Abstract
Dysregulation of the transcriptional repressor element-1 silencing transcription factor (REST)/neuron-restrictive silencer factor is important in a broad range of diseases, including cancer, diabetes, and heart disease. The role of REST-dependent epigenetic modifications in neurodegeneration is less clear. Here, we show that neuronal insults trigger activation of REST and CoREST in a clinically relevant model of ischemic stroke and that REST binds a subset of “transcriptionally responsive” genes (gria2, grin1, chrnb2, nefh, nfκb2, trpv1, chrm4, and syt6), of which the AMPA receptor subunit GluA2 is a top hit. Genes with enriched REST exhibited decreased mRNA and protein. We further show that REST assembles with CoREST, mSin3A, histone deacetylases 1 and 2, histone methyl-transferase G9a, and methyl CpG binding protein 2 at the promoters of target genes, where it orchestrates epigenetic remodeling and gene silencing. RNAi-mediated depletion of REST or administration of dominant-negative REST delivered directly into the hippocampus in vivo prevents epigenetic modifications, restores gene expression, and rescues hippocampal neurons. These findings document a causal role for REST-dependent epigenetic remodeling in the neurodegeneration associated with ischemic stroke and identify unique therapeutic targets for the amelioration of hippocampal injury and cognitive deficits.
Keywords: chromatin remodeling, global ischemia, CA1, synaptic plasticity
The transcriptional repressor element-1 (RE1) silencing transcription factor (REST)/neuron-restrictive silencer factor (NRSF) is a gene silencing transcription factor that is widely expressed during embryogenesis and is critical to elaboration of the neuronal phenotype (1–3). In pluripotent stem cells and neural progenitors, REST actively represses a large array of coding and noncoding neuron-specific genes important to synaptic plasticity and structural remodeling, including synaptic vesicle proteins, neuroreceptors and channels, and microRNAs that regulate networks of nonneuronal genes (1–4). In neural progenitor cells, REST is regulated at the level of protein stability by a balance between β-transducin repeat containing protein (β-TrCP)-mediated, ubiquitin-based proteasomal degradation (5, 6) and herpesvirus-associated ubiquitin-specific protease (HAUSP)-mediated deubiquitylation (7); during terminal neuronal differentiation, ubiquitin-based proteasomal degradation of REST is required for acquisition of the neural phenotype (8). Disruption or ectopic expression of REST during embryogenesis results in cellular apoptosis, aberrant differentiation, and lethality (9, 10). In mature neurons, REST is quiescent but can be activated in selectively vulnerable hippocampal neurons by insults, such as global ischemia (11, 12) and epileptic seizures (13), and aberrantly accumulates in selectively vulnerable striatal neurons in humans with Huntington disease (14, 15).
A fundamental mechanism by which REST silences target genes is that of epigenetic remodeling (16). REST binds the RE1 element of target genes and recruits C-terminal cofactor for REST (CoREST) (17, 18) and mSin3A (19–21), corepressor platforms that recruit histone deacetylases (HDACs) 1 and 2. HDACs deacetylate core histone proteins (16, 22). In addition, REST recruits the site-specific histone methyl-transferase G9a, which promotes dimethylation of histone 3 at lysine 9 (H3K9me2) via CoREST-dependent (8) and independent (23) mechanisms; the site-specific histone demethylase LSD1, which removes methyl groups from histone 3 mono- or dimethylated at lysine 4 (H3K4me1, HSK4me2) (24, 25); and methyl CpG binding protein 2 (MeCP2) (8, 26), a protein that reads epigenetic marks on core histones and hotspots of DNA methylation. Whereas histone deacetylation is primarily a mark of dynamic gene repression, histone and DNA methylation are implicated in long-term, stable gene repression (16, 22).
REST is essential for repressing neuronal genes in neural progenitors (1–3). A prevailing view is that down-regulation of REST during the late stages of neuronal differentiation is critical to acquisition and maintenance of the neuronal phenotype (8). An earlier paper by our group broadened this view (11). Experiments involving molecular and genetic approaches showed that REST is activated in mature hippocampal neurons in response to ischemic insults and that the increase in REST correlates with a decrease in histone acetylation and gene silencing of GluA2 (11). Acute knockdown of REST in hippocampal slices subjected to oxygen glucose deprivation (OGD), an in vitro model of ischemia, prevented GluA2 down-regulation and neuronal death (11). Although compelling, these findings raise new questions. Are other synaptic proteins regulated by REST in insulted neurons? Are REST and corepressors recruited to the promoters of target genes, and if so, does the corepressor complex orchestrate epigenetic remodeling and gene silencing? Is REST causally related to neuronal death in ischemic stroke?
These findings advance previous studies in that they show that in addition to gria2, other genes essential for synaptic function (e.g., grin1, trpv1, nfκb2; gene names are provided in Table 1) are targets of REST in postischemic neurons and document a causal role for REST in neuronal death in a clinically relevant model of global ischemia in vivo. We further show that REST recruits corepressors [CoREST, mSin3A, HDAC1 and HDAC2, G9a, and MeCP2] to the promoters of target genes, which together orchestrate epigenetic remodeling and gene silencing. Consistent with this, the HDAC inhibitor trichostatin A (TSA) rescues CA1 neurons, linking histone deacetylation and epigenetic remodeling to ischemia-induced neuronal death. RNAi-mediated silencing of REST or dominant-negative (dn) REST delivered directly into the hippocampus of live animals via the lentivirus expression system prevents epigenetic remodeling, restores gene expression, and rescues hippocampal neurons destined to die. These findings document a causal role for REST in epigenetic remodeling of plasticity genes and neuronal death, and identify therapeutic targets for amelioration of the neurodegeneration and cognitive deficits associated with ischemic stroke.
Table 1.
Target genes that exhibit REST enrichment at promoter sites in CA1 at 24 and 48 h after ischemia
 | |||
| Chr | Gene symbol | Gene name | Gene function |
| 1 | Peg12/Frat3 | Paternally expressed 12 | Imprinted gene that encodes a modulator of Wnt signaling; mutations in Frat3 are implicated in Angelman and Prader–Willi syndromes |
| 1 | Slc22a12† | Solute carrier family 22 (organic anion/urate transporter), member 12 | Uric acid transporter and uric acid-anion exchanger that regulates the level of uric acid in the blood |
| 1 | Nfκb2 | Nuclear factor of κ light chain enhancer in B cells 2, p49/p100 | Transcription factor implicated in neuronal survival, synaptic plasticity, and memory |
| 2 | Gria2*‡ | AMPAR GluA2 | AMPAR subunit that assembles with GluA1 or GluA3 subunit to form channels with reduced conductance, pronounced inward rectification, and low Ca2+ permeability; regulates AMPAR targeting to synapses |
| 2 | Chrnb2*‡ | Nicotinic cholinergic receptor β-2 (neuronal) | Nicotinic ACh receptor subunit expressed in neurons; mutations in Chrnb2 are associated with autosomal-dominant nocturnal frontal lobe epilepsy |
| 3 | Grin1 | NMDAR GluN1 | NMDAR subunit that assembles with GluN2 subunits to form channels with high Ca2+ permeability and voltage-dependent sensitivity to Mg2+ |
| 3 | Csrnp3 | Cysteine-serine-rich nuclear protein 3 | Transcription factor that binds to the consensus sequence 5′-AGAGTG-3′ and is implicated in apoptosis (TGF-β–induced apoptosis protein 3) |
| 5 | Nppa‡ | Natriuretic peptide precursor A | 13,000-Mr precursor protein that is processed to generate members of the natriuretic peptide family; brain natriuretic peptide is secreted by the ventricles of the heart in response to myocardial dysfunction, and its physiological action is to decrease systemic vascular resistance and central venous pressure and to increase natriuresis |
| 8 | Slc22a13† | Solute carrier family 22 (organic anion transporter), member 13 | Member of the organic-cation transporter family that mediates uptake of uric acid; a high-affinity nicotinic acid exchanger in kidneys and intestine |
| 9 | Scg2*‡ | Secretogranin II (chromogranin C) | Neuroendocrine secretory granule protein and precursor for biologically active peptides |
| 10 | Fdxr* | Ferredoxin reductase | Mitochondrial flavoprotein that initiates electron transport for cytochrome P450; accepts electrons from NADPH and catalyzes the reduction of ferredoxin |
| 14 | Nefh†‡ | Neurofilament, heavy polypeptide | Subunit that assembles to form neurofilaments, proteins that mediate intracellular transport in axons and dendrites; commonly used as a biomarker of neuronal damage; mutations in the NEFH gene are associated with susceptibility to ALS |
| X | Syp*‡ | Synaptophysin | Synaptic trafficking protein involved in exocytosis of synaptic vesicles at nerve terminals; important to short- and long-term synaptic plasticity |
Results
Global Ischemia Increases REST and CoREST but Not mSin3A Expression in Hippocampal CA1.
We first examined the impact of ischemia on members of the REST–corepressor complex in CA1 from animals subjected to a clinically relevant model of stroke. Transient forebrain or global ischemia in rats provides a well-established model of neuronal insult in which cell death occurs primarily in CA1 pyramidal neurons and is delayed by 3–4 d, allowing examination of molecular mechanisms that underlie cell death (27–29). Ischemia induced a marked up-regulation of REST (Fig. 1A) and CoREST (Fig. 1B) mRNA in vulnerable CA1 neurons. The increase in REST and CoREST mRNA was subfield-specific in that changes did not occur in resistant CA3 (Fig. 1 A and B). Ischemia caused a transient reduction in mSin3A mRNA in CA1 and CA3, evident at 12 h; by 24 h, mSin3A mRNA was at control levels in CA1 but slightly increased in CA3 (Fig. 1C). Because alterations in mRNA abundance do not necessarily predict alterations in protein, we next examined the impact of ischemia on levels of corepressor proteins. Ischemia induced an increase in REST in the nuclear fraction of CA1 (to ∼180% of control at 6 h; Fig. 1D) and in nuclei of individual pyramidal neurons (11). The increase in REST protein (Fig. 1D) preceded the increase in REST mRNA (Fig. 1A), consistent with regulation of REST at the level of protein stability (5–8). CoREST was also increased in the nuclear fraction of CA1 (to ∼150% of control at 24 h and ∼220% at 48 h) but with a delay relative to REST (Fig. 1E). mSin3A protein abundance was unchanged in the nuclear fraction of CA1 at all times examined (Fig. 1F).
Fig. 1.
Global ischemia increases REST and CoREST (but not mSin3A) expression in CA1. RT-qPCR shows a marked increase in REST (A) and CoREST (B) mRNA expression in selectively vulnerable CA1, but not resistant CA3, at times after ischemia (n = 3 animals per group). (C) mSin3A exhibited a small but significant increase in CA3 (but not CA1). Cycle threshold values of samples were normalized to hypoxanthine phosphoribosyl transferase (“housekeeping” mRNA) and to corresponding samples from control (sham) animals (relative expression ratio, normalized to sham ± 1). Representative Western blots show a marked increase in REST (D) and CoREST (E), but not mSin3A (F), in the nuclear (Nu), but not cytosolic (Cy), fraction of CA1, but not CA3 (REST), at times after ischemia (n = 6 animals per group). Values for ischemic animals were normalized to nucleolin or actin and to corresponding samples for control animals. (G) RE1-oligoprecipitation shows that REST (but not actin) binds the RE1 consensus sequence in CA1 (but not CA3) of ischemic (but not control) animals. Proteins bound to biotin-labeled RE1 oligonucleotide were captured with streptavidin beads and analyzed by Western blot. Immunolabeling of brain sections at the level of the dorsal hippocampus shows increased expression of CoREST (H), but not mSin3A (I), in nuclei of individual neurons in CA1 at times after ischemia at low (Left), intermediate (Center), and high (Right) magnification. Bars represent the mean ± SEM. Statistical significance was assessed by the randomization test. *P < 0.05; **P < 0.01; ***P < 0.001 in CA1 vs. CA3. #P < 0.05; ##P < 0.01; ###P < 0.001 in ischemia vs. sham. so, stratum oriens; sp, stratum pyramidale; sr, stratum radiatum.
To examine whether REST is functional, we performed oligoprecipitation with a biotinylated 21-bp oligonucleotide corresponding to the sequence of the RE1 regulatory element and probed Western blots for REST. Ischemia increased association of REST with the RE1 sequence in CA1 samples, evident at 12 h, 24 h, and 48 h (Fig. 1G), but not in CA3 samples. In contrast, ischemia did not increase association of β-actin (which lacks RE1 sites) with the RE1 oligonucleotide in CA1. These findings indicate target and subfield specificity of REST activity. To examine the spatial and temporal pattern of CoREST and mSin3A protein expression in CA1, we performed immunocytochemistry. CoREST was constitutively expressed in the nuclei of CA1 pyramidal neurons under physiological conditions; ischemia induced an increase in CoREST protein in nuclei of individual CA1 pyramidal neurons (Fig. 1H). mSin3A was abundantly expressed in the nuclei and cytosol of CA1 neurons, and was not altered by ischemia (Fig. 1I). Thus, ischemic insults up-regulate and activate REST and its corepressor CoREST (but not mSin3A) in the nuclei of selectively vulnerable CA1 neurons.
ChIP-on-Chip Analysis Reveals That REST Binds to a Discrete Subset of Target Genes in CA1.
To address recruitment of REST to target genes, we applied a targeted ChIP-on-chip approach to identify genes with altered REST occupancy in the selectively vulnerable CA1 following transient global ischemia. ChIP-on-chip analysis enables identification of loci with enhanced REST association and provides broad-based, genome-wide information at high resolution about epigenomic dysregulation of REST targets critical to ischemia-induced neurodegeneration. We used a custom tiling microarray, in which a total of 385,000 overlapping, isothermal probes interrogated 0.63% (17.3 Mb) of the rat genome encompassing 230 genes (Table 1). The genomic regions were selected to include 73 coding and 7 noncoding neuron-specific genes that contain RE1 sites and are validated targets of REST (of which 6 encode ionotropic glutamate receptors gria2, grin1, grin2B, grin2C, grik2, and grik5); an additional 8 genes that encode ionotropic glutamate receptors (of which some contain RE1 sites but are not, as yet, validated targets of REST); six “negative control” regions of the genome, including imprinted loci and immunoglobulins (which are not expressed in brain); and 136 additional coding genes with no known RE1 sites in regions adjacent to REST targets (Table 1 and Table S1). Thus, the microarray contained 14 of 18 genes (5.4 Mb) encoding ionotropic glutamate receptors.
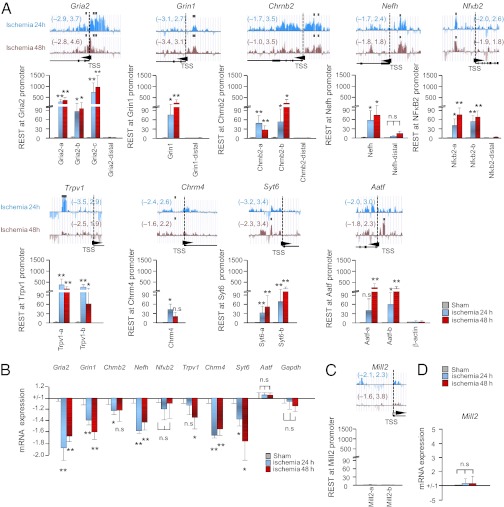
Loci with aberrant REST association in CA1 from animals subjected to global ischemia were compared with corresponding loci in samples of CA1 from control, sham-operated animals. ChIP-on-chip analysis revealed REST enrichment at the promoter regions (within 2 kb upstream or downstream from the transcriptional start sites) of 58 target genes at 24 h, 50 target genes at 48 h, and 13 target genes of 230 total genes at both 24 and 48 h after ischemia (Fig. 2A, Table 1, and Table S2). A list of the 13 genes exhibiting marked enrichment of REST in CA1 at both 24 and 48 h after ischemia, relative to that of control CA1, is presented in Table 1. Genes with marked REST enrichment at their promoters at 24 and/or 48 h after ischemia included gria2 [AMPA receptor (AMPAR), GluA2 subunit], grin1 [NMDA receptor (NMDAR), GluN1 subunit], chrnb2 (neuronal nicotinic AChR, β2 subunit), nefh (neurofilament, heavy polypeptide), nfκb2 (NF-κB2, a transcription factor), trpv1 [transient receptor potential cation channel, subfamily V, member 1 (TRPV1)], chrm4 (muscarinic AchR, M4 subunit), syt6 (synaptotagmin VI, a component of the SNARE complex), and slc22a12/13 (solute carrier family 22, member 12/13) (Fig. 2A and Table 1). Of note, REST occupancy was markedly enriched at three loci in close proximity to the RE1 site within the gria2 proximal promoter region (Fig. 2A). REST occupancy was also increased at the promoters of genes encoding other neurotransmitter receptors, such as the nicotinic receptor β2 and the muscarinic receptor M4, implicated in hippocampal synaptic plasticity; synaptotagmin VI, a component of the SNARE complex involved in exocytosis of synaptic vesicles at nerve terminals and implicated in short- and long-term synaptic plasticity; and NF-κB2, a transcription factor implicated in synaptic plasticity and memory. A modest increase in the μ-opioid receptor (Mor1) was observed (not illustrated) in confirmation of the findings of Formisano et al. (12). Searching the JASPAR CORE database (http://jaspar.genereg.net/) revealed an RE1-consensus sequence at each of these loci. Thus, REST associates with a discrete subset of target genes in postischemic neurons. REST enrichment localized not only to gene promoters but to inter- and intragenic regions (Table 1). Moreover, REST enrichment was not limited to loci with RE1 elements. These findings are consistent with a model whereby REST binding to DNA can occur via association with noncanonical RE1 motifs and/or with other DNA binding proteins, which recruit REST to their cognate recognition sequence (30–32).
Fig. 2.
REST regulates a subset of “transcriptionally responsive” target genes in CA1. (A) (Upper) REST profiles across a subset of validated target genes were assessed by ChIP-on-chip analysis in postischemic CA1. Altogether, 13 target genes exhibited REST enrichment at promoter sites at 24 h and 48 h after ischemia (Table 1). Numbers in parentheses denote log-fold changes in REST enrichment in ischemic vs. control CA1. Black squares denote “hot spots” of REST enrichment. Arrowheads denote transcriptional start sites (TSSs) and transcriptional direction. (Lower) Technical validation was assessed by single-locus ChIP, followed by qPCR, at sites upstream and downstream of TSSs of 9 genes with REST enrichment as assessed by ChIP-on-chip analysis (gria2, grin1, chrnb2, nefh, nfκb2, trpv1, chrm4, syt6, and aatf). (B) Genes that exhibited REST enrichment by ChIP-on-chip analysis exhibited reduced mRNA and protein expression (biological validation). Nfκb2 and Aatf are not significantly changed. (C) Single-locus ChIP-qPCR assay shows that Mill2, which contains an RE1 site, does not exhibit enhanced REST association. (D) Mill2 mRNA expression is not altered (negative control). Statistical significance was assessed by pairwise comparisons between experimental samples and corresponding control samples by means of the two-tailed Student t test. *P < 0.05; **P < 0.01; n.s., nonsignificant.
Neuron-Specific Genes with Enriched REST Exhibit Gene Silencing.
Validation experiments involving single-locus ChIP revealed REST enrichment at the promoters of the REST target genes gria2, grin1, chrnb2, nefh, nfκb2, trpv1, chrm4, syt6, and aatf in CA1 at 24 h and/or 48 h after ischemia relative to that in control CA1 (Fig. 2A). Of the genes examined, gria2 exhibited the most striking REST enrichment at 24 h and 48 h after ischemia. In contrast, REST was not enriched at sites 10 kb downstream from RE1 sites within the gria2, grin1, chrnb2, nefh, and nfκb proximal promoters or at the promoter of the β-actin gene, which lacks an RE1 site (Fig. 2A). These findings provide technical validation of the ChIP-on-chip analysis and indicate target specificity. To examine whether altered REST occupancy at target genes results in altered target gene expression, we performed a RT-quantitative PCR (qPCR) assay. Seven REST target genes with enhanced REST association (gria2, grin1, chrnb2, nefh, trpv1, chrm4, and syt6) exhibited decreased mRNA (Fig. 2B). The eighth gene, Nfκb2, exhibited a trend toward down-regulation that did not achieve significance. Two genes with enhanced REST association (Nfκb2 and Aatf) did not exhibit altered gene transcription. In addition, expression of gapdh (Fig. 2B), which does not contain an RE1 site, and Mill2, which contains an RE1 site but not enhanced REST association, was not altered. Because changes in mRNA do not necessarily predict changes in protein, we examined protein levels of three REST targets (GluA2, GluN1, and GluN2B) known to play roles in neuronal death in postischemic CA1 (33, 34). All three proteins exhibited down-regulation in CA1 at 48 h after ischemia (Fig. S1). Of the proteins examined, GluA2 exhibited the most pronounced down-regulation. Collectively, these findings provide technical and biological validation of the ChIP-on-chip analysis data and identify gria2, which encodes the AMPAR subunit GluA2, a neuron-specific gene involved in synaptic plasticity, structural remodeling, and neuronal death, as a leading target of REST in postischemic CA1.
REST and Corepressors Associate with the gria2 Promoter in CA1.
To address the mechanism by which REST orchestrates silencing of target genes, we performed a more in-depth analysis of GluA2 because it was the leading candidate in our Chip-on-chip analysis. Moreover, the role of GluA2 silencing and expression of GluA2-lacking, Ca2+-permeable AMPARs in global ischemia-induced neuronal death are well-established (33, 35–38). We first examined the physical association of members of the REST repressor complex with the gria2 promoter in postischemic CA1. Toward this end, we performed single-locus ChIP on cross-linked chromatin from CA1 and CA3 with antibodies to REST, CoREST, and mSin3A, followed by a qPCR assay to detect a region of the gria2 promoter within 150 bp of the RE1 site. In control CA1, REST occupancy was low but detectable. Ischemia induced enrichment of REST (shown as ratio of experimental to control) at the gria2 promoter in CA1 (but not in CA3; Fig. 3A). Ischemia did not detectably alter REST at sites either 10 kb upstream or downstream from the RE1 site or at the β-actin promoter, which lacks an RE1 site. These findings document target and subfield specificity of the interaction between REST and the GluA2 proximal promoter in CA1.
Fig. 3.
Ischemia promotes assembly of the REST–corepressor complex and epigenetic remodeling of the gria2 promoter. Single-locus ChIP performed on microdissected CA1 and CA3 tissue from control and experimental animals at times after ischemia shows assembly of the REST–corepressor complex (A–D) and epigenetic remodeling (E–G) of the gria2 promoter. Ischemia promotes association of REST (A), CoREST (B), mSin3A (C), MeCP2 (D), and H3K9me2 (F), but not H3K4me2 (G), as well as marked depression of H3K9/14ac (E), at the gria2 promoter in CA1 (but not CA3). (H) Representative gel showing enrichment of CoREST and mSin3A at the gria2 promoter and negligible signal with IgG (n = 3–6 animals per treatment group and time point). Cross-linked chromatin was immunoprecipitated, followed by amplification of a 150-bp region containing RE1 sites within the gria2 promoter. Cycle threshold values of immunoprecipitated samples were normalized to input and to corresponding samples from control (sham) animals (±1). Statistical analysis was by the two-tailed Student t test. *P < 0.05; **P < 0.01; ***P < 0.01; n.s., nonsignificant.
We next examined CoREST and mSin3A enrichment. Whereas CoREST mediates primarily long-term silencing of target genes, mSin3A mediates dynamic and reversible gene repression (8, 26). Ischemia induced a marked increase in association of CoREST with RE1 sites within the gria2 promoter in CA1 (but not CA3) to approximately fivefold that of control levels at 12 h after ischemia (Fig. 3 B and H). The finding that CoREST is markedly enriched at the target gene promoter at 12 h, a time when CoREST protein is not yet significantly increased, is consistent with the concept that CoREST is constitutively expressed in CA1 under physiological conditions and that its relative binding is increased after ischemia. Ischemia also increased association of mSin3A with RE1 sites within the gria2 promoter in CA1 (but not CA3), but with a delay relative to that of REST or CoREST (Fig. 3 C and H). In contrast, ischemia did not alter association of CoREST or mSin3A in CA3 (Fig. 3 B and C).
The REST corepressor complex also recruits MeCP2, which, in turn, recruits DNA methyl-transferases (39). DNA methylation is an epigenetic mark of relatively stable gene silencing. Global ischemia induced an increase in MeCP2 at the gria2 promoter in CA1, evident at 24 h (Fig. 3D). The time course of MeCP2 association paralleled that of mSin3A but not that of REST or CoREST. Thus, ischemia not only increases expression of REST and assembly of the corepressor complex but regulates dynamic interaction of corepressors within this complex. Because MeCP2 is associated with high methylation of CpG islands, we examined methylation in a region of the gria2 proximal promoter within 300 bp of the RE1. Ischemia did not significantly alter the methylation status of CpG islands at the gria2 promoter, assessed in CA1 at 24 h after ischemia by bisulfite pyrosequencing of a −270- to +25-bp region (Fig. S2). Thus, REST represses gria2 promoter activity via epigenetic modifications of histone but not DNA methylation within the proximal promoter region. These findings do not, however, rule out the possibility of altered methylation status in other regions of the GluA2 gene.
REST–Corepressor Complex Orchestrates Epigenetic Remodeling at the gria2 Promoter in CA1.
CoREST and mSin3A serve as corepressor platforms that recruit HDAC1 and HDAC2, which remove acetyl groups from lysines on core histone proteins, and thereby promote gene repression (16, 40). Whereas acetylation of lysines 9 and 14 on histone 3 (H3K9/14ac) is an epigenetic mark of open chromatin and active gene transcription, deacetylation of lysines 9 and 14 is a mark of gene repression (16, 40). To evaluate HDAC activity, we examined the acetylation status of H3K9 and H3K14 associated with the gria2 promoter in ischemic vs. control CA1. Ischemia induced a marked decrease in H3K9/14ac, evident at 12, 24, and 48 h (Fig. 3E). These findings are consistent with a model whereby REST acts, at least in part, via HDAC activity to repress GluA2 expression in insulted neurons.
The REST–corepressor complex also recruits the histone methyltransferase G9a (23), which promotes site-specific monomethylation and dimethylation of lysine 9 on histone 3 (H3K9me1 and H3K9me2, respectively), and LSD1 (24), which removes methyl residues from lysine 4 on histone 3 (H3K4me1 and H3K4me2). Whereas dimethylation of H3K4 is an epigenetic mark of open chromatin and active gene transcription, dimethylation of H3K9 is a mark of gene repression (40, 41). To evaluate G9a and LSD1 activity, we examined the methylation status of H3K4 and H3K9 in ischemic vs. control CA1. In control CA1, the level of H3K4me2 was high, whereas H3K9me2 was low, an epigenetic signature of active gene transcription. Ischemia induced a marked enrichment in H3K9me2 (Fig. 3F), with no significant change in H3K4me2 (Fig. 3G), an epigenetic signature of gene repression, in CA1 at 12 h and 24 h after insult; by 48 h, H3K4me2 abundance was modestly enhanced.
Dimethylation can occur only on lysine residues that are deacetylated (42). To address whether acetylation and methylation occur in the same population of histone 3 proteins, we performed double-ChIP experiments on microdissected CA1 at times after ischemia. Loci with increased H3K9/14ac exhibited high H3K4me2 but little or no H3K9me2 (Fig. S3), consistent with the notion that ischemia promotes deacetylation and dimethylation of H3K4 in the same population of histone 3 proteins. Together, these findings indicate that the REST complex is active in insulted CA1 and document epigenetic remodeling of the REST target GluA2 in neurons destined to die.
HDAC Inhibitor TSA Affords Neuroprotection.
The findings thus far demonstrate that assembly of the REST complex triggers epigenetic modifications in neurons destined to die but do not establish that remodeling leads to neuronal death. To address this issue, we performed two experiments. First, we examined the impact of the broad-spectrum HDAC inhibitor TSA on survival of insulted CA1 neurons in vitro. OGD in organotypically cultured hippocampal slices is a well-established in vitro model of global ischemia. OGD (30 min) elicited delayed, selective death of CA1 and, to a lesser extent, dentate gyrus neurons, as assessed by uptake of the DNA-indicator dye propidium iodide (Fig. 4A). TSA (0.5 μM applied immediately after ischemia) markedly reduced ischemia-induced neuronal death (Fig. 4 A and B). Second, we examined the impact of TSA on neuronal survival in an in vivo model of ischemic stroke. Global ischemia induced extensive neurodegeneration in the CA1 pyramidal cell layer, as assessed by Fluoro-Jade staining, an indicator of cells undergoing neurodegeneration, at 6 d after surgery (Fig. 4 C and D). TSA (1 mg/kg administered i.p. at 0 h after ischemia) did not detectably affect neurons in sham-operated animals but afforded marked protection of CA1 neurons in postischemic animals (Fig. 4 C and D). These findings indicate a requirement for HDAC activity in neuronal death but do not preclude a role for off-target activity at nonhistone proteins.
Fig. 4.
HDAC inhibitor TSA affords protection of CA1 neurons against ischemia-induced cell death in vitro and in vivo. (A) Fluorescence images show uptake of propidium iodide, an indicator of cell death, in organotypically cultured hippocampal slices subjected to normoxia (control), OGD, or OGD + TSA (0.5 μM, applied immediately after insult) at 72 h after insult. TSA reduces death of CA1 neurons subjected to OGD. (B) Summary data (n = 12 slices in 4 animals). (C) Fluorescence images show Fluoro-Jade (FJ) staining, an indicator of cells undergoing neurodegeneration, of brain sections from animals subjected to sham operation (first column), to global ischemia (second column), to ischemia followed by TSA (third column), or by saline injection (fourth column). TSA (1 mg/kg) administered i.p. to animals immediately after ischemia afforded substantial protection of CA1 neurons. (D) Summary data (n = 8–12 sections in 4–6 animals). The statistical analysis was assessed by the two-tailed Student t test. *P < 0.05; **P < 0.01; n.s., nonsignificant. so, stratum oriens; sp, stratum pyramidale; sr, stratum radiatum.
RNAi-Mediated Depletion of REST Rescues CA1 Neurons from Postischemic Death.
To establish a causal relation between REST-dependent epigenetic remodeling and neuronal death in a clinically relevant model, we delivered lentivirus carrying short interfering REST (siREST) to REST unilaterally into the hippocampus; 14 d later, we subjected rats to global ischemia (Fig. 5A). The lentivirus expression system allows for stable, long-lasting expression of engineered siRNA sequences, which are processed in vivo, and is a useful method for delivery of DNA and RNA to postmitotic mammalian cells, such as neurons, with an exceedingly low incidence of toxicity (43, 44). We designed three siRNA sequences directed to REST (Fig. 5A). To validate their specificity and effectiveness, we performed several control experiments. First, we documented the ability of these siRNA constructs targeting distinct sequences in rat REST (rREST) to silence REST in HEK293 cells. We identified two siRNA constructs that were effective and one that was not (Fig. 5B). Second, neither nontargeting (NT) siRNA nor scrambled siRNA detectably altered REST expression in control conditions (Fig. 5B). Third, we examined the impact of rREST siRNA on expression of human REST (hREST), which differs from rREST in the region targeted by REST siRNA-2. Whereas REST siRNA-2 knocked down rREST, hREST rescued REST expression (Fig. S4 A and B). Fourth, we examined the impact of REST siRNA-2 on the REST corepressors mSinA and CoREST. REST siRNA did not detectably alter CoREST or mSin3A expression in HEK293 cells or CA1 neurons in vivo (Fig. S4C). These findings document target specificity of the siRNA. Fifth, we monitored the impact of REST siRNA on the interferon (IFN) response, which would promote off-target effects. REST siRNA did not detectably alter expression of the IFN-responsive gene 2′,5′-oligoadenylate synthetase-1 (AS1) in CA1 (Fig. S4D).
Fig. 5.
REST is critical to ischemia-induced neuronal death. (A) Target constructs (Upper) and time line (Lower). (B) Representative Western blots probed for REST, EGFP, and β-actin. On this and subsequent figures, GFP is used as the abbreviation for EGFP. (C) EGFP (green), Nissl (red), and DAPI (blue) stains are expressed in brain sections at the level of the dorsal hippocampus from rats that were unilaterally transduced in the right hippocampus with REST siRNA-2 or NT siRNA and, 14 d later, subjected to global ischemia. ipsi, ipsilateral; contra, contralateral. (D) Fluoro-Jade (FJ) label of rats injected with siRNA (Upper) and REST siRNA (Lower) 6 d after ischemia. (E and F) Summary of data in D (n = 12 animals per group). In F, Fluoro-Jade–positive cells for the ipsilateral (I) and contralateral (C) sides of the same animal are shown. Error bars represent the mean ± SEM. Statistical significance was assessed by the Student t test. ***P < 0.001; n.s., nonsignificant. so, stratum oriens; sp, stratum pyramidale; sr, stratum radiatum.
Lentiviral expression was robust in the ipsilateral hippocampus at 14 d after transduction, as assessed by EGFP fluorescence (Fig. S5). In NT-siRNA–injected rats, ischemia induced bilateral neuronal loss in the CA1 pyramidal layer, as assessed by Nissl staining at 6 d after ischemia (Fig. 5C). REST siRNA fluorescence was prominent within the CA1 pyramidal layer and the dentate gyrus, as assessed by EGFP fluorescence (Fig. 5C and Fig. S6). REST siRNA promoted striking protection in the ipsilateral CA1, as assessed by Nissl staining (Fig. 5C and Fig. S6). The area showing protection (Fig. 5C, row 3) appeared greater than that expressing siRNA (Fig. 5C, row 2), consistent with a possible “bystander effect,” in which cells not expressing siRNA are protected indirectly by contact with neighboring cells expressing siRNA (Fig. S6) (45). DAPI staining revealed an intact CA1 in both hemispheres (Fig. 5C and Fig. S6). Complementary results were observed with Fluoro-Jade staining (Fig. 5 D–F). In NT-siRNA–injected rats, Fluoro-Jade staining was prominent in CA1 in both hemispheres (Fig. 5D, rows 1 and 2). REST siRNA afforded protection in the ipsilateral CA1, as demonstrated by reduced Fluoro-Jade staining (Fig. 5D, rows 3–5). The dentate gyrus, which is resistant to ischemic cell death, exhibited striking expression of REST siRNA on the ipsilateral side but no neuronal loss in either hemisphere of sham or postischemic animals (not illustrated), indicating that siREST does not itself cause neuronal death. Thus, REST silencing or inhibition rescues CA1 neurons, indicating a causal role for REST in ischemia-induced neuronal death.
RNAi-Mediated Depletion of REST Prevents Epigenetic Remodeling of the GluA2 Promoter.
If REST induces neuronal death via silencing of GluA2, REST siRNA should prevent epigenetic remodeling of the GluA2 gene and GluA2 down-regulation in CA1. We assessed the impact of REST siRNA injected unilaterally into the hippocampus of living rats on REST occupancy and epigenetic marks at the gria2 promoter by ChIP at 24 h after ischemia (Fig. 6A). In NT-siRNA–injected rats, ischemia increased REST and CoREST at the gria2 promoter in CA1 (Fig. 6 B and C). REST siRNA had little or no effect on REST occupancy at the gria2 promoter in CA1 of sham-operated control animals, presumably because of the low rate of REST synthesis or degradation under physiological conditions (Fig. S7), but markedly reduced or reversed enrichment of REST and CoREST at the gria2 promoter in the ipsilateral CA1 of animals subjected to ischemia (Fig. 6 B and C). In NT-siRNA–injected and nontransduced rats, ischemia induced a modest decrease in H3K9/14ac (Fig. 6D) and increase in H3K9me2 (Fig. 6E), marks of gene silencing, at the gria2 promoter in CA1. REST siRNA blunted these changes on the ipsilateral side (Fig. 6 D and E) and completely blocked the decrease in acetylation on the contralateral side. This result might be attributable to some spread of the siRNA to the contralateral side or to biological variability. These findings indicate that epigenetic changes are REST-dependent.
Fig. 6.
REST siRNA attenuates ischemia-induced epigenetic remodeling and GluA2 silencing in CA1. (A) Time line. REST siRNA blunts epigenetic remodeling (B–E) and GluA2 silencing (F–I). REST siRNA attenuates association of REST (B), CoREST (C), H3K9/14ac (D), and H3K9me2 (E) at the gria2 promoter in the ipsilateral CA1, as assessed by ChIP-qPCR at 24 after ischemia (n = 8 animals per group). REST siRNA blunts the ischemia-induced increase in REST (F) and decrease in GluA2 (G) mRNA expression in CA1, as assessed by qPCR at 48 h after ischemia. REST-siRNA blunts the increase in REST protein (H) and decrease in GluA2 protein (I) in CA1 at 48 h after ischemia (n = 3–4 animals per group). Values for the ipsilateral and contralateral CA1 from REST- and NT siRNA–transduced animals at times after ischemia were compared with those for corresponding samples from control (nontransduced, sham-operated) animals. Statistical significance was assessed by the Student t test. *P < 0.05; **P < 0.01; n.s., nonsignificant. I, ipsilateral; C, contralateral.
Finally, we assessed the impact of REST siRNA on GluA2 expression in CA1. In NT-siRNA rats, ischemia induced a marked increase in REST and decrease in GluA2 mRNAs in CA1, as assessed by qPCR assay (Fig. 6 F and G). REST siRNA attenuated the increase in REST and decrease in GluA2 mRNA in the ipsilateral CA1 (Fig. 6 F and G). In NT-siRNA–injected rats, ischemia markedly increased REST and decreased GluA2 protein in CA1 (Fig. 6 H and I). REST siRNA had little or no effect on basal GluA2 protein expression in CA1 of sham-operated control animals, presumably because of the low occupancy by REST and CoREST at the gria2 promoter under physiological conditions (Fig. S7), but markedly attenuated the increase in REST and decrease in GluA2 in CA1 of animals subjected to ischemia (Fig. 6 H and I). These findings indicate a causal link between REST activation and GluA2 silencing in postischemic CA1.
dnREST Promotes Survival of Insulted CA1 Neurons.
To examine a possible causal relation between REST and ischemia-induced neuronal death further, we assessed the impact of dnREST on ischemia-induced epigenetic remodeling, gene silencing, and neuronal death. dnREST has the main DNA binding domain of full-length REST but lacks the N-terminal and C-terminal repressor domains (Fig. 7A) (46). Lentiviral vector expressing myc-tagged dnREST and EGFP afforded efficient transduction in HEK-293 cells and in neurons in vitro (Fig. 7B). We injected lentivirus expressing dnREST and EGFP or EGFP alone unilaterally into the right hippocampus of rats; 14 d later, we subjected the rats to bilateral global ischemia or sham operation (Fig. 7C). dnREST was prominently expressed on the injected side only, as evidenced by immunolabeling with an anti-myc antibody at 6 d after surgery (Fig. 7C). Within the CA1 pyramidal cell layer, not all cells were immunopositive, indicating that they did not all express dnREST (Fig. 7C). In uninjected rats (not illustrated) or rats expressing EGFP alone, ischemia induced bilateral neurodegeneration in CA1 pyramidal cells, as assessed by Fluoro-Jade label at 6 d after ischemia (Fig. 7 D and E). dnREST dramatically reduced ischemia-induced neuronal death in CA1 pyramidal neurons on the ipsilateral, but not the contralateral, side (Fig. 7 D and E). These findings demonstrate that inhibition of REST promotes survival of neurons destined to die and implicate REST as causally related to the neuronal death associated with ischemic stroke, in confirmation of the REST siRNA data.
Fig. 7.
dnREST suppresses ischemia-induced CA1 death in vivo. (A) (Upper) WT and dnREST constructs. (Lower) Lentiviral vectors encoding EGFP and myc-tagged dnREST. (B) (Left) Representative Western blots were probed for myc to detect REST (Upper) and EGFP (Lower). (Right) Images of hippocampal neurons expressing myc-tagged dnREST (Upper) and EGFP (Lower). (C) Time line. Images show myc-tagged dnREST on the injected side (Left) with little labeling on the contralateral side (Right). (D) Fluoro-Jade (FJ) staining of adjacent sections from rats that had been unilaterally transduced in the right hippocampus with EGFP (Upper) and dnREST (Lower) at 14 d before bilateral global ischemia and killed at 6 d after bilateral global ischemia. dnREST (Lower), but not EGFP alone (Upper), markedly reduced ischemia-induced neuronal death in the ipsilateral CA1. (E) Summary of data in D (n = 10–12 animals per group). dnREST afforded robust neuroprotection of the ipsilateral CA1 and a small degree of protection on the contralateral side. Error bars represent the mean ± SEM. Statistical significance was assessed by the Student t test. *P < 0.05; ***P < 0.001; n.s., nonsignificant. C, contralateral; I, ipsilateral; so, stratum oriens; sp, stratum pyramidale; sr, stratum radiatum.
dnREST Silencing Prevents Epigenetic Remodeling at the GluA2 Promoter.
We reasoned that if REST induces neuronal death via silencing of GluA2 expression, inhibition of REST should prevent ischemia-induced epigenetic remodeling at the GluA2 promoter and GluA2 down-regulation in CA1. We injected lentiviral vector carrying dnREST or EGFP into the hippocampus and subjected rats to ischemia or sham operation 14 d later. We assessed REST occupancy and epigenetic marks over the gria2 promoter at 24 h after surgery (Fig. 8A). In EGFP-injected rats, ischemia induced a marked enrichment in REST (Fig. 8B) and CoREST (Fig. 8C) in the ipsilateral and contralateral CA1. dnREST attenuated the ischemia-induced enrichment of REST (Fig. 8B) and CoREST (Fig. 8C) in the ipsilateral (but not the contralateral) CA1. This finding demonstrates that the presence of CoREST at the RE1 sites is attributable to its recruitment by endogenous REST. In EGFP-injected rats, ischemia induced a marked reduction in H3K9/14ac (Fig. 8D), an epigenetic mark of gene repression, in CA1 of both hemispheres. dnREST blunted the ischemia-induced decrease in H3K9/14ac in the ipsilateral CA1 and somewhat attenuated the decrease in acetylation in the contralateral CA1 (Fig. 8D). These findings establish a causal link between REST activation and epigenetic remodeling of GluA2 transcription in insulted CA1 neurons.
Fig. 8.
dnREST attenuates ischemia-induced epigenetic remodeling and GluA2 silencing in CA1. (A) Time line. dnREST blunts epigenetic remodeling (B–D) and GluA2 silencing (E) in the ipsilateral CA1, assessed by ChIP-qPCR at 24 h after ischemia. dnREST attenuates the ischemia-induced increase in REST (B) and CoREST (C) and decrease in H3K9/14ac (D) association with the gria2 promoter in the ipsilateral CA1, with little or no change in the contralateral CA1. EGFP did not significantly alter REST or CoREST abundance or epigenetic marks (n = 8 animals per group). (E) dnREST blunts the ischemia-induced decrease in GluA2 mRNA (Upper) and protein (Lower) expression in the ipsilateral (but not the contralateral) CA1 (n = 3–4 animals). Values for the ipsilateral and contralateral CA1 from dnREST- and EGFP-transduced animals at times after ischemia were compared with corresponding values for control (nontransduced, sham-operated) animals. Statistical significance was assessed by the Student t test. *P < 0.05; n.s., nonsignificant.
Finally, we assessed the impact of dnREST on GluA2 mRNA and protein expression in CA1 at 48 h after insult (Fig. 8E). In EGFP-injected rats, ischemia induced a marked decrease in GluA2 mRNA on both the ipsilateral and contralateral sides. dnREST attenuated the decrease in GluA2 mRNA on the ipsilateral side, with less effect on the contralateral side. GluA2 protein levels were higher after ischemia on the side injected with dnREST compared with the contralateral side and compared with both sides expressing EGFP. Similar results were obtained in animals injected with REST-VP16, which also acts in a dn manner. REST-VP16 depressed ischemia-induced neuronal death and attenuated epigenetic remodeling of the gria2 promoter in CA1 in vivo (Figs. S8 and S9). Collectively, these findings provide compelling evidence for a causal link between REST activation and gria2 silencing in CA1 of postischemic animals.
Discussion
Dysregulation of the transcriptional repressor REST is important in a broad range of diseases, including cancer, diabetes, and heart disease (3). Using a targeted ChIP-on-chip analysis containing nearly all known, functionally validated targets of REST and tissue samples from animals subjected to global ischemia in vivo, we show that ischemia triggers activation of not only REST but corepressors. The REST–corepressor complex binds and orchestrates epigenetic remodeling of a subset of transcriptionally responsive target genes (gria2, grin1, chrnb2, nefh, nfκb2, trpv1, chrm4, and syt6), of which the AMPAR gene GluA2 is a top hit. Targets in CA1 with enriched REST exhibited decreased mRNA and protein expression. We further show that REST and corepressors (CoREST, mSin3A, HDAC1 and HDAC2, G9a, and MeCP2) are recruited to the gria2 promoter, where they orchestrate epigenetic remodeling and gene silencing in insulted hippocampal neurons. A single, acute injection of TSA, an inhibitor of key components of the REST–corepressor complex, HDAC1 and HDAC2, administered to animals after an ischemic episode ameliorates neuronal injury. This finding has important clinical implications and suggests that HDAC inhibitors may be a promising avenue for intervention in the neurodegeneration associated with ischemic stroke. RNAi-mediated depletion of REST (“silencing the silencer”) or lentiviral-mediated delivery of dnREST into the hippocampus before ischemia prevented epigenetic modifications, rescued GluA2 expression, and ameliorated hippocampal injury. These findings advance previous studies in that they show that other genes, in addition to gria2, essential for synaptic function (e.g., grin1, trpv1, nfκb2) are targets of REST in postischemic neurons and document a causal role for REST-dependent epigenetic remodeling in neuronal death in a clinically relevant model of global ischemia in vivo.
This broad-spectrum REST binding study is unique in that it examines REST binding in the hippocampal CA1 from animals subjected to ischemia in vivo. Although an unbiased, genome-wide study of genes enriched for REST has yet to be performed, in the present study, targeting ChIP-on-chip profiling and bioinformatics analysis, reveals a set of REST targets that exhibit profound alterations in response to ischemic insults. Several of these hits are genes implicated in excitotoxic cell death but not yet identified as therapeutic targets in stroke. Of 13 target genes enriched for REST at 24 h and 48 h after ischemia (Table 1), 6 are implicated in ischemia-induced neuronal death (gria2, chrnb2, grin1, nppa, scg2, and syp); 2 are implicated in neuronal death but not in ischemia (nfkb2 and nefh); 1 is implicated in cell death but not in neuronal death (fdxr); and 4 are not yet linked to any form of cell death (peg12/frat3, csrnp3, slc22a12, and slc22a13). Of note, REST was enriched at the promoters of genes encoding the AMPAR subunit GluA2 (gria2), the NMDAR subunit GluN1 (grin1), and the TRPV1 channel (trpv1). Whereas NMDARs and GluA2-lacking AMPARs mediate toxic Ca2+ entry into neurons, TRPV1 promotes excitability of pyramidal neurons. Excessive activation of the TRPV1 channel is thought to contribute to dysregulation of neural circuits during epileptic activity (47). We predict that REST-dependent silencing of TRPV1, by analogy to silencing of the μ-opioid receptor (12), leads to enhanced inhibitory synaptic input to selectively vulnerable CA1 pyramidal neurons, and thus represents a failed attempt to promote survival of pyramidal neurons.
It is noteworthy that the transcriptionally responsive REST targets identified in our study differ from target genes identified by unbiased, genome-wide approaches, such as, for example, ChIP serial analysis of chromatin occupancy in a mouse kidney cell line (32), large-scale ChIP-seq in Jurkat cells (30), and ChIP-on-chip in mouse neural stem cells (48) and parietal cortex tissue from postmortem Huntington disease brain (15). In the present study, of 13 target genes enriched for REST at 24 h and 48 h after ischemia, 10 overlap with targets identified in at least one other study (Table 1). Six genes (gria2, chrnb2, nppa, scg2, nefh, and syp) are targets identified in Jurkat cells (30), 3 genes (slc22a12, slc22a13, and nefh) are targets identified in a mouse kidney cell line (32), but only 1 gene (nefh) overlaps with genes identified in both Jurkat (30) and mouse kidney (32) cells. Interestingly, even though it is the only other study to use brain tissue, only 1 gene identified by us as a positive hit at both 24 h and 48 h (chrnb2) and 1 identified at 24 h but not 48 h (chrm4) overlap with positive hits in postmortem Huntington disease brain (15). These findings indicate that under different conditions, in different cell types, and during different stages of development, REST regulates different networks of target genes. Moreover, whereas those studies address REST occupancy under basal conditions, our study focused on genes that undergo dynamic changes in REST occupancy following ischemic insult.
In the present study, we show that REST assembles with CoREST, mSinA, HDACs, G9a, and MeCP2 at the promoter of a representative target gene, gria2, and orchestrates epigenetic remodeling of target genes. The REST–corepressor complex confers site-specific epigenetic marks of gene repression (deacetylation and methylation) to core histone proteins, which drive gene silencing. It is of note that REST assembles with different corepressors to orchestrate epigenetic remodeling and silencing of target genes in different cell types and at different developmental stages. Whereas REST is abundant in ES cells, in neural progenitors, REST is maintained at low levels by ubiquitin-based proteasomal degradation, consistent with a chromatin status poised for gene activation (8). As neural progenitors differentiate into neurons, REST and its corepressors depart the RE1 site of selected neuronal genes, triggering transcriptional activation. In newborn neurons, the level of expression of REST targets is adjusted further by CoREST–MeCP2 repressor complexes, which remain bound after REST (8, 49). A recent study suggests that MeCP2 localizes to nearly every nucleosome and, as such, may not bind preferentially to individual genes (50). Although not addressed by the present study, MeCP2 may be widespread throughout chromatin in the brain.
Bioinformatics analysis predicts nearly 2,000 REST target genes within the mammalian genomes (51) and in immortalized Jurkat cells (31). What then determines the specificity of interaction between REST and target genes? An attractive scenario is that on transition from neural progenitors to newborn neurons, genes critical to elaboration of the neuronal phenotype acquire epigenetic marks that maintain them in a state of stable activation (52). An example is trimethylation of core histone 3 at lysine 36 (H3K36me3). Such marks might serve to oppose REST-dependent gene silencing. Another possibility is that other DNA binding proteins may influence recruitment and/or stabilization of REST at promoters of target genes. Polycomb group proteins serve as global enforcers of epigenetically repressed states in an array of cell types, including neurons (53). Recent studies indicate that polycomb repressive complex 2 (PRC2) is recruited to RE1-containing genes by REST via the noncoding RNA HOTAIR (54) and that PRC1 interacts with REST at RE1 sites (55). Moreover, polycomb proteins are activated and afford neuroprotection in the setting of ischemic preconditioning (56).
In summary, findings in the present study demonstrate activation of the REST corepressor complex and REST-dependent epigenetic remodeling of a subset of transcriptionally responsive target genes, of which the AMPAR gene gria2 is a top hit. We further show a causal relation between REST activation and neuronal death in a clinically relevant model of global ischemia in vivo. Whereas epigenetic modifications are known to play a role in brain development and cognition, a role for epigenetics in neurodegenerative disorders has remained unclear (57). Dysregulation of REST and repression of REST target genes are implicated in the pathogenesis of epilepsy (13); Huntington disease (14, 15); Down syndrome (58); medulloblastoma (59); and, more recently, SMCX-associated X-linked mental retardation (60). Findings in the present study add ischemic stroke to the growing list of diseases involving dysregulation of REST and have broad implications for our understanding of the molecular mechanisms underlying neurodegenerative disorders and diseases.
Materials and Methods
Detailed methods can be found in SI Materials and Methods.
Animals and Global Ischemia.
Male Sprague–Dawley rats (150–200 g; Charles River Laboratories) were subjected to transient global ischemia or sham operation by four-vessel occlusion as described (11, 61).
ChIP-on-Chip Analysis and Single-Locus ChIP Assays.
For ChIP-on-chip experiments, the CA1 subfield was microdissected and cross-linked adducts were sonicated to shear chromosomal DNA to a size of ∼300 bp. Samples were subjected to immunoprecipitation with an antibody directed against REST (anti-REST; Upstate Biotechnology), and immunocomplexes were collected on magnetic beads. ChIP output of anti-REST–precipitated chromatin and total chromatin were subjected to whole-genome amplification and labeled with different fluorophores. For single-locus ChIP-qPCR assays, ChIP analysis was performed with the same antibodies as above, followed by qPCR assay.
In Vivo Delivery of Viral Constructs.
REST siRNA-2, siRNA-3, NT siRNA, dnREST, or EGFP was delivered into the hippocampus of live rats by stereotaxic injection 14 d before global ischemia or sham surgery as described (62). Viral solution (4.0 μL) was injected into the right hippocampus by means of a 10-μL Hamilton syringe with a 34-gauge needle driven by a Quintessential Stereotaxic Injector (Stoelting Company). To monitor the time course of siRNA or dnREST expression, control (sham-operated) rats were killed at indicated times and EGFP fluorescence was assessed in brain sections at the level of the hippocampus.
Histology.
Histological analysis of Nissl- or Fluoro-Jade–stained brain sections was performed at 6 d after ischemia was induced. In brief, coronal sections (30 μm) were cut at the level of the dorsal hippocampus with a cryotome and processed for staining with Nissl, DAPI, or Fluoro-Jade stain. Number of cells per 600 μm length of medial CA1 were counted.
Supplementary Material
Acknowledgments
We thank Dr. David Anderson (Howard Hughes Medical Institute, California Institute of Technolgy, Pasadena, CA) for a generous gift of dnREST and Dr. Sadhan Majumder (MD Anderson Cancer Center, University of Texas, Houston, TX) for a generous gift of REST-VP16. We thank Adrianna Latuszek for technical assistance. This work was supported by National Institutes of Health Grants NS 46742 (to R.S.Z.) and NS 55363 (to M.V.L.B.), a McKnight Foundation Brain Disorders Award (to R.S.Z.), and a generous grant from the F. M. Kirby Foundation (to R.S.Z.). M.V.L.B. is the Sylvia and Robert S. Olnick Professor of Neuroscience and Distinguished Professor of the Albert Einstein College of Medicine. R.S.Z. is the F. M. Kirby Professor in Neural Repair and Protection.
Footnotes
The authors declare no conflict of interest.
See Author Summary on page 5928 (volume 109, number 16).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121568109/-/DCSupplemental.
References
- 1.Roopra A, Huang Y, Dingledine R. Neurological disease: Listening to gene silencers. Mol Interv. 2001;1:219–228. [PubMed] [Google Scholar]
- 2.Ballas N, Mandel G. The many faces of REST oversee epigenetic programming of neuronal genes. Curr Opin Neurobiol. 2005;15:500–506. doi: 10.1016/j.conb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Ooi L, Wood IC. Chromatin crosstalk in development and disease: Lessons from REST. Nat Rev Genet. 2007;8:544–554. doi: 10.1038/nrg2100. [DOI] [PubMed] [Google Scholar]
- 4.Qureshi IA, Mehler MF. Regulation of non-coding RNA networks in the nervous system—What's the REST of the story? Neurosci Lett. 2009;466:73–80. doi: 10.1016/j.neulet.2009.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westbrook TF, et al. SCFbeta-TRCP controls oncogenic transformation and neural differentiation through REST degradation. Nature. 2008;452:370–374. doi: 10.1038/nature06780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guardavaccaro D, et al. Control of chromosome stability by the beta-TrCP-REST-Mad2 axis. Nature. 2008;452:365–369. doi: 10.1038/nature06641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Z, et al. Deubiquitylase HAUSP stabilizes REST and promotes maintenance of neural progenitor cells. Nat Cell Biol. 2011;13:142–152. doi: 10.1038/ncb2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121:645–657. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Chen ZF, Paquette AJ, Anderson DJ. NRSF/REST is required in vivo for repression of multiple neuronal target genes during embryogenesis. Nat Genet. 1998;20:136–142. doi: 10.1038/2431. [DOI] [PubMed] [Google Scholar]
- 10.Paquette AJ, Perez SE, Anderson DJ. Constitutive expression of the neuron-restrictive silencer factor (NRSF)/REST in differentiating neurons disrupts neuronal gene expression and causes axon pathfinding errors in vivo. Proc Natl Acad Sci USA. 2000;97:12318–12323. doi: 10.1073/pnas.97.22.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calderone A, et al. Ischemic insults derepress the gene silencer REST in neurons destined to die. J Neurosci. 2003;23:2112–2121. doi: 10.1523/JNEUROSCI.23-06-02112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Formisano L, et al. Ischemic insults promote epigenetic reprogramming of mu opioid receptor expression in hippocampal neurons. Proc Natl Acad Sci USA. 2007;104:4170–4175. doi: 10.1073/pnas.0611704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palm K, Belluardo N, Metsis M, Timmusk T. Neuronal expression of zinc finger transcription factor REST/NRSF/XBR gene. J Neurosci. 1998;18:1280–1296. doi: 10.1523/JNEUROSCI.18-04-01280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuccato C, et al. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat Genet. 2003;35:76–83. doi: 10.1038/ng1219. [DOI] [PubMed] [Google Scholar]
- 15.Zuccato C, et al. Widespread disruption of repressor element-1 silencing transcription factor/neuron-restrictive silencer factor occupancy at its target genes in Huntington's disease. J Neurosci. 2007;27:6972–6983. doi: 10.1523/JNEUROSCI.4278-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60:961–974. doi: 10.1016/j.neuron.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrés ME, et al. CoREST: A functional corepressor required for regulation of neural-specific gene expression. Proc Natl Acad Sci USA. 1999;96:9873–9878. doi: 10.1073/pnas.96.17.9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballas N, et al. Regulation of neuronal traits by a novel transcriptional complex. Neuron. 2001;31:353–365. doi: 10.1016/s0896-6273(01)00371-3. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Myers SJ, Dingledine R. Transcriptional repression by REST: Recruitment of Sin3A and histone deacetylase to neuronal genes. Nat Neurosci. 1999;2:867–872. doi: 10.1038/13165. [DOI] [PubMed] [Google Scholar]
- 20.Naruse Y, Aoki T, Kojima T, Mori N. Neural restrictive silencer factor recruits mSin3 and histone deacetylase complex to repress neuron-specific target genes. Proc Natl Acad Sci USA. 1999;96:13691–13696. doi: 10.1073/pnas.96.24.13691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimes JA, et al. The co-repressor mSin3A is a functional component of the REST-CoREST repressor complex. J Biol Chem. 2000;275:9461–9467. doi: 10.1074/jbc.275.13.9461. [DOI] [PubMed] [Google Scholar]
- 22.Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 23.Roopra A, Qazi R, Schoenike B, Daley TJ, Morrison JF. Localized domains of G9a-mediated histone methylation are required for silencing of neuronal genes. Mol Cell. 2004;14:727–738. doi: 10.1016/j.molcel.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Lee MG, Wynder C, Cooch N, Shiekhattar R. An essential role for CoREST in nucleosomal histone 3 lysine 4 demethylation. Nature. 2005;437:432–435. doi: 10.1038/nature04021. [DOI] [PubMed] [Google Scholar]
- 25.Shi YJ. Regulation of LSD1 histone demethylase activity by its associated factors. Mol Cell. 2005;19:1–8. doi: 10.1016/j.molcel.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 26.Lunyak VV, et al. Corepressor-dependent silencing of chromosomal regions encoding neuronal genes. Science. 2002;298:1747–1752. doi: 10.1126/science.1076469. [DOI] [PubMed] [Google Scholar]
- 27.Liou AK, Clark RS, Henshall DC, Yin XM, Chen J. To die or not to die for neurons in ischemia, traumatic brain injury and epilepsy: A review on the stress-activated signaling pathways and apoptotic pathways. Prog Neurobiol. 2003;69:103–142. doi: 10.1016/s0301-0082(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 28.Ofengeim D, Miyawaki T, Zukin RS. In: Stroke: Pathophysiology, Diagnosis and Management. Mohr JP, et al., editors. Philadelphia: Churchill Livingstone Elsevier; 2011. pp. 75–106. [Google Scholar]
- 29.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: Mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson DS, Mortazavi A, Myers RM, Wold B. Genome-wide mapping of in vivo protein-DNA interactions. Science. 2007;316:1497–1502. doi: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
- 31.Mortazavi A, Leeper Thompson EC, Garcia ST, Myers RM, Wold B. Comparative genomics modeling of the NRSF/REST repressor network: From single conserved sites to genome-wide repertoire. Genome Res. 2006;16:1208–1221. doi: 10.1101/gr.4997306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otto SJ, et al. A new binding motif for the transcriptional repressor REST uncovers large gene networks devoted to neuronal functions. J Neurosci. 2007;27:6729–6739. doi: 10.1523/JNEUROSCI.0091-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30:126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 35.Liu S, et al. Expression of Ca(2+)-permeable AMPA receptor channels primes cell death in transient forebrain ischemia. Neuron. 2004;43:43–55. doi: 10.1016/j.neuron.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Noh KM, et al. Blockade of calcium-permeable AMPA receptors protects hippocampal neurons against global ischemia-induced death. Proc Natl Acad Sci USA. 2005;102:12230–12235. doi: 10.1073/pnas.0505408102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oguro K, et al. Knockdown of AMPA receptor GluR2 expression causes delayed neurodegeneration and increases damage by sublethal ischemia in hippocampal CA1 and CA3 neurons. J Neurosci. 1999;19:9218–9227. doi: 10.1523/JNEUROSCI.19-21-09218.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwak S, Weiss JH. Calcium-permeable AMPA channels in neurodegenerative disease and ischemia. Curr Opin Neurobiol. 2006;16:281–287. doi: 10.1016/j.conb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Kimura H, Shiota K. Methyl-CpG-binding protein, MeCP2, is a target molecule for maintenance DNA methyltransferase, Dnmt1. J Biol Chem. 2003;278:4806–4812. doi: 10.1074/jbc.M209923200. [DOI] [PubMed] [Google Scholar]
- 40.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 41.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 42.Rea S, et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406:593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- 43.Naldini L, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 44.Dittgen T, et al. Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc Natl Acad Sci USA. 2004;101:18206–18211. doi: 10.1073/pnas.0407976101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin JH, et al. Gap-junction-mediated propagation and amplification of cell injury. Nat Neurosci. 1998;1:494–500. doi: 10.1038/2210. [DOI] [PubMed] [Google Scholar]
- 46.Tapia-Ramírez J, Eggen BJ, Peral-Rubio MJ, Toledo-Aral JJ, Mandel G. A single zinc finger motif in the silencing factor REST represses the neural-specific type II sodium channel promoter. Proc Natl Acad Sci USA. 1997;94:1177–1182. doi: 10.1073/pnas.94.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron. 2008;57:746–759. doi: 10.1016/j.neuron.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abrajano JJ, et al. Corepressor for element-1-silencing transcription factor preferentially mediates gene networks underlying neural stem cell fate decisions. Proc Natl Acad Sci USA. 2010;107:16685–16690. doi: 10.1073/pnas.0906917107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mandel G, et al. Repressor element 1 silencing transcription factor (REST) controls radial migration and temporal neuronal specification during neocortical development. Proc Natl Acad Sci USA. 2011;108:16789–16794. doi: 10.1073/pnas.1113486108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thambirajah AA, et al. MeCP2 binds to nucleosome free (linker DNA) regions and to H3K9/H3K27 methylated nucleosomes in the brain. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruce AW, et al. Genome-wide analysis of repressor element 1 silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) target genes. Proc Natl Acad Sci USA. 2004;101:10458–10463. doi: 10.1073/pnas.0401827101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vakoc CR, Sachdeva MM, Wang H, Blobel GA. Profile of histone lysine methylation across transcribed mammalian chromatin. Mol Cell Biol. 2006;26:9185–9195. doi: 10.1128/MCB.01529-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zukin RS. Eradicating the mediators of neuronal death with a fine-tooth comb. Sci Signal. 2010;3:pe20. doi: 10.1126/scisignal.3125pe20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsai MC, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ren X, Kerppola TK. REST interacts with Cbx proteins and regulates polycomb repressive complex 1 occupancy at RE1 elements . Mol Cell Biol. 2011;31:2100–2110. doi: 10.1128/MCB.05088-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stapels M, et al. Polycomb group proteins as epigenetic mediators of neuroprotection in ischemic tolerance. Sci Signal. 2010;3:ra15. doi: 10.1126/scisignal.2000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abel T, Zukin RS. Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr Opin Pharmacol. 2008;8:57–64. doi: 10.1016/j.coph.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bahn S, et al. Neuronal target genes of the neuron-restrictive silencer factor in neurospheres derived from fetuses with Down's syndrome: A gene expression study. Lancet. 2002;359:310–315. doi: 10.1016/S0140-6736(02)07497-4. [DOI] [PubMed] [Google Scholar]
- 59.Lawinger P, et al. The neuronal repressor REST/NRSF is an essential regulator in medulloblastoma cells. Nat Med. 2000;6:826–831. doi: 10.1038/77565. [DOI] [PubMed] [Google Scholar]
- 60.Tahiliani M, et al. The histone H3K4 demethylase SMCX links REST target genes to X-linked mental retardation. Nature. 2007;447:601–605. doi: 10.1038/nature05823. [DOI] [PubMed] [Google Scholar]
- 61.Calderone A, et al. Late calcium EDTA rescues hippocampal CA1 neurons from global ischemia-induced death. J Neurosci. 2004;24:9903–9913. doi: 10.1523/JNEUROSCI.1713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miyawaki T, et al. The endogenous inhibitor of Akt, CTMP, is critical to ischemia-induced neuronal death. Nat Neurosci. 2009;12:618–626. doi: 10.1038/nn.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]