FPKMs (SI Materials and Methods).
Shake Flask Studies.
Cultures were grown in 1% sucrose for 24 h followed by the addition of 2% sucrose or 0.2% cellobiose. Supernatant was harvested after 24 h (WT, Δ3βG, and Δ3βGΔcre) or 72 h (Δ3βG). The WT Avicel culture was grown for 5 d on 2% Avicel, Δ3βG was grown in 1% sucrose for 24 h followed by 48 h in 1% Avicel and Δ3βGΔcre was grown in 1% sucrose for 24 h followed by 24 h in 1% Avicel.
Bioreactor Studies.
Cellulase production was carried out in a 3.7 L bioreactor (BioEngineering AG) at an operating volume of 1 L. Following 24 h initial growth on 1% wt/vol sucrose as the sole carbon source, cellulase production was induced with either cellobiose or Avicel added to a final concentration of 0.2% wt/vol (SI Materials and Methods).
Enzymatic Assays.
Total secreted proteins were measured using the BioRad Protein Assay kit (BioRad). Total Avicel hydrolysis activity was performed at 50 °C with 1% cellulose (Avicel) in 50 mM (pH 5.0) sodium acetate. Sugars released were monitored by a DIONEX ICS-3000 HPLC (Dionex) using a CarboPac PA20 column (SI Materials and Methods).
Mass Spectrometry.
Trypsin-digested proteins were analyzed using an orthogonal acceleration quadrupole time-of-flight (Q-TOF) mass spectrometer connected to an ultraperformance liquid chromatograph (UPLC). Peptides were separated using a nanoAcquity UPLC (Waters) equipped with C18 trapping and analytical columns connected to a Universal NanoFlow Sprayer nanoelectrospray ionization (nanoESI) emitter mounted in the nanoflow ion source (Q-TOF Premier, Waters). Resulting data were processed using ProteinLynx Global Server and searched against the N. crassa protein database (Broad Institute) (SI Materials and Methods).
Supplementary Material
Acknowledgments.
We thank Mara Bryan, Stefan Bauer, and Ana Belén Ibáñez for technical assistance. We thank Morgann Reilly for her comments on the manuscript. This work was supported by a grant from Energy Biosciences Institute (N.L.G. and J.H.D.C). Liquid chromatography-mass spectrometry instrumentation was acquired with National Institutes of Health Grant 1S10RR022393-01. We are pleased to acknowledge use of materials generated by P01 GM068087 Functional analysis of a model filamentous fungus.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: All profiling data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE36719).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118440109/-/DCSupplemental.
References
- 1.Rubin EM. Genomics of cellulosic biofuels. Nature. 2008;454:841–845. doi: 10.1038/nature07190. [DOI] [PubMed] [Google Scholar]
- 2.Himmel ME, et al. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science. 2007;315:804–807. doi: 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- 3.Cherry JR, Fidantsef AL. Directed evolution of industrial enzymes: An update. Curr Opin Biotechnol. 2003;14:438–443. doi: 10.1016/s0958-1669(03)00099-5. [DOI] [PubMed] [Google Scholar]
- 4.Kubicek CP, et al. The Trichoderma cellulase regulatory puzzle: From the interior life of a secretory fungus. Enzyme Microb Technol. 1993;15:90–99. doi: 10.1016/0141-0229(93)90030-6. [DOI] [PubMed] [Google Scholar]
- 5.Vaheri MP, et al. Formation and release of cellulolytic enzymes during growth of Trichoderma reesei on cellobiose and glycerol. Appl Microbiol Biotechnol. 1979;8:73–80. [Google Scholar]
- 6.Mandels M, Reese ET. Induction of cellulase in fungi by cellobiose. J Bacteriol. 1960;79:816–826. doi: 10.1128/jb.79.6.816-826.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaheri MP, et al. Formation and release of cellulolytic enzymes during growth of Trichoderma reesei on cellobiose and glycerol. Appl Microbiol Biotechnol. 1979;8:73–80. [Google Scholar]
- 8.Chikamatsu G, et al. Structure and expression properties of the endo-beta-1,4-glucanase A gene from the filamentous fungus Aspergillus nidulans. FEMS Microbiol Lett. 1999;175:239–245. doi: 10.1111/j.1574-6968.1999.tb13626.x. [DOI] [PubMed] [Google Scholar]
- 9.Nevalainen KM, et al. Heterologous protein expression in filamentous fungi. Trends Biotechnol. 2005;23:468–474. doi: 10.1016/j.tibtech.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki H, et al. Cellotriose and cellotetraose as inducers of the genes encoding cellobiohydrolases in the basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 2010;76:6164–6170. doi: 10.1128/AEM.00724-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaheri M, et al. Transglycosylation products of cellulase system of Trichoderma reesei. Biotechnol Lett. 1979;1:41–46. [Google Scholar]
- 12.Mandels M, et al. Sophorose as an inducer of cellulase in Trichoderma viride. J Bacteriol. 1962;83:400–408. doi: 10.1128/jb.83.2.400-408.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sternberg D, Mandels GR. Induction of cellulolytic enzymes in Trichoderma reesei by sophorose. J Bacteriol. 1979;139:761–769. doi: 10.1128/jb.139.3.761-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sternberg D, Mandels GR. Regulation of the cellulolytic system in Trichoderma reesei by sophorose: Induction of cellulase and repression of beta-glucosidase. J Bacteriol. 1980;144:1197–1199. doi: 10.1128/jb.144.3.1197-1199.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gielkens MM, et al. Two cellobiohydrolase-encoding genes from Aspergillus niger require d-xylose and the xylanolytic transcriptional activator XlnR for their expression. Appl Environ Microbiol. 1999;65:4340–4345. doi: 10.1128/aem.65.10.4340-4345.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulmer DC, et al. Possible induction of the ligninolytic system of Phanerochaete chrysosporium. J Biotechnol. 1984;1:13–24. [Google Scholar]
- 17.Fritscher CC. Cellobiose metabolism and cellobiohydrolase I biosynthesis by Trichoderma reesei. Exp Mycol. 1990;14:405–415. [Google Scholar]
- 18.Reese ET, et al. Nojirimycin and d-glucono-1,5-lactone as inhibitors of carbohydrases. Carbohydr Res. 1971;18:381–388. [Google Scholar]
- 19.Woodward J, Arnold SL. The inhibition of β-glucosidase activity in Trichoderma reesei C30 cellulase by derivatives and isomers of glucose. Biotechnol Bioeng. 1981;23:1553–1562. [Google Scholar]
- 20.Tian C, et al. Systems analysis of plant cell wall degradation by the model filamentous fungus Neurospora crassa. Proc Natl Acad Sci USA. 2009;106:22157–22162. doi: 10.1073/pnas.0906810106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun J, Glass NL. Identification of the CRE-1 cellulolytic regulon in Neurospora crassa. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galazka JM, et al. Cellodextrin transport in yeast for improved biofuel production. Science. 2010;330:84–86. doi: 10.1126/science.1192838. [DOI] [PubMed] [Google Scholar]
- 23.Maddi A, et al. Trifluoromethanesulfonic acid-based proteomic analysis of cell wall and secreted proteins of the ascomycetous fungi Neurospora crassa and Candida albicans. Fungal Genet Biol. 2009;46:768–781. doi: 10.1016/j.fgb.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bohlin C, et al. A comparative study of activity and apparent inhibition of fungal beta-glucosidases. Biotechnol Bioeng. 2010;107:943–952. doi: 10.1002/bit.22885. [DOI] [PubMed] [Google Scholar]
- 25.Seiboth B, et al. Lactose metabolism and cellulase production in Hypocrea jecorina: The gal7 gene, encoding galactose-1-phosphate uridylyltransferase, is essential for growth on galactose but not for cellulase induction. Mol Genet Genomics. 2002;267:124–132. doi: 10.1007/s00438-002-0654-9. [DOI] [PubMed] [Google Scholar]
- 26.Portnoy T, et al. The CRE1 carbon catabolite repressor of the fungus Trichoderma reesei: A master regulator of carbon assimilation. BMC Genomics. 2011;12:269–281. doi: 10.1186/1471-2164-12-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamayo EN, et al. CreA mediates repression of the regulatory gene xlnR which controls the production of xylanolytic enzymes in Aspergillus nidulans. Fungal Genet Biol. 2008;45:984–993. doi: 10.1016/j.fgb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Nakari-Setala T, et al. Genetic modification of carbon catabolite repression in Trichoderma reesei for improved protein production. Appl Environ Microbiol. 2009;75:4853–4860. doi: 10.1128/AEM.00282-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cantarel BL, et al. The carbohydrate-active enzymes database (CAZy): An expert resource for glycogenomics. Nucleic Acids Res. 2009;37:D233–D238. doi: 10.1093/nar/gkn663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen H, et al. Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc. 2007;2:953–971. doi: 10.1038/nprot.2007.131. [DOI] [PubMed] [Google Scholar]
- 31.Ruepp A, et al. The FunCat, a functional annotation scheme for systematic classification of proteins from whole genomes. Nucleic Acids Res. 2004;32:5539–5545. doi: 10.1093/nar/gkh894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noguchi Y, et al. Genes regulated by AoXlnR, the xylanolytic and cellulolytic transcriptional regulator, in Aspergillus oryzae. Appl Microbiol Biotechnol. 2009;85:141–154. doi: 10.1007/s00253-009-2236-9. [DOI] [PubMed] [Google Scholar]
- 33.Portnoy T, et al. Differential regulation of the cellulase transcription factors XYR1, ACE2, and ACE1 in Trichoderma reesei strains producing high and low levels of cellulase. Eukaryot Cell. 2011;10:262–271. doi: 10.1128/EC.00208-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goncalves RD, et al. A genome-wide screen for Neurospora crassa transcription factors regulating glycogen metabolism. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M111.007963. 10.1074/mcp.M111.007963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips CM, et al. A quantitative proteomic approach for cellulose degradation by Neurospora crassa. J Proteome Res. 2011;10:4177–4185. doi: 10.1021/pr200329b. [DOI] [PubMed] [Google Scholar]
- 36.Ilmen M, et al. Regulation of cellulase gene expression in the filamentous fungus Trichoderma reesei. Appl Environ Microbiol. 1997;63:1298–1306. doi: 10.1128/aem.63.4.1298-1306.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine SE, et al. A mechanistic model for rational design of optimal cellulase mixtures. Biotechnol Bioeng. 2011;108:2561–2570. doi: 10.1002/bit.23249. [DOI] [PubMed] [Google Scholar]
- 38.Gibbs PA, et al. Growth of filamentous fungi in submerged culture: Problems and possible solutions. Crit Rev Biotechnol. 2000;20:17–48. doi: 10.1080/07388550091144177. [DOI] [PubMed] [Google Scholar]
- 39.Schmoll M, et al. Cloning of genes expressed early during cellulase induction in Hypocrea jecorina by a rapid subtraction hybridization approach. Fungal Genet Biol. 2004;41:877–887. doi: 10.1016/j.fgb.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Sipos B, et al. Characterization of specific activities and hydrolytic properties of cell-wall-degrading enzymes produced by Trichoderma reesei Rut C30 on different carbon sources. Appl Biochem Biotechnol. 2010;161:347–364. doi: 10.1007/s12010-009-8824-4. [DOI] [PubMed] [Google Scholar]
- 41.Kubicek CP, et al. Triggering of cellulase biosynthesis by cellulose in Trichoderma reesei. Involvement of a constitutive, sophorose-inducible, glucose-inhibited beta-diglucoside permease. J Biol Chem. 1993;268:19364–19368. [PubMed] [Google Scholar]
- 42.Ha SJ, et al. Engineered Saccharomyces cerevisiae capable of simultaneous cellobiose and xylose fermentation. Proc Natl Acad Sci USA. 2011;108:504–509. doi: 10.1073/pnas.1010456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langmead B, et al. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts A, et al. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011;12:R22. doi: 10.1186/gb-2011-12-3-r22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.