Abstract
How the functional activity of the brain is altered during aging to cause age-related memory impairments is unknown. We used functional cellular imaging to monitor two different calcium-based memory traces that underlie olfactory classical conditioning in young and aged Drosophila. Functional imaging of neural activity in the processes of the dorsal paired medial (DPM) and mushroom body neurons revealed that the capacity to form an intermediate-term memory (ITM) trace in the DPM neurons after learning is lost with age, whereas the capacity to form a short-term memory trace in the α′/β′ mushroom body neurons remains unaffected by age. Stimulation of the DPM neurons by activation of a temperature-sensitive cation channel between acquisition and retrieval enhanced ITM in aged but not young flies. These data indicate that the functional state of the DPM neurons is selectively altered with age to cause an age-related impairment of ITM, and demonstrate that altering the excitability of DPM neurons can restore age-related memory impairments.
Memory impairments that occur with age decrease the quality of life for elderly subjects (1). The forms of memory impaired, the brain regions most affected, and the neurophysiological processes that become impaired with age remain as issues without complete answers. Functional imaging studies of the aged human brain recently revealed a reduction in the coordinated activity between different brain regions that is associated with poor performance in several cognitive domains (2). Neural activity also becomes less localized in some regions of the aged brain, particularly the prefrontal cortex, during the performance of executive level tasks (3, 4). These studies have supported the possibility that altered functional activation of brain regions and their coordination during aging may cause, in part, age-related memory impairment. However, this broad and somewhat obvious possibility lacks the concrete information necessary to gain real traction for the problem of age-related memory impairment.
Age-related memory impairment occurs in many and perhaps all higher organisms, including Caenorhabditis elegans, Drosophila, the mouse, and humans (1). A major limitation in performing studies of age-related memory impairment is the long lifespan of many animal models. However, Drosophila has a relative short lifespan and is highly suited for genetic and behavioral analyses of aged individuals. Furthermore, genetic and gene expression analyses have revealed strong conservation of “age-related” genes (5).
Olfactory memory is the most widely studied form of memory exhibited by Drosophila. Classical aversive olfactory conditioning involves pairing an odor conditioned stimulus (CS) with electric foot shocks as the unconditioned stimulus. One conditioning cycle (1×) produces short-term memory (STM) that persists for ∼30 min and intermediate-term memory (ITM) that persists between 30 min and several hours after conditioning. All performance gains obtained after one cycle of conditioning decay to near zero within 24 h after training (6). Similar to vertebrate memory, STM and earlier phases of ITM are labile and can be disrupted with anesthesia (cold shock for Drosophila), whereas the later phases of ITM are consolidated into an anesthesia-resistant form of memory (7). Anesthesia-resistant memory (ARM) can also persist more than 24 h as a long-term form of memory that is independent of protein synthesis when generated by five to 10 training cycles with no rest interval between the training trials (i.e., massed conditioning). A protein synthesis-dependent form of long-term memory (LTM) persisting for 4 to 7 d is generated by five to 10 training cycles with rest intervals between the training trials (i.e., spaced training) (7, 8).
Functional imaging approaches have recently been applied in Drosophila for discovering and visualizing cellular memory traces, which are defined as the physiologic changes that occur in neurons as a result of learning (9). Several memory traces discovered so far form in the olfactory nervous system after olfactory conditioning using transgenic flies that express G-CaMP, an EGFP molecule that provides a visual assay for calcium influx. The earliest memory traces that may underlie STM include traces that form in the antennal lobe projection neurons, the GABAergic APL neuron, and the α′/β′ mushroom body (MB) neurons (10–12). ITM is associated with a memory trace that forms in dorsal paired medial (DPM) neurons (13). LTM generated by spaced conditioning may be underlain by at least two memory traces: one that forms in the α/β MB neurons and exists between 9 and 24 h after conditioning (8), and one that forms in the MB γ-neurons and exists between 18 and 48 h after conditioning (14).
The cellular memory trace that forms in the DPM neurons is of particular interest. DPM neurons have no obvious dendritic elements but have two major branches of their single neurite that innervate the horizontal and vertical lobes of the MB (13, 15), suggesting that DPM neurons may participate in reciprocal interactions with MB neurons to support olfactory memory (13). Behavior studies have shown that blocking neurotransmission from DPM neurons after training but before testing impairs ITM (15, 16), indicating that these neurons exhibit postconditioning activity and that this activity is required for the formation of normal levels of ITM (9, 15, 16). Our functional imaging studies established that a single aversive conditioning trial produces an ITM trace, detected as an increased influx of calcium in response to the learned odor, that forms in these neurons between 15 and 30 min after conditioning and persists to approximately 1 h (9, 13). This trace fails to form in amnesiac (amn) mutants that acquire odor memories at the same rate as normal flies but have an ephemeral ITM (13, 17). The amn gene is hypothesized to encode a putative neuropeptide that is preferentially expressed and required in the DPM neurons for normal ITM (15, 18). Thus, the amn mutants have been considered to be impaired in ITM, with the DPM neuron trace supporting memory across this time window (9).
The background set by progress from functional cellular imaging studies offers a unique opportunity to probe the effects that aging has on behavioral memory and the neurophysiological fingerprints of memory, revealed as the aforementioned cellular memory traces. Here, we combine behavioral experiments to describe the effects of aging on the various temporal forms of memory with functional cellular imaging to examine the status of the cellular memory traces across age, and with newly developed genetic tools to alter the activity state of the neurons that form memory traces. Our behavioral experiments show that ITM decreases as a function of age, confirming and extending the conclusion of Tamura and colleagues (19). Functional imaging experiments show that the ITM trace that normally forms in DPM neurons degrades with age, whereas an STM trace remains unaffected. Furthermore, we show that increasing the excitability of DPM neurons with artificial stimulation between training and testing improves ITM in aged but not young flies. Overall, these data indicate that the ITM trace and functional state of the DPM neurons is particularly vulnerable to the adverse effects of age.
Results
Behavioral Memory as a Function of Age.
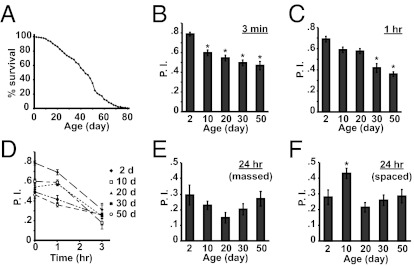
We assayed memory after a single cycle of olfactory classical conditioning at 3 min, 1 h, and 3 h in flies of several different age groups. Such conditioning produces STM measured at 3 min and ITM measured at 1 h after conditioning. Under our rearing conditions, the average lifespan of normal flies was 45 d after eclosion (Fig. 1A). Flies 2, 10, 20, 30, and 50 d of age were used for behavioral experiments. The behavior analysis demonstrated that (i) STM was impaired statistically in flies of 10 d of age and older (Fig. 1B), (ii) 1-h memory was severely impaired in flies older than 30 d of age (Fig. 1C), and (iii) 3-h performance scores were unaffected by age (Fig. 1D). LTM generated by massed conditioning and measured at 24 h was unaltered across the age groups examined (Fig. 1E). The 24-h performance score after spaced conditioning for flies 10 d of age was significantly higher than that for flies 2 d or 20 d of age (Fig. 1F), suggesting that protein synthesis-dependent LTM remains immature in adult flies within 2 d after eclosion and requires a few days of adult life to develop competence to form. Despite this maturation, protein synthesis-dependent LTM deteriorates between 10 and 20 d of age.
Fig. 1.
Age-related changes in memory expression. (A) Survival curve of w(CS10) obtained from 600 flies. The w(CS10) flies carry the white-eyed mutation in a Canton-S genetic background. The average lifespan was 45 d. (B and C) The 3-min (B) and 1-h (C) performance indices (P.I.) after 1× paired conditioning of w(CS10) flies at 2, 10, 20, 30, and 50 d of age. Asterisks denote significant differences from the performance index at 2 d of age (*P < 0.0001, Tukey post hoc following significant differences after one-way ANOVA; n ≥ 12 for all groups). (D) Memory retention across age. The retention curves were generated from 3 min, 1 h, and 3 h performance indices after 1× paired conditioning of w(CS10) flies at 2, 10, 20, 30, and 50 d of age. (E and F) Age-related changes in 24-h memory after massed (E) or spaced conditioning (F). There was no significant difference among the age groups at 24 h after massed conditioning (Tukey post hoc following significant differences after one-way ANOVA; n ≥ 6). Significant differences were observed in 24 h performance indices after spaced conditioning between 2 and 10 d of age (P = 0.040) and between 10 and 20 d of age (P = 0.001, Tukey post hoc following significant differences after one-way ANOVA; n = 12; *P < 0.05). All error bars indicate SEM.
Odor Avoidance and Shock Reactivity Is Impaired in Aged Flies.
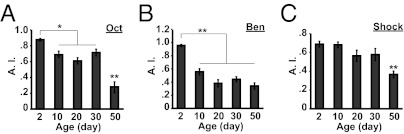
Impairments in performance can be observed after olfactory conditioning if the flies used are defective in task-related skills, including the perception and avoidance of odor or electric shock stimuli used for conditioning. We measured avoidance for the odors used for conditioning [3-octanol (Oct) and benzaldehyde (Ben)] and shock reactivity in flies 2, 10, 20, 30, and 50 d of age. Avoidance of air laced with Oct or Ben decreased significantly between 2 and 10 d of age, with no further decrements between 10 d and 30 d of age (Fig. 2 A and B). Flies 50 d of age showed a severe impairment in avoidance of Oct but not Ben compared with flies 30 d of age (Fig. 2 A and B). Shock reactivity was not significantly altered across the first 30 d of age; 50-d-old flies exhibited a severe impairment in shock reactivity (Fig. 2C). These results, along with the conditioning data shown earlier, present the possibility that the decrease in 3-min memory at 10 d of age might result from an age-related impairment in odor perception. To study this further, we measured 3-min memory in 2- and 10-d-old flies by using a high concentration of odors that both age groups avoid equally. Under these conditions, the performance of 10-d-old flies was still poorer than that of 2-d-old flies (Fig. S1). In addition, an earlier study showed that acquisition is slightly reduced in 10-d-old flies, but memory stability is comparable to that in very young flies (19). The combined data indicate that the poor performance in 3-min memory for flies of 10 d of age is caused by a defect in STM acquisition rather than odor perception and avoidance. The impairment in 1-h memory in flies 30 d of age cannot be explained by an age-related reduction in task-related skills relative to 10-d-old flies, as odor avoidance and shock reactivity are not significantly different between these two age groups.
Fig. 2.
Aversive olfactory avoidance and shock reactivity in aged flies. (A) Olfactory avoidance indices (A.I.) for Oct at the odor concentration used for training and testing (0.12%) of w(CS10) flies at 2, 10, 20, 30, and 50 d of age. (B) Olfactory avoidance indices for Ben at the odor concentration used for training and testing (0.05%) of w(CS10) flies at 2, 10, 20, 30, and 50 d of age. (C) Shock avoidance at the voltage used for olfactory conditioning (90 V) of w(CS10) flies at 2, 10, 20, 30, and 50 d of age. Data were evaluated via Tukey post hoc following significant differences after one-way ANOVA (n ≥ 10 for all groups; *P < 0.05, **P < 0.0001). All error bars indicate SEM.
ITM Trace That Forms in DPM Neurons Deteriorates with Age.
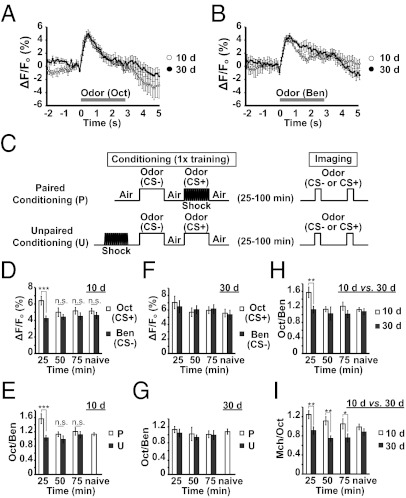
We performed functional cellular imaging to probe the integrity of the DPM neurons and the ITM trace that forms in these neurons in flies of different ages. We selected the DPM neurons for our initial studies because flies 10 d and 30 d of age exhibit a distinct difference in 1-h memory, a time at which the DPM neuron trace is robust. Flies expressing the calcium reporter transgene uas-G-CaMP from the DPM neuron driver c316-Gal4 were prepared for in vivo functional imaging by mounting them stably under a laser-scanning confocal microscope to detect basal fluorescence and the change in fluorescence with the presentation of odor. We collected imaging data across time at the depth in the brain where the dorsal neurite branch of the DPM neurons innervates the vertical lobes of the MBs. The DPM neurons in flies 10 d and 30 d of age responded with a transient and equal response to the presentation of Oct or Ben (Fig. 3 A and B). Our data show that the change in fluorescence is measurable and reproducible with maximum amplitudes typically between 4% and 5% in flies of both ages.
Fig. 3.
DPM neuron ITM trace across age. (A and B) Time course for the percent change in fluorescence (ΔF) relative to baseline (Fo) of G-CaMP in response to Oct (A) or Ben (B), measured in the distal portion of the vertical lobes of the MBs in flies 10 d or 30 d of age. The horizontal axis represents the time after odor presentation. (C) Diagram illustrating two conditioning protocols used for imaging experiments. Flies received paired or unpaired conditioning with 1 min of odor CS+ along with 12 electric shock pulses. After conditioning, each fly was mounted in a pipette tip and prepared for imaging. The calcium response to Oct was assayed first by imaging across a 3-s odor exposure. After a 3-min interval, the calcium response to Ben or Mch was assayed in an identical way. The imaging was performed across 25 to 99 min after conditioning in three time interval groups: 25, 25 to 49 min; 50, 50 to 74 min; 75, 75 to 99 min after conditioning. (D) Calcium responses in the vertical lobes at various times after conditioning using 10-d-old flies trained with Oct. As a control, imaging was performed using flies without conditioning (i.e., naive). A significant increase in %ΔF/Fo in response to CS+ odor (Oct) was detected in DPM neurons at 25 to 49 min after conditioning compared with naive flies [Fisher least significant difference (LSD) following ANOVA, P = 0.044]. The CS+ response for all other time intervals was not significantly different from the naive response (Fisher LSD following ANOVA, P ≥ 0.825). No significant difference was observed in the response magnitudes to the CS− odor across time (Fisher LSD following ANOVA, P ≥ 0.499). Given the constancy in CS− response, we also compared the CS+ response vs. the CS− response within each time interval group. The CS+ response was significantly higher than the CS− response at 25 to 49 min after conditioning; however, no significant difference was observed at 50 min after training and thereafter (Tukey post hoc following significant differences after one-way ANOVA: 25–49 min, P = 0.003; 50–74 min, P = 0.955; 75–99 min, P = 0.955; naive, P = 0.994). Asterisks indicate a statistically significant difference (n = 9–14 for all groups). (E) The ratio of the response to the CS+ odor (Oct) relative to the CS− odor (Ben) in flies receiving paired vs. unpaired conditioning. As the CS− responses were constant across time intervals (D), this allows the CS+ response to be normalized within each fly to the CS− response. The 25- to 49-min time interval showed a marked difference in CS+/CS− response in the paired group vs. unpaired group (Tukey post hoc following significant differences after one-way ANOVA, P = 0.003). No significant difference was observed at 50 min after training and thereafter (Tukey post hoc following significant differences after one-way ANOVA, P ≥ 0.929). Asterisks indicate a statistically significant difference (n = 9–14 for all groups). (F) Calcium responses in the vertical lobes of the MBs at various times after conditioning with 30-d-old flies trained with Oct. As a control, imaging was performed with flies without conditioning (i.e., naive). The CS+ response for all time intervals was not significantly different from the naive response (Tukey post hoc following significant differences after one-way ANOVA, P ≥ 0.817). No significant difference was observed in the response magnitudes to the CS− odor across time (Tukey post hoc following significant differences after one-way ANOVA, P ≥ 0.958). Given the constancy in CS− response, we also compared the CS+ response to the CS− response within each time interval group. No significant difference was observed across time (Tukey post hoc following significant differences after one-way ANOVA, P ≥ 0.998; n = 9–19 for all groups). (G) The ratio of the response to the CS+ odor (Oct) relative to the CS− odor (Ben) in flies receiving paired vs. unpaired conditioning. Because the CS− responses were constant across time intervals (F), this allows the CS+ response to be normalized within each fly to the CS− response. No significant difference in CS+/CS− response was observed with pairing compared with unpaired group across time intervals (Tukey post hoc following significant differences after one-way ANOVA, P ≥ 0.996). Asterisks indicate a statistically significant difference (n = 6–19 for all groups). (H) The ratio of CS+/CS− responses across time interval groups for 10- and 30-d-old flies. The 25- to 49-min time interval showed a robust difference in CS+/CS− response between in 10- and 30-d-old flies (Tukey post hoc following significant differences after one-way ANOVA, P = 0.014). (I) Flies 10 d or 30 d of age were trained with Mch. The ratio in the response to the CS+ odor (Mch) relative to the CS− odor (Oct) is plotted. The 25- to 49-min time interval after conditioning showed a marked increase in CS+/CS− response compared with naive flies in 10-d-old flies (Fisher LSD following ANOVA, P = 0.020), but not in 30-d-old flies (Fisher LSD following ANOVA, P = 0.788). The difference in CS+/CS− response between in 10- and 30-d-old flies was observed at 25 to 49 min (Fisher LSD following ANOVA, P = 0.002), 50 to 74 min (Fisher LSD following ANOVA, P = 0.001), and 75 to 99 min (Fisher LSD following ANOVA, P = 0.020; n = 10–12 for all groups). All error bars indicate SEM.
To measure the DPM ITM trace, groups of flies 10 d or 30 d of age expressing G-CaMP in their DPM neurons were trained with paired or unpaired conditioning and then prepared for functional imaging (Fig. 3C). The imaging was performed between 25 and 100 min after training and divided into three time interval groups: 25 to 49 min, 50 to 74 min, and 75 to 99 min after training (Fig. 3C). There was no significant difference in calcium influx for the CS+ and CS− odors in naive flies of either age group (Fig. 3 D and F). However, we observed a marked increase in calcium response to the CS+ odor in the 25- to 49-min conditioning group of 10- but not 30-d-old flies (Fig. 3 D and F), indicative of an ITM trace registered as increased calcium influx in response to the conditioned odor. As the calcium response to the CS− odor was stable across time after conditioning of both age groups (Fig. 3 D and F), we also normalized the CS+ response to the CS− response within each time interval group. This comparison again showed that the DPM ITM trace is present in 10-d-old flies and absent in 30-d-old flies (Fig. 3H). Moreover, the formation of the DPM ITM trace required paired conditioning of 10-d-old flies, as unpaired conditioning was without effect (Fig. 3 E and G). We also observed a deteriorated DPM ITM trace in 30-d-old flies when using 4-methylcyclohexanol (Mch) as the CS+ and Oct as the CS− (Fig. 3I), although the duration of the trace in 10-d-old flies with Mch used as the CS+ was longer (Fig. 3I) than when Oct was used as the CS+ (Fig. 3H). The differential duration may depend on the odors used for conditioning. Nevertheless, there remains an age dependency in ability to form olfactory memory traces.
STM Trace That Forms in α′/β′ MB Neurons Remains Unaltered with Age.
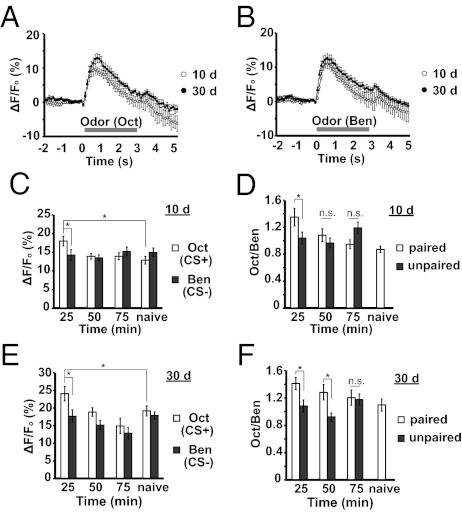
We next examined whether older flies form a STM memory trace in their α′/β′ MB neurons like young flies by using the c305a-gal4 driver along with uas-G-CaMP. The presentation of Oct or Ben elicited a measurable and reproducible calcium influx into the α′/β′ MB neurons of naive flies 10 d and 30 d of age (Fig. 4 A and B). The responses of flies of 30 d of age were slightly increased vs. flies 10 d of age (Fig. 4 A and B). To measure the memory trace that forms in the α′/β′ MB neurons, flies were trained by using paired conditioning or unpaired conditioning, and the posttraining calcium responses to CS+ and CS− odors were measured (Fig. 4 C–F). There was no significant difference in calcium response between the CS+ and CS− odor in naive flies, yet flies both 10 d and 30 d of age exhibited a quantitatively similar increase in calcium response to the CS+ odor at 25 to 49 min after conditioning (Fig. 4 C and E). This increase was observed only during the 25 to 49 min window for 10- and 30-d-old flies and not thereafter (Fig. 4 C and E), and only in flies that received paired and not unpaired conditioning (Fig. 4 D and F). Thus, a memory trace forms in the α′/β′ MB neurons across the 25- to 49-min time window in young and aged flies. Unexpectedly, we observed that the memory trace persisted into the 50- to 74-min time window for the 30-d-old flies when assessed as the ratios of responses between flies receiving paired vs. unpaired conditioning (Fig. 4F). Whether this is a real effect caused by aging or results from the low unpaired ratio for the 50- to 74-min time window compared with unpaired ratios for other time windows is not known.
Fig. 4.
The α′/β′ MB neuron STM memory trace across age. (A and B) Time course for the percent change in fluorescence of G-CaMP in the α′/β′ MB neurons in response to Oct (A) or Ben (B) in flies 10 d and 30 d of age. (C) Calcium responses in the α′/β′ MB neurons at various times after conditioning by using 10-d-old flies trained with Oct. As a control, imaging was performed with flies without conditioning (i.e., naive). A significant increase in %ΔF/Fo in response to CS+ odor (Oct) was detected in the α′/β′ MB neurons at 25 to 49 min after conditioning vs. naive flies. (Fisher LSD following ANOVA, P = 0.010). The CS+ response for all other time intervals was not significantly different from the naive response (Fisher LSD following ANOVA, P ≥ 0.511). No significant difference was observed in the response magnitudes to the CS− odor across time. (Fisher LSD following ANOVA, P ≥ 0.328). Given the constancy in CS− response, we also compared the CS+ response vs. the CS− response within each time interval group. The CS+ response was significantly higher than the CS− response at 25 to 49 min after conditioning; however, no significant difference was observed at 50 min after training and thereafter (Fisher LSD following ANOVA: 25–49 min, P = 0.010; 50–74 min, P = 0.717; 75–99 min, P = 0.422; naive, P = 0.240). Asterisks indicate a statistically significant difference (n = 8–14 for all groups). (D) The ratio of the response to the CS+ odor (Oct) to the CS− odor (Ben). As the CS− responses were constant across time intervals (C), this allows the CS+ response to be normalized within each fly to the CS− response. The 25- to 49-min time interval showed a robust difference in CS+/CS− response with pairing compared with the unpaired group (Fisher LSD following ANOVA, P = 0.022). No significant difference was observed at 50 min after training and thereafter (Fisher LSD following ANOVA: 50–74 min, P = 0.339; 75–99 min, P = 0.074). Asterisks indicate a statistically significant difference (n = 8–14 for all groups). (E) Calcium responses in the α′/β′ MB neurons at various times after conditioning by using 30-d-old flies trained with Oct. As a control, imaging was performed with flies without conditioning (naive). A significant increase in %ΔF/Fo in response to CS+ odor (Oct) was detected in the α′/β′ MB neurons at 25 to 49 min after conditioning compared with naive flies (Fisher LSD following ANOVA, P = 0.043). The CS+ response for all other time intervals was not significantly different from the naïve response (Fisher LSD following ANOVA: 50–74 min, P = 0.863; 75–99 min, P = 0.078). No significant difference was observed in the response magnitudes to the CS− odor across time to 75 min, although the response during the 75- to 99-min time window was decreased (Fisher LSD following ANOVA: 25–49 min, P = 0.928; 50–74 min, P = 0.279; 75–99 min, P = 0.037). We also compared the CS+ response to the CS− response within each time interval group. The CS+ response was significantly higher than the CS− response at 25 to 49 min after conditioning; however, no significant difference was observed at 50 min after training and thereafter (Fisher LSD following ANOVA: 25–49 min, P = 0.007; 50–74 min, P = 0.161; 75–99 min, P = 0.375; naive, P = 0.578). Asterisks indicate a statistically significant difference (n = 8–10 for all groups). (F) The ratio of the response to the CS+ odor (Oct) relative to the CS− odor (Ben). As the CS− responses were usually constant across time intervals (E), this allows the CS+ response to be normalized within each fly to the CS− response. The 25- to 49-min and 50- to 74-min time interval showed a marked difference in CS+/CS− response with the paired group compared with unpaired group (Fisher LSD following ANOVA: 25–49 min, P = 0.017; 50–74 min, P = 0.026). No significant difference was observed at 75 to 99 min after training (Fisher LSD following ANOVA: 75–99 min, P = 0.573). Asterisks indicate a statistically significant difference (n = 6–10 for all groups). All error bars indicate SEM.
Artificial Activation of DPM Neurons Enhances ITM in Aged Flies.
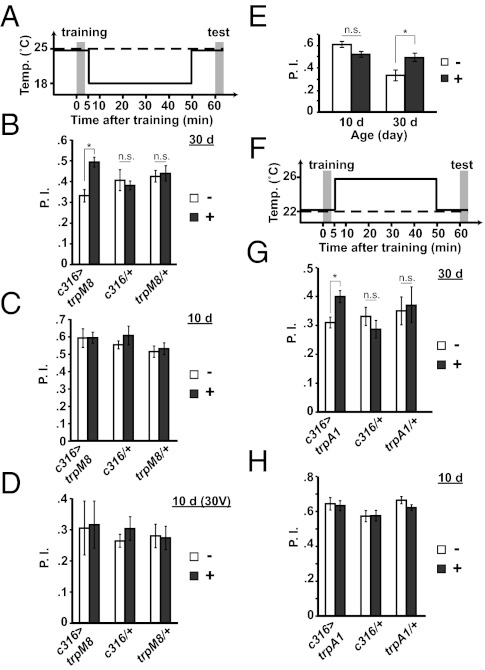
We performed a series of experiments to determine whether the age-related deterioration of the DPM neuron trace could be circumvented to restore ITM in aged flies. We hypothesized that aging decreases the excitability of the DPM neurons, leading to a loss of the DPM neuron memory trace, and that increasing the excitability of the neurons after training would restore ITM. We increased the excitability of these neurons by expressing and activating the cold-sensitive cation channel, TrpM8, for the 45-min period between training and testing by using a temperature shift from 25 °C to 18 °C (Fig. 5A). The TrpM8 channel is activated at lower than 18 °C (20–22). We tested 1-h memory of flies expressing uas-trpM8 in DPM neurons, those expressing only the c316-Gal4 driver, and flies carrying only the uas-trpM8 transgene. Cold treatment had no significant effect on memory of c316-Gal4/+ or uas-trpM8/+ flies at 30 d of age (Fig. 5B). However, cold-stimulated expression of TrpM8 in the DPM neurons produced a remarkable 40% enhancement of 1-h memory compared with flies of the same genotype that received no stimulation (Fig. 5B). This memory enhancement occurred only in flies 30 d of age; parallel experiments using flies 10 d of age failed to demonstrate a similar enhancement (Fig. 5C). To eliminate the possibility that the lack of enhancement using 10 d old flies was caused by a ceiling level performance, we repeated the experiment with less intense electric shock stimulation to lower the performance scores. Under these conditions, which reduced the performance scores of all groups, flies 10 d of age still failed to exhibit a performance enhancement (Fig. 5D).
Fig. 5.
Artificial stimulation of DPM neurons in aged animals. (A) The conditioning protocol for flies carrying one or three copies of trpM8. Flies were trained at 25 °C and shifted immediately after training to 18 °C for 45 min. They were then returned to 25 °C and tested for 1 h aversive odor memory at 25 °C (solid trace). Flies without treatment were trained and tested for 1 h memory at 25 °C as a control (dashed trace). (B) Activating DPM neurons between training and testing enhanced 1 h memory in c316>trpM8 flies (Tukey post hoc following significant differences after one-way ANOVA, P = 0.019), but not in c316/+ or trpM8/+ at 30 d of age (Tukey post hoc following significant differences after one-way ANOVA, P ≥ 0.998). Asterisks indicate a statistically significant difference (n ≥ 9 for all groups). (C) Activating DPM neurons between training and testing did not alter 1-h memory in c316>trpM8, c316/+, or trpM8/+ flies 10 d of age (Tukey post hoc following significant differences after one-way ANOVA, P ≥ 0.690; n ≥ 6 for all groups). (D) Activating DPM neurons between training and testing did not alter 1 h memory in c316>trpM8, c316/+, or trpM8/+ flies 10 d of age, when flies were trained with weak electric shock (30 V; Tukey post hoc following significant differences after one-way ANOVA, P ≥ 0.994; n ≥ 8 for all groups). (E) Activating DPM neurons between training and testing enhanced 1-h memory in c316>trpM8 (three copies) flies at 30 d of age (Tukey post hoc following significant differences after one-way ANOVA, P = 0.028), but not at 10 d of age (Tukey post hoc following significant differences after one-way ANOVA, P = 0.169). Asterisks indicate a statistically significant difference (n = 11 and n = 6 at 10 d and 30 d, respectively). (F) Conditioning protocol for trpA1 flies. Flies were trained at 22 °C and immediately shifted to 26 °C for 45 min. They were then returned to 22 °C and tested for 1-h aversive odor memory at 22 °C (solid trace). Flies without treatment were trained and tested for 1 h memory at 22 °C (dashed trace). (G) Activating DPM neurons between training and testing enhanced 1-h memory in c316>trpA1 flies (Fisher LSD following ANOVA, P = 0.020), but not in c316/+, trpA1/+, or w(CS10) flies 30 d of age (Fisher LSD following ANOVA, P ≥ 0.395; n ≥ 7 for all groups). (H) Activating DPM neurons between training and testing did not affect 1-h memory in c316>trpA1, c316/+, or trpA1/+ flies 10 d of age (Tukey post hoc following significant differences after one-way ANOVA, P ≥ 0.912; n ≥ 7 for all groups). All error bars indicate SEM.
To reinforce and confirm the conclusion that artificial stimulation of the DPM neurons after conditioning enhances the performance at 1 h of 30- but not 10-d-old flies, and also to test whether the performance enhancement was limited by the level of TrpM8 expression, we repeated the experiment using both age groups but with the experimental genotype containing three copies of uas-trpM8 (Fig. 5E). Although there was no significant difference in 1-h memory using flies 10 d of age with vs. without cold stimulation, an enhancement of 1-h memory was observed in flies 30 d of age (Fig. 5E). However, the degree of enhancement was similar in flies carrying one or three copies of uas-trpM8 (compare Fig. 5E vs. 5B).
We also stimulated the DPM neurons in aged animals with the more robust, heat-sensitive TrpA1 cation channel (23). Heat treatment of only 60 s incapacitates flies expressing TrpA1 throughout the nervous system (23), whereas 5 min of cold treatment at 15 °C is required to incapacitate flies expressing one copy of TrpM8 (22). Initially, we stimulated the DPM neurons by incubating flies expressing TrpA1 in the DPM neurons at 32 °C between training and testing. Surprisingly, we observed a complete loss of 1 h memory instead of an enhancement by this robust stimulation. Subsequent experiments using milder stimulation of the neurons with 26 °C treatment revealed that this also enhanced the performance of 30- but not 10-d-old flies (Fig. 5 F–H). This enhancement required the presence of the gal4 driver and uas-trpA1 (Fig. 5G). The data from these three experiments together, using inducible channels of different operators (cold and heat), offer compelling evidence that increasing the excitability of DPM neurons after conditioning restores the age-related impairment of ITM for 30- but not 10-d-old flies.
We also examined whether a similar increase in the excitability of the MB α′/β′ neurons might alter STM in 10-d-old flies. However, the stimulation of the MB α′/β′ neurons with heat and TrpA1, or cold and TrpM8, failed to alter STM in a genotype-specific way (Fig. S2), suggesting that the memory restoration observed with artificial stimulation of neurons is specific to the age-related loss of ITM and DPM neurons.
Discussion
Age-related memory impairment occurs in many species ranging from Drosophila to humans (1). Understanding the brain systems altered by aging and how these impact different forms of memory is essential for understanding the molecular mechanisms by which aging alters brain function. However, this issue represents the intersection of two very complex problems in themselves: how memories are formed, stored, and retrieved and how aging alters brain function underlying these processes. Because of this complexity, we currently have little concrete information in our knowledge set to understand specifically how aging can impair memory formation. The studies presented here contribute to this knowledge set using Drosophila as a model by demonstrating that (i) aging impairs ITM between 20 and 30 d of age; (ii) the formation of protein synthesis-dependent LTM exhibits a complex relationship with age, increasing in early adulthood and subsequently decreasing between 10 and 20 d of age; and (iii) protein synthesis-independent LTM (massed conditioning) is unaffected by age. Interestingly, spatial memory becomes severely impaired at approximately 10 to 12 mo after birth in mice (24) and 23 to 27 mo in rats (25), perhaps reflecting a similar middle-age impairment that we observe here with flies.
We have used the technique of functional cellular imaging to assay the integrity of cellular memory traces in aged animals. This has yielded two important conclusions. First, the DPM neuron ITM trace is impaired in flies 30 d of age, but is unaffected in young flies 10 d of age. Second, the earlier forming trace of the α′/β′ MB neurons appears normal in flies of both ages. It is logical to conclude that the behavioral impairment in ITM in aged flies is caused by the failure of the DPM ITM trace. Four different lines of evidence support this conclusion. First, synaptic transmission from DPM neurons is required during the interval between training and testing for the normal expression of 3-h memory (13, 15, 16). Second, an ITM impairment is characteristic of the amn mutant, whose gene product is required in DPM neurons for normal memory (13, 15). Flies carrying mutations in the amn gene acquire conditioned behavior at the same rate as control flies by using short, repeated training trials, but forget faster than control animals after reaching similar levels of acquisition (13). Third, amn mutants do not form the DPM neuron memory trace, indicating that the DPM trace requires the expression of the amn gene in DPM neurons (13). Fourth, enhancing the activity of the DPM neurons in aged flies enhances ITM performance (Fig. 5). Thus, the excitable state of DPM neurons, which influences levels of synaptic activity after training, appears to facilitate memory retention at intermediate times after conditioning and aging deteriorates the level of excitability.
The artificial activation experiments provide evidence that stimulation of DPM neurons between training and testing enhances ITM in 30-d-old flies. There are a few earlier studies that showed improvement of memory in aged animals through modulation of oxidative stress pathway (26), caloric restriction pathway (27), and PKA-cAMP pathway (28–30) by using principally pharmacological approaches. Our approach used “thermogenetics” delimited to a single neuron in the fly brain.
The enhancement of ITM by stimulation of DPM neurons in aged flies might occur for two different reasons: the stimulation of DPM neurons may lead to the formation of additional ITM or it may rescue the age-dependent loss of ITM. Our experiments reveal that the enhancement of ITM by activation of DPM neurons was not observed in young flies even after weak training, strongly suggesting that the enhancement in aged flies is caused by the recovery of the memory normally lost through the aging process.
These results shine a spotlight on the DPM neurons and their physiological status for the processes underlying cognitive aging. There are at least two general possibilities for how aging might alter DPM neuron physiology, the DPM neuron memory trace, and subsequent memory stability. First, older flies may have altered synaptic connectivity between the DPM neurons and their synaptic partners in the MB lobes, including the MB neurons. Our stimulation experiments indicate that old flies still have the ability to express ITM, indicating that synaptic connections may not be permanently altered during aging. A recent study argued that the DPM neurons release serotonin (i.e., 5HT) onto the MB neurons to promote the formation of ARM (31), along with the amn gene product, a putative neuropeptide, for the promotion of anesthesia-sensitive memory formation (15, 18). As massed conditioning promotes the formation of ARM, and 24-h memory after mass conditioning was unaltered across age (Fig. 1E), the age-dependent impairment in the DPM neurons may alter the amnesiac pathway rather than 5HT. A second possibility not mutually exclusive with the first is that aging alters gene expression in the DPM neurons and this alters their cellular role in memory stability. Although earlier reports revealed no change in the expression of the amn gene in fly heads across age (19), a definitive answer to this question will only come through transcriptome analysis of the DPM neurons themselves.
Materials and Methods
Fly Stocks.
Cantonized w1118 [w(CS10)] flies were used as normal control animals in our experiments. All fly stocks were maintained at 25 °C and 70% humidity under a 12:12 h light/dark cycle. For behavioral studies, approximately 50 flies each were divided into aliquots into food vials and transferred to fresh food vials every 3 or 4 d until the age for training and testing was reached. For longevity assays, 600 flies were collected and cultured in vials containing 20 flies each. The flies were transferred to fresh food vials every 3 or 4 d, and deaths were scored every other day.
Behavior.
Training and testing were performed under dim red light at 25 °C and 70% relative humidity by using previously described procedures (32) (SI Materials and Methods).
Functional Cellular Imaging.
Functional imaging experiments were performed as described previously (8) (SI Materials and Methods). Flies carrying a uas transgene containing G-CaMP (33, 34) along with the DPM neuron driver, c316-Gal4 (15), or the α′/β′ MB neuron driver, c305a-gal4 (35), were used.
Supplementary Material
Acknowledgments
This work was supported by a Senior Scholar Award from the Ellison Medical Foundation (to R.L.D.). A.T. is a Japan Society for the Promotion of Science postdoctoral fellow for research abroad.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. L.C.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118126109/-/DCSupplemental.
References
- 1.Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464:529–535. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews-Hanna JR, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- 4.Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 5.Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis RL. Olfactory memory formation in Drosophila: From molecular to systems neuroscience. Annu Rev Neurosci. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- 7.Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 8.Yu D, Akalal DB, Davis RL. Drosophila α/β mushroom body neurons form a branch-specific, long-term cellular memory trace after spaced olfactory conditioning. Neuron. 2006;52:845–855. doi: 10.1016/j.neuron.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis RL. Traces of Drosophila memory. Neuron. 2011;70:8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Davis RL. The GABAergic anterior paired lateral neuron suppresses and is suppressed by olfactory learning. Nat Neurosci. 2009;12:53–59. doi: 10.1038/nn.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Mamiya A, Chiang AS, Zhong Y. Imaging of an early memory trace in the Drosophila mushroom body. J Neurosci. 2008;28:4368–4376. doi: 10.1523/JNEUROSCI.2958-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu D, Ponomarev A, Davis RL. Altered representation of the spatial code for odors after olfactory classical conditioning; memory trace formation by synaptic recruitment. Neuron. 2004;42:437–449. doi: 10.1016/s0896-6273(04)00217-x. [DOI] [PubMed] [Google Scholar]
- 13.Yu D, Keene AC, Srivatsan A, Waddell S, Davis RL. Drosophila DPM neurons form a delayed and branch-specific memory trace after olfactory classical conditioning. Cell. 2005;123:945–957. doi: 10.1016/j.cell.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 14.Akalal DB, Yu D, Davis RL. A late-phase, long-term memory trace forms in the γ neurons of Drosophila mushroom bodies after olfactory classical conditioning. J Neurosci. 2010;30:16699–16708. doi: 10.1523/JNEUROSCI.1882-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waddell S, Armstrong JD, Kitamoto T, Kaiser K, Quinn WG. The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell. 2000;103:805–813. doi: 10.1016/s0092-8674(00)00183-5. [DOI] [PubMed] [Google Scholar]
- 16.Keene AC, et al. Diverse odor-conditioned memories require uniquely timed dorsal paired medial neuron output. Neuron. 2004;44:521–533. doi: 10.1016/j.neuron.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Quinn WG, Sziber PP, Booker R. The Drosophila memory mutant amnesiac. Nature. 1979;277:212–214. doi: 10.1038/277212a0. [DOI] [PubMed] [Google Scholar]
- 18.Feany MB, Quinn WG. A neuropeptide gene defined by the Drosophila memory mutant amnesiac. Science. 1995;268:869–873. doi: 10.1126/science.7754370. [DOI] [PubMed] [Google Scholar]
- 19.Tamura T, et al. Aging specifically impairs amnesiac-dependent memory in Drosophila. Neuron. 2003;40:1003–1011. doi: 10.1016/s0896-6273(03)00732-3. [DOI] [PubMed] [Google Scholar]
- 20.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 21.Peier AM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 22.Peabody NC, et al. Characterization of the decision network for wing expansion in Drosophila using targeted expression of the TRPM8 channel. J Neurosci. 2009;29:3343–3353. doi: 10.1523/JNEUROSCI.4241-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamada FN, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bach ME, et al. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci USA. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Issa AM, Rowe W, Gauthier S, Meaney MJ. Hypothalamic-pituitary-adrenal activity in aged, cognitively impaired and cognitively unimpaired rats. J Neurosci. 1990;10:3247–3254. doi: 10.1523/JNEUROSCI.10-10-03247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu R, et al. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci USA. 2003;100:8526–8531. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eckles-Smith K, Clayton D, Bickford P, Browning MD. Caloric restriction prevents age-related deficits in LTP and in NMDA receptor expression. Brain Res Mol Brain Res. 2000;78:154–162. doi: 10.1016/s0169-328x(00)00088-7. [DOI] [PubMed] [Google Scholar]
- 28.Ramos BP, et al. Dysregulation of protein kinase a signaling in the aged prefrontal cortex: New strategy for treating age-related cognitive decline. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- 29.Yamazaki D, et al. The Drosophila DCO mutation suppresses age-related memory impairment without affecting lifespan. Nat Neurosci. 2007;10:478–484. doi: 10.1038/nn1863. [DOI] [PubMed] [Google Scholar]
- 30.Wang M, et al. Neuronal basis of age-related working memory decline. Nature. 2011;476:210–213. doi: 10.1038/nature10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee PT, et al. Serotonin-mushroom body circuit modulating the formation of anesthesia-resistant memory in Drosophila. Proc Natl Acad Sci USA. 2011;108:13794–13799. doi: 10.1073/pnas.1019483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng Y, et al. Drosophila fasciclinII is required for the formation of odor memories and for normal sensitivity to alcohol. Cell. 2001;105:757–768. doi: 10.1016/s0092-8674(01)00386-5. [DOI] [PubMed] [Google Scholar]
- 33.Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, et al. Stereotyped odor-evoked activity in the mushroom body of Drosophila revealed by green fluorescent protein-based Ca2+ imaging. J Neurosci. 2004;24:6507–6514. doi: 10.1523/JNEUROSCI.3727-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during drosophila odor memory processing. Neuron. 2007;53:103–115. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.