Abstract
The in vivo biological activities of IgG antibodies result from their bifunctional nature, in which antigen recognition by the Fab is coupled to the effector and immunomodulatory diversity found in the Fc domain. This diversity, resulting from both amino acid and glycan heterogeneity, is translated into cellular responses through Fcγ receptors (FcγRs), a structurally and functionally diverse family of cell surface receptors found throughout the immune system. Although many of the overall features of this system are maintained throughout mammalian evolution, species diversity has precluded direct analysis of human antibodies in animal species, and, thus, detailed investigations into the unique features of the human IgG antibodies and their FcγRs have been limited. We now report the development of a mouse model in which all murine FcγRs have been deleted and human FcγRs, encoded as transgenes, have been inserted into the mouse genome resulting in recapitulation of the unique profile of human FcγR expression. These human FcγRs are shown to function to mediate the immunomodulatory, inflammatory, and cytotoxic activities of human IgG antibodies and Fc engineered variants and provide a platform for the detailed mechanistic analysis of therapeutic and pathogenic IgG antibodies.
The IgG Fc receptor (FcγR) system is composed of both activating and inhibitory receptors expressed on a diversity of immune cells whose signals must be appropriately integrated to regulate the outcome of inflammation and immunity and to maintain tolerance (1). Defects in the correct functioning of this complex system can result in a variety of autoimmune and inflammatory conditions, on the one hand, or defective host defense responses on the other. Importantly, the biological activities of the various IgG Fc subclasses are critically dependent on their relative affinities for activating and inhibitory FcγRs (2). Thus, the in vivo activity of an Ab in mediating antitumor responses or conventional neutralization of a virus or toxin, for example, requires Ab engagement of both the appropriate epitope and FcγR. For example, activating FcγRs are vital during cancer immunotherapy using mAbs such as anti-CD20 mAb, anti-Her2neu, and anti-EGFR (3–6). Fc interactions with activating FcγRs are also critical for the neutralization of bacterial toxins by mAb (7) and during Ab-mediated neutralization of viral pathogens (8–10). By contrast, inhibitory FcγR engagement by passively-administered agonistic anti-CD40 mAb or similar antibodies reactive with other members of the TNFR superfamily are required for their ability to enhance cellular immune responses by inducing adjuvant or apoptotic effects (11).
It has not been possible to accurately predict the consequences of engaging activating or inhibitory human (hu)FcγRs by IgGs for a particular biological response, either for its therapeutic or adverse effects, using currently available in vitro or in vivo model systems. Although murine and nonhuman primate models can be informative, they do not mirror the structural diversity or unique expression patterns observed for huFcγRs on human cells (12). For example, humans express a single-chain activation receptor, FcγRIIA, on dendritic cells (DCs), monocytes, and neutrophils, as well as a glycosylphosphatidylinositol (GPI)-anchored FcγRIIIB exclusively on neutrophils. Both of these receptors are lacking in mice (1). Expression patterns of FcγRs also differ between mice and humans; for example, monocyte-derived DCs from mice express FcγRI, FcγRIIB, FcγRIII, and FcγRIV, whereas human monocyte-derived DCs express only FcγRIIA and FcγRIIB. It is also apparent that the IgG subclasses and FcγRs has coevolved for a particular species, such that the absolute affinities of IgG subclasses for their cognate FcγRs cannot be extrapolated between species, even for recently diverged human and primate species (1, 12). This situation is further complicated by the existence of polymorphisms in the human population for FcγRIIA and FcγRIIIA that result in different affinities for huIgGs (13–16), as well as polymorphisms in FcγRIIB regulating its level of expression or signaling (17). Attempts to model huIgG interactions with human FcγR-expressing cells in vitro fail to mirror the diversity of cellular populations that may be required for an in vivo response. Therefore, new systems to study the in vivo function of the huFcγR system and the biological effects of engaging the activating and inhibitory huFcγRs by IgG are required. Furthermore, the increasing number of Ab-based therapeutics being developed for the treatment of neoplastic, infectious, and autoimmune diseases requires a system in which evaluation of the consequences of huFcγR interactions be addressed. We describe the generation and characterization of an FcγR humanized mouse generated through the transgenic expression of the entire huFcγR family, under the control of their human regulatory elements, on a genetic background lacking all mouse FcγRs. These mice are viable, breed normally, demonstrate normal lymphoid tissue development, and generate normal immune responses. The FcγR humanized mice recapitulate huFcγR expression patterns and expression levels and are functional in a variety of huIgG-mediated models of inflammation, cytotoxicity, and tumor clearance. HuIgG1 mAbs engineered for selectively enhanced Fc-huFcγR affinities demonstrate enhanced in vivo responses in the FcγR humanized mouse. This mouse model, thus, provides an in vivo system to address the functional contributions of huFcγRs to Ab-mediated biological responses.
Results
huFcγR Expression in FcγR Humanized Mice.
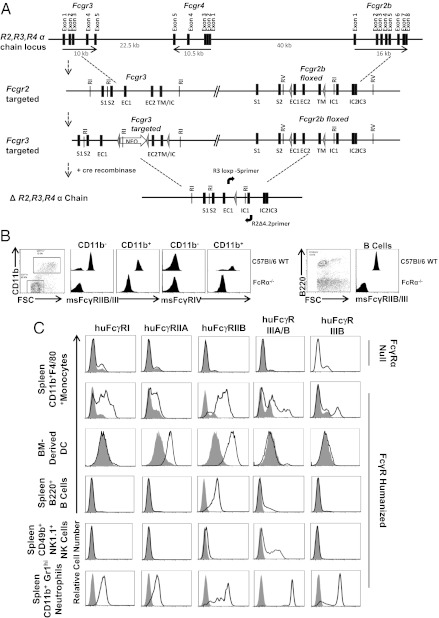
FcγRα−/− mice lacking mouse FcγRIIB, FcγRIII, and FcγRIV were generated by conditionally targeting the low and intermediate affinity mouse FcγRα locus found on chromosome 1 (Fig. 1A) using a sequential strategy of integration of loxP sites into the mouse Fcgr3 and Fcgr2b genes which flank this locus. Cre recombinase-mediated deletion in the C57BL/6 ES cells harboring this dual loxP integration resulted in an intragenic deletion of 95 kb with resulting inactivation of the murine Fcgr2b, Fcgr3, and Fcgr4 genes. Flow cytometric analysis confirmed that mouse FcγRIIB, FcγRIII, and FcγRIV were undetectable on the surface of CD11b+ cells or B220+ B cells in FcRα−/− mice (Fig. 1B). To generate a strain lacking all mouse FcγR α-chain genes, the FcRα−/− mice were crossed to a strain deleted for the mouse high affinity FcγRI α-chain found on mouse chromosome 4, and the resulting mice were bred to homozygosity and named FcRα null mice. These FcRα null mice were than bred to a series of human transgenic lines expressing either huFCGR1A (18), huFCGR2AR131 (19), huFCGR2BI232 (generated for the purposes of this current study; see materials and methods), huFCRG3AF158 (20), and huFCGR3B (20) α-chain genes under the control of their endogenous human regulatory elements. The huFcγRI and huFcγRIIIA α-chains pair with the mouse Fc γ-chain, which is intact in the FcRα null strain. Thus, FcRα null mice that express human FcγRI, FcγRIIA, FcγRIIB, FcγRIIIA, and FcγRIIIB have been designated as FcγR humanized mice. These mice are fertile, develop normally, generate normal immune responses (see below) and do not appear to have any evidence of spontaneous pathology under the specific pathogen-free conditions maintained in the Rockefeller University animal facility.
Fig. 1.
Generation of FcRα−/− mice and huFcγR expression analysis in FcγR humanized mice. (A) Conditional targeting of Fcgr2b and Fcgr3 genes (arrows indicate the coding and noncoding regions of the genes) on the murine α-chain locus and deletion by Cre recombinase of 95 kb of genomic DNA between the two most distal LoxP sites (triangles). RI and RV indicate the EcoRI and EcoRV restriction sites, respectively, used in the Southern blotting analysis. (B) Mouse FcγRIIB, FcγRIII, and FcγRIV are not detectable in FcRα−/− mice. Spleen CD11b+ cells and B220+ B cells from C57BL/6 wild-type (upper graphs) and FcRα−/− (lower graphs) mice were stained for surface mouse FcγR expression. Representative flow cytometric histograms are shown. (C) HuFcγR expression in FcγR humanized mice. The indicated cell types from the indicated tissues were analyzed for huFcγR expression (heavy lines) by immunofluorescence staining and flow cytometric analysis. Shaded histograms show background staining by isotype control mAbs, except for the FcγRIIIB histograms, where shaded histograms show FcγRIIIB staining on cells from FcRα null mice.
To determine whether the cell type-specific expression pattern of huFcγRs in FcγR humanized mice recapitulated FcγR expression patterns normally found in humans, huFcγR expression was examined by immunofluorescence staining and flow cytometric analysis of specific immune cell populations in the FcγR humanized mice. HuFcγRI was detected on monocytes and neutrophils in the spleen, blood, and bone marrow of the FcγR humanized mice (Fig. 1C and Fig. S1), recapitulating huFcγRI expression on human blood monocytes and neutrophils. HuFcγRI was not detected on NK cells or B cells in FcγR humanized mice, but was induced on monocytes and neutrophils by in vitro treatment with IFN-γ (Fig. S2), as occurs with human-derived cells (21). HuFcγRIIA was expressed on neutrophils, monocytes, eosinophils, platelets, and dendritic cells (DCs) in the spleen, blood, and bone marrow of FcγR humanized mice (Fig. 1C and Figs. S1 and S3), consistent with huFcγRIIA expression patterns in humans. No huFcγRIIA expression was detected on NK cells or B cells in FcγR humanized mice or human blood. HuFcγRIIB was detected on B cells in FcγR humanized mice, as well as on eosinophils, monocytes, neutrophils, and DCs. Importantly, bone marrow-derived DCs expressed only huFcγRIIA and huFcγRIIB, as occurs in human monocyte-derived DCs. HuFcγRIIIA was detected on monocytes and NK cells while only neutrophils expressed huFcγRIIIB in humanized mice. No huFcγRs were detected on T cells. Thus, cell type-specific huFcγR expression patterns in FcγR humanized mice recapitulated expression patterns in humans. Table S1 summarizes the expression pattern of FcγR genes on human immune cell populations compared with the FcγR humanized mice. Although definitive quantitation of the absolute levels of expression of the human FcγR proteins on the immune cell populations in the FcγR humanized mice has not been performed, it appears to be qualitatively consistent with the levels reported for these proteins in normal human donors.
Immune System in FcγR Humanized Mice Develops and Functions Normally.
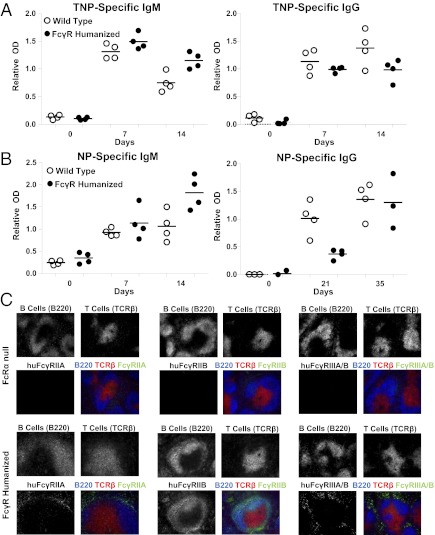
To assess whether the FcγR humanized mice are capable of mounting immune responses to antigenic challenges that are comparable to wild-type B6 mice, humoral immunity elicited against model antigens was compared between wild-type C57BL/6 and FcγR humanized mice. Immunization with the thymic-independent antigen trinitrophenyl (TNP)-LPS resulted in TNP-specific IgM and IgG Ab responses that were similar between wild-type and FcγR humanized mice at both 7 and 14 d after immunization (Fig. 2A). Similarly, Ab responses to the thymic-dependent antigen 4-hydroxy-3-nitrophenyl (NP)–ovalbumin (OVA) resulted in NP-specific IgM levels that were also similar between wild-type and FcγR humanized mice at both days 7 and 14 after immunization (Fig. 2B). NP-specific IgG levels were modestly decreased in FcγR humanized mice compared with wild-type mice 21 d after immunization. However, recall responses 7 d after boosting were similar between both groups. Thus, FcγR humanized mice generate normal Ab responses to both thymic-dependent and thymic-independent antigens.
Fig. 2.
FcγR humanized mice generate normal immune responses and develop normal lymphoid structures. (A and B) Wild-type (WT), C57BL/6 (open circles), and FcγR humanized mice (filled circles) were immunized i.p. with TNP-LPS (A) or NP-OVA (B), followed by a boost with NP-OVA in PBS on day 28. Serum was harvested at the indicated time points and analyzed for TNP- or NP-specific IgM and IgG levels by ELISA. Horizontal bars indicate mean relative OD. (C) FcRα null (Upper) and FcγR humanized (Lower) mouse spleen sections were examined by immunohistochemistry with fluorescence microscopy analysis for B220 (B cells), TCRβ (T cells), huFcγRIIA, huFcγRIIB, and huFcγRIIIA/B expression. Representative images are shown.
Whether lymphoid architecture was normal and huFcγR expression recapitulated human cell type-specific expression patterns in FcγR humanized mice was assessed by immunohistochemistry with fluorescence microscopy analysis. Normal spleen B-cell and T-cell zones were visualized in both FcRα null and FcγR humanized mice (Fig. 2C). As expected, huFcγRIIB expression colocalized with B cells (Fig. 2C), whereas huFcγRIIA and huFcγRIIIA/B were not detected on B cells or T cells (Fig. 2C). Further, huFcγRIIA, huFcγRIIB, and huFcγRIIIA/B were detected on myeloid cells in the red pulp. Thus, the lymphoid architecture was normal and huFcγR expression patterns recapitulated human expression patterns in FcγR humanized mice.
Activating FcγR-Mediated Cytotoxic Effector Functions Are Normal in FcγR Humanized Mice.
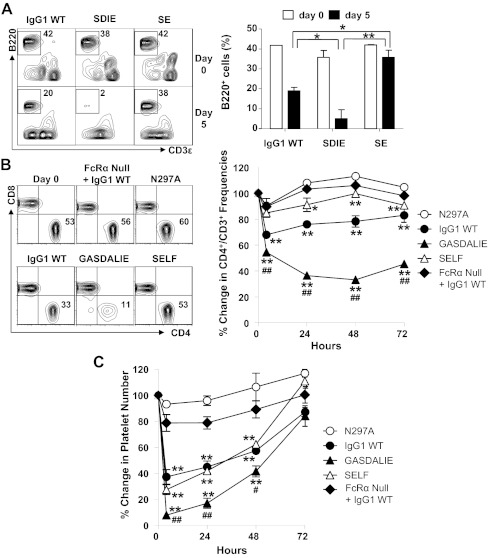
The ability of the humanized FcγR system to mediate cellular cytotoxicity triggered by human IgG was assessed in three in vivo systems: anti-CD40 mAb-mediated B-cell depletion, anti-CD4 mAb-mediated T-cell depletion, and anti-platelet mAb-mediated platelet depletion. Chimeric mAbs reactive with CD40, CD4, or platelets with huIgG1 Fc's in place of their mouse Fc's were generated and expressed. In addition to these wild-type chimeric antibodies, mutant versions of these chimeric mAbs with point mutations in the human IgG1 Fc were generated that either selectively enhanced the affinity of the Fc for activating FcγRIIAR131, activating FcγRIIIAF158, or inhibitory FcγRIIB (Table S2). These point mutations in the huIgG1 Fc did not affect the affinity of mAbs for their respective antigens (Fig. S4).
FcγR humanized mice were treated either with wild-type huIgG1 anti-CD40 mAb, an anti-CD40 mAb mutant that engages hFcγRIIIAF158 with 31-fold increased affinity (SDIE mutant) (22), or an anti-CD40 mAb mutant that engages FcγRIIB with 30-fold increased affinity (SE mutant) (Table S2). Five days after respective mAb treatment, B220+ B cells were analyzed in the peripheral blood of the FcγR humanized mice. B220+ frequencies were decreased by 55% in mice receiving wild-type huIgG1 mAb (Fig. 3A), whereas FcγR humanized mice treated with an Ab whose human Fc was engineered for enhanced huFcγRIIIA binding were depleted by 86% (1.6-fold enhancement) in mice receiving the SDIE mutant mAb. In contrast, treatment of FcγR humanized mice with an anti-CD40 Ab whose human Fc was engineered to enhance binding to the inhibitory huFcγRIIB receptor and abolish binding to huFcγRIIIA (SE mutant) was ineffective in peripheral B-cell depletion.
Fig. 3.
FcγR humanized mice perform normal effector functions during in vivo models of mAb-mediated cytotoxicity. (A) B-cell depletion with CD40 mAb Fc variants. FcγR humanized mice received CD40 mAb with the indicated Fc. The frequency of blood B220+CD3− B cells was analyzed before (day 0) and 5 d after mAb treatment by immunofluorescence staining with flow cytometric analysis. Representative flow cytometric dot plots are shown. Values in the graph represent means (± SEM) frequencies of B cells in mice receiving the indicated treatment at the indicated time point (n ≥ 2 for all groups). Significant differences between the indicated sample means are indicated: *P < 0.05; **P < 0.01. (B) T-cell depletion with CD4 mAb Fc variants. The indicated mice received CD4 mAb with the indicated Fc. FcRα null mice received huIgG1 WT mAb. The frequency of blood CD4+ T cells was analyzed before and 2 d after mAb treatment by immunofluorescence staining with flow cytometric analysis. Representative flow cytometric dot plots are shown. Values in the graph represent the mean (± SEM) percentage change in the frequency of CD4+ T cells relative to the prebleed at 0 h (n ≥ 3 for all groups). (C) Platelet depletion with 6A6 mAb Fc variants. The indicated mice received 6A6 mAb with the indicated Fc. FcRα null mice received huIgG1 WT mAb. Platelet numbers were analyzed at the indicated time points, and values represent the mean (± SEM) percentage change in platelet number relative to the prebleed at 0 h (n ≥ 3 for all groups). (B and C) Significant differences between means from the indicated sample and the N297A group are indicated: **P < 0.01. Significant differences between sample means from the GASD/ALIE group and the IgG1 WT group are indicated: #P < 0.05; ##P < 0.01.
Similar studies were performed using anti-CD4 mAbs with wild-type human IgG1 Fc or modified variants of human IgG1 and in vivo T-cell depletion was assessed. FcγR humanized mice were treated with anti-CD4 mAbs containing either a wild-type huIgG1, a huIgG1 Fc mutant that does not engage FcγRs (N297A mutant), a human Fc that engages hFcγRIIAR131 and hFcγRIIIAF158 with 25- and 29.5-fold increased affinities (GASDALIE mutant), or an anti-CD4 mAb with a human Fc that engages huFcγRIIB with 166-fold increased affinity (SELF mutant) (Table S2). Blood CD4+ T-cell frequencies were decreased by 22% in mice receiving wild-type huIgG1 CD4 mAb 48 h after mAb treatment (Fig. 3B). However, CD4+ T-cell frequencies were decreased by 66% (threefold enhancement) in mice receiving the GASDALIE mutant mAb, whereas frequencies were not affected in mice receiving the N297A or SELF mutant mAbs. CD4+ T-cell frequencies were also not affected in FcRα null mice receiving wild-type IgG1 CD4 mAb.
Anti-platelet mAbs chimerized using this same panel of Fc mutants were also tested for their ability to deplete platelets in FcγR humanized mice. Platelets were decreased by 63% in mice receiving wild-type huIgG1 6A6 mAb 4 h after mAb treatment (Fig. 3C). Platelets were decreased by 92% (1.5-fold enhancement) in mice receiving the GASDALIE mutant 6A6 mAb. However, in contrast to what was observed for T-cell depletion with this mutant Fc, platelet depletion was similar between mice receiving wild-type huIgG1 mAb and the SELF mutant. This difference between T-cell depletion and platelet depletion is likely the result of the expression of FcγRIIA on platelets in the FcγR humanized mice and the ability of the SELF mutant to activate platelets through its enhanced FcγRIIA binding Fc, thereby resulting in platelet consumption. Platelet numbers were not affected in FcγR humanized mice receiving the N297A mutant mAb or in FcRα null mice receiving wild-type IgG1 6A6 mAb. Collectively, these results demonstrate that in vivo cellular cytotoxic functions are normal in FcγR humanized mice. Furthermore, enhancing the affinity of the Fc of a mAb for the activating FcγRs (FcγRIIA and FcγRIIIA) enhanced cellular cytotoxicity, whereas augmenting Fc affinity for inhibitory FcγRIIB led to impaired cytotoxic function.
FcγR-Mediated Antitumor Immunity Functions Normally in FcγR Humanized Mice.
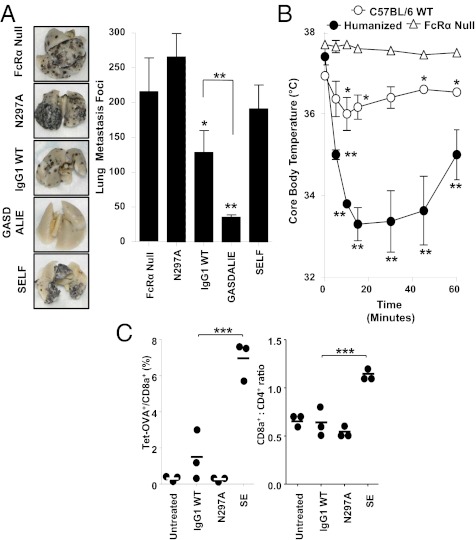
To determine if mAb-mediated tumor clearance through ADCC mediated by activating FcγRs functioned normally in FcγR humanized mice, the B16 metastatic melanoma tumor model was studied in these mice. Chimeric TA99 mAbs using the same panel of huIgG1 Fc mutants used in the platelet and T-cell depletion studies were also tested for their ability to modulate lung metastasis in FcγR humanized mice. Lung metastasis foci were decreased by 41% in mice treated with wild-type huIgG1 mAb, but were decreased by 84% (2.1-fold enhanced clearance) in mice treated with the GASDALIE mutant (Fig. 4A). No tumor clearance was noted in FcγR humanized mice receiving SELF or N297A mutant mAbs, or in FcRα null mice receiving the wild-type huIgG1 mAb. Thus, in vivo mAb-mediated tumor clearance was intact in FcγR humanized mice and functioned in an activating FcγR-dependent manner. Furthermore, enhancing the interactions between huIgG1 Fc and activating huFcγRs augmented tumor clearance.
Fig. 4.
Tumor clearance, immune complex-mediated anaphylaxis, and FcγRIIB-mediated enhancement of immunization by CD40 mAb is effective in FcγR humanized mice. (A) B16 melanoma model. FcγR humanized mice or FcRα−/− mice were injected i.v. with B16-F10 cells and received TA99 mAb with the indicated Fc (n ≥ 4 for all groups) on days 0, 2, 4, 7, 9, and 11. FcRα null mice received huIgG1 WT mAb. On day 13, lungs were harvested and metastasis foci were counted. Pictures show representative lungs from mice receiving the indicated treatment. Values in the graph represent the mean (± SEM) number of lung metastasis foci from mice receiving the indicated treatment. Significant differences between sample means and the N297A group, or between the indicated groups are shown: *P < 0.05; **P < 0.01. (B) Immune complex-mediated anaphylaxis model. Wild-type (WT) C57BL/6, FcRα null, and FcγR humanized mice (n = 3 for each group) were injected i.v. with heat-aggregated human IVIG. Values represent the mean (± SEM) mouse core body temperature measured at the indicated time points. Significant differences between sample means from the indicated time point and time 0 are shown: *P < 0.05; **P < 0.01. (C) Inhibitory FcγR-mediated enhancement of immunization by CD40 mAb. FcγR humanized mice (n = 3 per group) were treated with DEC-OVA and CD40 mAbs with the indicated Fcs. The frequencies of OVA-specific CD8+ T cells [identified by SIINFEKL H-2b tetramer (Tet-OVA) staining; Left] and the ratios of CD8+ T cells to CD4+ T cells (Right) were analyzed in the peripheral blood 7 d later. Each point represents data from an individual mouse, with bars showing mean values. Significant differences between the indicated sample means are shown: ***P < 0.001.
Immune Complex-Mediated Anaphylaxis in FcγR Humanized Mice.
The administration of immune complexes in vivo induces systemic anaphylaxis in an FcγR-dependent manner (23). Therefore, to test whether treatment with human IgG immune complexes induced anaphylaxis in the FcγR humanized model system, wild-type C57BL/6, FcγR humanized, and FcRα null mice were injected with heat-aggregated human IgG as a model immune complex. Core body temperatures in FcγR-humanized mice decreased by 11% by 15 min after heat-aggregated IgG administration but returned to near-normal levels after 60 min (Fig. 4B). However, core body temperatures remained unchanged in FcRα null mice and were only modestly decreased in wild-type B6 mice. Thus, immune complex administration induces FcγR-dependent systemic anaphylaxis in FcγR humanized mice.
FcγRIIB-Dependent Vaccination Functions Normally in FcγR Humanized Mice.
Agonistic CD40 mAb cotreatment in mice immunized with a chimeric DEC205 Ab fused to OVA (DEC-OVA) generates an OVA-specific CD8+ T-cell immune response that is dependent on interactions between the CD40 mAb Fc and the inhibitory FcγRIIB (11). To confirm that requirement for huFcγRIIB in enhancing CD40-mediated immune activation, FcγR humanized mice were immunized with DEC-OVA and cotreated with CD40 mAb containing either a wild-type huIgG1 Fc or a mutated huIgG1 Fc that abolishes FcγR binding (N297A mutant) or enhances FcγRIIB binding (SE mutant). After 7 d, blood OVA-specific T-cell frequencies were increased 4.6-fold in mice receiving SE mutant CD40 mAb compared with wild-type huIgG1 mAb (Fig. 4C). The CD8+:CD4+ T-cell ratio was increased 1.8-fold in SE mutant CD40 mAb-treated mice, compared with mice receiving wild-type huIgG1 CD40 mAb. Thus, enhancement of CD8+ T-cell immune responses through the engagement of FcγRIIB by CD40 mAb was intact in FcγR humanized mice.
Discussion
The emergence of Fc receptors as a central determinant in the in vivo function of IgG antibodies for activities as diverse as cytotoxicity, neutralization, immunomodulation, and anti-inflammatory responses has highlighted the need for model systems in which to investigate these diverse functions. In vitro assays have proven to be of limited value in defining the specific receptors and cell types that may be critical for a particular biological function mediated by an IgG Ab. The species divergence of the receptors and their complex, cell type-specific regulation has also compromised the predictive value of nonhuman primate species in determining either the potential toxicities or therapeutic activities of IgG antibodies destined for human use. Thus, antibodies deemed safe based on nonhuman primate species evaluation have shown dramatic toxicity in humans, contributed in part by differences in FcγR affinities and distribution between the species (24). Similarly, the therapeutic activity of human IgG antibodies assessed in vitro or in nonhuman primates fails to accurately predict in vivo behavior.
We have addressed this issue by developing an in vivo model that represents a closer approximation of human FcγR structural and functional diversity by establishing a stable and reproducible humanized mouse model for assessing human IgG Ab interactions with human FcγRs in the context of an intact immune system. The use of human transgenes, rather than replacement of mouse genes, has ensured that the human pattern of expression and cell type specificity is recapitulated in this humanized mouse model. The human receptors retain their signaling capacity by integrating into mouse intracellular signaling systems, coupling the specificity of human IgG antibodies binding to human FcγR with mouse activating or inhibitory signaling pathways. This was accomplished by retaining the immunoreceptor tyrosine-based activation motif (ITAM)-containing mouse common γ-chain of the FcγR family and allowing it to assemble with the human FcγR α-chains. For receptors that do not use this chain, such as human FcγRIIA or FcγRIIB, the human ITAM or immunoreceptor tyrosine-based inhibitory motif (ITIM) found in the cytoplasmic domains of their α-chains, respectively, appear to couple to mouse kinases and phosphatases, respectively, to mediate appropriate signaling functions. In addition, the FcRα null mouse developed for this study will serve as an important tool for understanding the roles of and requirements for FcγRs in vivo because it offers advantages over the use of Fc γ-chain−/− mice; FcRα null mice lack both activating and inhibitory FcγRs on a pure C57BL/6 genetic background but retain a functional Fc γ-chain, which pairs with and is required for the function of other immune system components.
Although many of these huFcγR transgenes have been expressed as single or even multiple genes in mouse backgrounds lacking some or all of the endogenous mouse FcγRs genes, the current model reproduces the full array of human FcγRs in an immunologically intact mouse background lacking confounding mouse FcγRs. It thus permits the evaluation of normal as well as modified human IgG Fc domains and their impact on an in vivo response. Unique aspects of the human FcγR system, such as the expression of an activating FcγR on platelets, are retained in these mice and function to activate those cells upon engagement by an appropriately engineered human Fc. Similarly, expression of the GPI-anchored decoy receptor, FcγRIIIB, is found exclusively on neutrophils and thereby permits detailed exploration of its in vivo function. The FcγR humanized system will allow the contributions of activating FcγR expression (huFcγRIIIA) on NK cells during Ab-mediated cellular cytotoxicity to be assessed in vivo, because murine NK cells only express the mouse FcγRIII activating receptor and lack the FcγRIV activating receptor, which is the mouse ortholog of huFcγRIIIA. How DC activation and function are regulated by the activating and inhibitory huFcγRIIA and huFcγRIIB can also now be assessed in vivo. Inhibitory FcγR functions normally in FcγR humanized mice because Ab responses to model antigens, which are regulated by the inhibitory FcγRIIB, are normal and CD40 mAb-mediated enhancement of cellular immune responses are intact. Thus, the FcγR humanized mouse will serve as a platform to not only to measure activating FcγR-mediated immunity and therapeutics, but also novel therapeutics aimed at inhibiting immunity through targeting huFcγRIIB, such as mAb-mediated coengagement of FcγRIIB and B-cell surface antigens to suppress humoral immunity (25).
Although the current model is a significant improvement over previous attempts, the presence of mouse IgG may offer some unnecessary complication in the evaluation of a human IgG response. Future iterations of this model could incorporate human IgG Fc in place of the murine locus to allow for evaluation of an exogenous human IgG Ab in the context of human serum IgG antibodies to both mimic the clinical environment more closely and tolerize the animal to the introduction of human IgG to facilitate the analysis of chronic Ab administration. The incorporation of additional huFc binding molecules, like huFcRn and DC-SIGN (Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin), will extend this system further as a surrogate for the in vivo evaluation of human IgG antibodies for therapeutic and pathological activities.
Materials and Methods
Generation of FcRα−/−, FcRα Null, and FcγR Humanized Mice.
The FcRα−/− mouse was designed to contain a 95-kb deletion spanning the Fcgr2b, Fcgr3, and Fcgr4 loci on chromosome 1 (Figs. S5 and S6). This mouse was crossed with Fcgr1−/− mice, and the resulting FcRα−/−FcγRI−/− progeny were termed FcRα null mice. Transgenic mice expressing huFcγRI, huFcγRIIAR131, huFcγRIIBI232, huFcγRIIIAF158, and huFcγRIIIB were individually mated together to create a mouse containing the full repertoire of human FcγRs. This mouse was then mated with the FcRα null mouse to establish the FcγR humanized mouse. C57BL/6 mice were purchased from Jackson Labs. All mice were maintained in a specific pathogen-free facility at the Rockefeller University, and all studies were approved by the Rockefeller University Institutional Animal Care and Use Committee. Detailed methods for FcRα−/−, FcRα null, and FcγR humanized mouse generation are included in SI Materials and Methods.
Antibodies and Reagents.
Descriptions of all Abs and other reagents used in this study are included in the Supplementary Methods.
Immunofluorescence Staining, Bone Marrow DC Cultures, Flow Cytometry, and Histology.
Single-cell suspensions of cells were stained on ice using predetermined optimal concentrations of each primary and secondary Ab, and analyzed on FACSCalibur or LSR-II flow cytometers (BD Biosciences). DCs were cultured from mouse tibia and femur bone marrow cells as described (26). Briefly, 1 × 106 cells/mL were plated in 24-well plates with DMEM supplemented with 10% (vol/vol) FBS and 10 ng/mL mouse granulocyte macrophage colony stimulating factor (GM-CSF; Peprotech). On day 6, loosely adherent cells were collected by gentle pipetting, and were subjected to flow cytometric analysis. Frozen tissue sections (8 μm) were fixed in ice-cold acetone for 3 min, rehydrated with PBS for 10 min, and stained with predetermined optimal concentrations of each Ab for 60 min in PBS-Tween containing 5% goat serum. Tissue staining was visualized using a fluorescence microscope.
Immunizations and ELISA.
Mice were immunized i.p. with TNP-LPS (50 μg; Biosearch Technologies) in 200 μL of PBS or NP-OVA (50 μg; Biosearch Technologies) in 200 μL of Alum (Thermo Scientific). Some mice were boosted i.p. with 50 μg of NP-OVA in 200 μL of PBS 28 d after primary immunization. TNP- and NP-specific Ab levels in individual serum samples were determined in duplicate using Ab isotype-specific ELISA. Sera were diluted for analysis using ELISA plates coated with TNP-BSA or NP-BSA (Biosearch Technologies), and plates were developed with AP-conjugated goat anti-mouse IgM or IgG Abs.
In Vivo Model Systems.
For the B-cell and CD4+ T-cell depletion models, mice were injected i.v. with 100 μg of recombinant IC10 huIgG1 mAb variants or i.p. with 50 μg of recombinant GK1.5 huIgG1 mAb variants diluted in 200 μL of PBS. The mice were bled at various time points to analyze B220+CD3− B-cell or CD4+CD8− T-cell frequencies, respectively, by immunofluorescence staining with flow cytometric analysis. Platelet depletion model: Mice were injected i.v. with 10 μg of recombinant 6A6 huIgG1 mAb variants diluted in 200 μL PBS. Mice were bled at the indicated time points before and after mAb injection, and platelet counts were measured using an Advia 120 hematology system (Bayer Healthcare). For the B16-F10 lung metastasis model, mice were injected i.v. with 1 × 106 B16-F10 tumor cells and received 100 μg of recombinant TA99 huIgG1 mAb variants i.p. on days 0, 2, 4, 7, 9, and 11. On day 13 after tumor cell injection, mice were killed and lungs were analyzed for the presence of surface metastasis foci by using a dissecting microscope. For the immune complex-mediated anaphylaxis model, mice were injected i.v. with 250 μg of human IVIG that was previously heat-aggregated at 63 °C for 60 min. Mouse core body temperature was measured using a rectal thermoprobe. DEC-OVA immunizations: DEC-OVA was generated as described (11). Mice were treated with 5 μg of DEC-OVA and 30 μg of recombinant CD40 mAb 7 d before blood T-cell analysis.
Statistics.
All data are shown as means ± SEM. Significant differences in the CD40 mAb-mediated B-cell depletion and DEC-OVA CD8+ T-cell expansion experiments were determined by using a one-way ANOVA with Tukey post hoc. The significance of differences between all other sample means was determined using a two-tailed Student t test.
Supplementary Material
Acknowledgments
We thank Dr. J. G. J. van de Winkel for supplying huFcγRI transgenic mice and Dr. P. Mark Hogarth for supplying FcgR1−/− mice. We acknowledge support from the Rockefeller University Flow Cytometry, Imaging, and Gene Targeting Resource Centers. We also thank Jaqueline Carroll, Kensuke Horiuchi, Mayumi Kibe, and Ruben Peraza for technical assistance. This work was supported by grants from the National Institutes of Health and the Bill and Melinda Gates Foundation (to J.V.R.). D.J.D. is a fellow of the Leukemia and Lymphoma Society and received support from the New York Community Trust, F.L. is supported in part by the Paralyzed Veterans of America Research Foundation, and S.B. is supported by funds from a National Institute of Allergy and Infectious Diseases Northeast Biodefense Center Career Development Award.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203954109/-/DCSupplemental.
References
- 1.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 2.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 3.Cartron G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 4.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 5.Musolino A, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 6.Overdijk MB, et al. Epidermal growth factor receptor (EGFR) antibody-induced antibody-dependent cellular cytotoxicity plays a prominent role in inhibiting tumorigenesis, even of tumor cells insensitive to EGFR signaling inhibition. J Immunol. 2011;187:3383–3390. doi: 10.4049/jimmunol.1003926. [DOI] [PubMed] [Google Scholar]
- 7.Abboud N, et al. A requirement for FcγR in antibody-mediated bacterial toxin neutralization. J Exp Med. 2010;207:2395–2405. doi: 10.1084/jem.20100995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baum LL, et al. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol. 1996;157:2168–2173. [PubMed] [Google Scholar]
- 9.Hessell AJ, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 10.Corti D, et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333:850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- 11.Li F, Ravetch JV. Inhibitory Fcγ receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 2011;333:1030–1034. doi: 10.1126/science.1206954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers KA, Scinicariello F, Attanasio R. IgG Fc receptor III homologues in nonhuman primate species: Genetic characterization and ligand interactions. J Immunol. 2006;177:3848–3856. doi: 10.4049/jimmunol.177.6.3848. [DOI] [PubMed] [Google Scholar]
- 13.van Sorge NM, van der Pol WL, van de Winkel JG. FcgammaR polymorphisms: Implications for function, disease susceptibility and immunotherapy. Tissue Antigens. 2003;61:189–202. doi: 10.1034/j.1399-0039.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- 14.Ravetch JV, Perussia B. Alternative membrane forms of Fc gamma RIII(CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med. 1989;170:481–497. doi: 10.1084/jem.170.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warmerdam PA, van de Winkel JG, Gosselin EJ, Capel PJ. Molecular basis for a polymorphism of human Fc gamma receptor II (CD32) J Exp Med. 1990;172:19–25. doi: 10.1084/jem.172.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salmon JE, Edberg JC, Brogle NL, Kimberly RP. Allelic polymorphisms of human Fc gamma receptor IIA and Fc gamma receptor IIIB. Independent mechanisms for differences in human phagocyte function. J Clin Invest. 1992;89:1274–1281. doi: 10.1172/JCI115712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su K, et al. Expression profile of FcgammaRIIb on leukocytes and its dysregulation in systemic lupus erythematosus. J Immunol. 2007;178:3272–3280. doi: 10.4049/jimmunol.178.5.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heijnen IA, et al. Antigen targeting to myeloid-specific human Fc gamma RI/CD64 triggers enhanced antibody responses in transgenic mice. J Clin Invest. 1996;97:331–338. doi: 10.1172/JCI118420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKenzie SE, et al. The role of the human Fc receptor Fc gamma RIIA in the immune clearance of platelets: A transgenic mouse model. J Immunol. 1999;162:4311–4318. [PubMed] [Google Scholar]
- 20.Li M, Wirthmueller U, Ravetch JV. Reconstitution of human Fc gamma RIII cell type specificity in transgenic mice. J Exp Med. 1996;183:1259–1263. doi: 10.1084/jem.183.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jayaram Y, Buckle AM, Hogg N. The Fc receptor, FcRI, and other activation molecules on human mononuclear phagocytes after treatment with interferon-gamma. Clin Exp Immunol. 1989;75:414–420. [PMC free article] [PubMed] [Google Scholar]
- 22.Lazar GA, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci USA. 2006;103:4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finkelman FD, Rothenberg ME, Brandt EB, Morris SC, Strait RT. Molecular mechanisms of anaphylaxis: Lessons from studies with murine models. J Allergy Clin Immunol. 2005;115:449–457, quiz 458. doi: 10.1016/j.jaci.2004.12.1125. [DOI] [PubMed] [Google Scholar]
- 24.Suntharalingam G, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 25.Horton HM, et al. Antibody-mediated coengagement of FcγRIIb and B cell receptor complex suppresses humoral immunity in systemic lupus erythematosus. J Immunol. 2011;186:4223–4233. doi: 10.4049/jimmunol.1003412. [DOI] [PubMed] [Google Scholar]
- 26.Inaba K, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.