Abstract
We propose a model wherein chronic stress results in glucocorticoid receptor resistance (GCR) that, in turn, results in failure to down-regulate inflammatory response. Here we test the model in two viral-challenge studies. In study 1, we assessed stressful life events, GCR, and control variables including baseline antibody to the challenge virus, age, body mass index (BMI), season, race, sex, education, and virus type in 276 healthy adult volunteers. The volunteers were subsequently quarantined, exposed to one of two rhinoviruses, and followed for 5 d with nasal washes for viral isolation and assessment of signs/symptoms of a common cold. In study 2, we assessed the same control variables and GCR in 79 subjects who were subsequently exposed to a rhinovirus and monitored at baseline and for 5 d after viral challenge for the production of local (in nasal secretions) proinflammatory cytokines (IL-1β, TNF-α, and IL-6). Study 1: After covarying the control variables, those with recent exposure to a long-term threatening stressful experience demonstrated GCR; and those with GCR were at higher risk of subsequently developing a cold. Study 2: With the same controls used in study 1, greater GCR predicted the production of more local proinflammatory cytokines among infected subjects. These data provide support for a model suggesting that prolonged stressors result in GCR, which, in turn, interferes with appropriate regulation of inflammation. Because inflammation plays an important role in the onset and progression of a wide range of diseases, this model may have broad implications for understanding the role of stress in health.
Keywords: lymphocytes, receptor sensitivity, psychological stress, cortisol, hypothalamic–pituitary–adrenocortical axis
Chronic psychological stress is associated with a greater risk of depression, cardiovascular disease (CVD), diabetes, autoimmune diseases, upper respiratory infections (URIs), and poorer wound healing (1). Although these associations are often attributed to stress-induced dysregulation of the hypothalamic–pituitary–adrenocortical axis (HPA) (e.g., refs. 2, 3), few human studies include assessments of stressful events, HPA response, and a disease outcome in the same subjects. The lack of such studies is partly attributable to the as yet incomplete understanding of the effects of prolonged stress on the HPA in human subjects (4) and on determining which stress-induced changes in HPA play a downstream role in disease risk. The simple notion that chronic stress acts through the direct effects of elevated circulating cortisol is becoming less likely (5, 6). What may matter more is how target tissues respond to cortisol, rather than levels of the hormone per se.
GCR refers to a decrease in the sensitivity of immune cells to glucocorticoid hormones that normally terminate the inflammatory response (6–9). Evidence for GCR in response to chronic stress has been found in parents of children with cancer (10), spouses of brain-cancer patients (11) and in persons reporting high levels of loneliness (5). Without sufficient glucocorticoid regulation, the duration and/or intensity of the inflammatory response increases, heightening risk for acute exacerbations such as occur in asthma and autoimmune diseases, as well as for the onset and progression of chronic inflammatory diseases such as CVD, and type II diabetes (12).
In the common cold, the typical signs and symptoms of illness are primarily caused by the release of the proinflammatory cytokines produced in response to infection (13). A series of studies have shown that chronic stress is associated with increased susceptibility to developing a common cold among persons experimentally exposed to an upper respiratory virus (e.g., refs. 14–17). This association has been replicated in cross-sectional and prospective studies conducted in natural settings (18). Although attempts to identify behavioral pathways linking stress to cold susceptibility have not been especially successful (14, 15), there is evidence consistent with stress effects resulting in increased disease risk because of their association with exaggerated local (in the nose) release of inflammatory cytokines (16). The explanation for this association has been that stress disrupts the HPA response and that the regulation of inflammation is under HPA control. However, cortisol levels based on 24-h urine (15) and waking diurnal assessments in saliva (19, 20) do not play a part in this process. Alternatively, what matters may be how the target tissue responds to cortisol, rather than the levels of the hormone per se. To the extent that chronic stress results in GCR, one might expect insufficient control over the inflammatory response to the infection, and consequently a greater expression of the signs and symptoms of disease.
The viral-challenge paradigm provides an ideal context to study the general model of stress leading to disease via effects on the HPA and inflammatory regulation. In these studies, stress and GCR are assessed before subjects are exposed to a virus and followed in quarantine to determine whether they develop a clinical illness (infection plus signs of illness). Here we conduct secondary analyses of two independent studies (15, 21), each using a different means of assessing GCR, to address the potential role of target tissue sensitivity to cortisol in linking stress to disease. In study 1, we attempt to replicate earlier evidence that stress exposure is associated with increased GCR (5, 6, 9–11) and test whether GCR prospectively predicts who will develop a cold when exposed to a rhinovirus. In study 2, we address whether GCR is prospectively associated with the magnitude of the local inflammatory cytokine response to being infected by a cold virus. We predict that GCR will be associated with experiencing a long-term threatening stressful experience, will interfere with the down-regulation of proinflammatory cytokine response, and will increase illness expression among persons infected with a cold virus.
Results
All analyses include seven covariates: prechallenge viral-specific antibody titers, age, body mass index [BMI: weight (kg)/height (m)2], season (spring or fall), race (white or not), sex, and education (study 1: less than high school, high school graduate, some college, bachelor's degree or greater; study 2: years of education). In study 1, where subjects receive one of two viruses, analyses also control for virus type [rhinovirus (RV)21 or RV39].
Study 1.
Stress and colds.
As reported in similar analyses of these data (14), in a multiple logistic regression, exposure to a recent major stressful life event was associated with an increased risk for developing a cold following exposure to RV (OR = 1.99, CI = 1.04, 3.08). This association was similar across the two viruses (P > 0.16 for stress-by-virus interaction).
Stress, GCR, and colds.
Greater levels of glucocorticoid are associated with higher numbers of circulating neutrophils, lower numbers of circulating lymphocytes, and a lower neutrophil-to-lymphocyte (N/L) ratio—an overall marker of the trafficking of these cells (e.g., refs. 22, 23). Cole and his colleagues (5, 24) showed that this association can be used to indirectly assess GCR. The logic of the measure is that there is a strong physiologic correlation between cortisol levels and the number of circulating leukocytes only if leukocyte glucocorticoid receptors are sensitive (i.e., signaling cells to redistribute).
Stress and GCR.
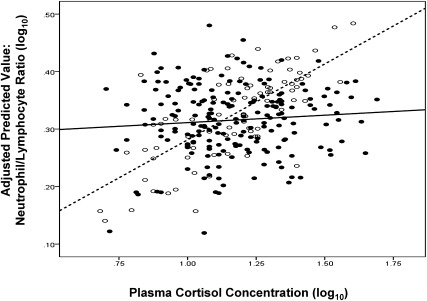
We found the expected associations between leukocyte numbers and cortisol among those who did not experience a major stressor (n = 83). In contrast, consistent with the hypothesis that stress results in GCR, there were no associations between cortisol and leukocytes among those who did experience a stressor (n = 193). Fig. 1 displays these findings graphically using the relation of cortisol to N/L ratio as an example. That the slopes of the stressed and nonstressed subjects were different from each other is supported by stress-by-cortisol level interactions found in linear regressions predicting lymphocytes (P = 0.03), neutrophils (P = 0.03) and the N/L ratio (P = 0.03). For all three analyses, simple slopes for those without stress were significantly different from 0, whereas slopes for those with stress were not (Table 1). These analyses support GCR emergence among those experiencing major stressors.
Fig. 1.
Association between plasma cortisol and the ratio of neutrophils to lymphocytes for subjects experiencing and not experiencing a significant stressful life event during the last year. Lack of correlation is a marker of GCR. Filled circles/solid line, major stressful life event; open circles/dashed line, no stressful event.
Table 1.
Simple effect associations of stress status with lymphocyte subsets
| % lymphocytes | % neutrophils | Neutrophils/lymphocytes | ||||
| β | SE β | β | SE β | β | SE β | |
| No stress | −0.27** | 0.09 | 0.24** | 0.09 | 0.26** | 0.09 |
| Stress | −0.01 | 0.09 | −0.02 | 0.09 | −0.00 | 0.09 |
Analyses control for age, sex, race, body mass index, education, prechallenge viral-specific antibody titers, season, and virus type. **P < 0.01.
GCR and colds.
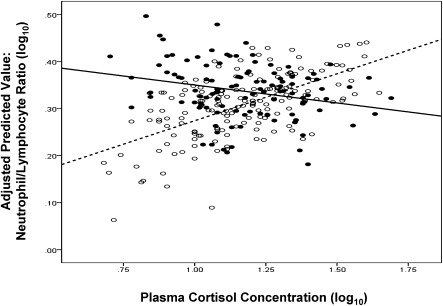
Similarly we find the expected associations between cortisol and leukocyte counts for persons who did not develop a cold following viral challenge (n = 167). In contrast, and consistent with GCR putting people at greater risk for illness, there were no associations between cortisol and leukocyte counts among those who did develop a cold (n = 109). (Fig. 2, N/L ratio.) Here linear regressions indicate stress-by-cortisol interactions for lymphocytes (P = 0.02), neutrophils (marginal at P = 0.08), and N/L ratio (P = 0.02). For all three analyses, simple slopes among those who did not develop a cold were significantly different from 0, whereas slopes for those who did develop a cold were not (Table 2). As expected, plasma cortisol levels did not predict who developed a cold (OR = 1.27, CI = 0.20, 7.94). Together, results from these prospective analyses support the hypothesis that GCR predicts an increased risk for colds following viral exposure, whereas levels of circulating cortisol do not.
Fig. 2.
Association between plasma cortisol and the ratio of neutrophils to lymphocytes for subjects who developed and did not develop a cold after exposure to a rhinovirus. Lack of correlation is a marker of GCR. Filled circles/solid line, meets cold criterion; open circles/dashed line, does not meet cold criterion.
Table 2.
Simple effect associations of cold status with lymphocyte subsets
| % lymphocytes | % neutrophils | Neutrophils/lymphocytes | ||||
| β | SE β | β | SE β | β | SE β | |
| No cold | −0.27** | 0.08 | 0.21* | 0.08 | 0.26** | 0.08 |
| Cold | 0.01 | 0.09 | −0.01 | 0.09 | −0.01 | 0.09 |
Analyses control for age, sex, race, body mass index, education, prechallenge viral-specific antibody titers, season, and virus type. *P < 0.05, **P < 0.01.
Consideration of alternative explanations.
To evaluate the possibility that sympathetic nervous system hormones that often increase with cortisol may be driving the redistribution of cells, we conducted all of the same analyses described earlier replacing cortisol with epinephrine (E) and with norepinephrine (NE). These hormones were not associated with numbers of any of the cell types in relevant subgroups of subjects defined by either stress or colds.
Study 2.
GCR and nasal cytokines.
We suggest that the association of GCR with colds reflects inadequate control of local inflammatory responses to the virus. To test this hypothesis, we examine whether GCR predicts the production of local proinflammatory cytokines when subjects are exposed to a rhinovirus. Data for this study were collected from a subsample of subjects in a larger viral-challenge trial (15). Here GCR was assessed before viral challenge using a standard ex vivo model (6), wherein leukocytes were coincubated with lipopolysaccharide and dexamethasone, and proinflammatory cytokine production measured in supernatants. The logic behind this assay is that dexamethasone should inhibit cytokine production only to the extent that leukocytes are sensitive to its effects. This study had enough subjects with the required data to examine cytokine response (a continuous variable) but not colds (a dichotomous outcome).
Table 3 presents the partial correlations between GCR and proinflammatory cytokine levels in the nasal cavity during the 5 d following viral challenge. For both IL-6 and TNF-α, increases in the resistance curve were associated with increased production of the matching local inflammatory cytokine. Greater IL-1β GCR was also associated with greater local IL-6 production. The correlation of IL-1β GCR with local IL-1β release was in the expected direction, but was not significant. All of these results are the same whether the analyses include all subjects, or are limited to those infected by the virus. Cortisol levels as assessed by multiple samples of saliva collected across the day before viral challenge were unrelated to any of the three GCR measures (all P > 0.20) or to proinflammatory cytokine levels (all P > 0.40). Overall, these data are consistent with the hypothesis that a decreased sensitivity of leukocytes to the inhibitory effects of cortisol (increased GCR) reflects a decreased ability of cortisol to regulate the production of local proinflammatory cytokines in response to infection.
Table 3.
Partial correlations of glucocorticoid resistance curves (AUC) with nasal cytokine AUCs: Entire sample and infected subset
| Nasal cytokine AUCs |
||||||
| Sample (n = 75) | Infected (n = 68) | |||||
| Glucocorticoid resistance AUC | IL-6 | TNF-α | IL-1β | IL-6 | TNF-α | IL-1β |
| IL-6 curve | 0.314*** | 0.238* | 0.177 | 0.348** | 0.247* | 0.198 |
| TNF-α curve | 0.231* | 0.283** | 0.234* | 0.238* | 0.276** | 0.241* |
| IL-1β curve | 0.282** | 0.193 | 0.173 | 0.317** | 0.179 | 0.169 |
Analyses control for age, sex, race, years of education, body mass index, prechallenge antibody, and season. *P < 0.10, **P < 0.05, ***P < 0.01.
Discussion
We proposed that exposure to a major stressful life event can result in GCR, which, in turn, would interfere with HPA down-regulation of local proinflammatory cytokine response to an infectious agent. Without appropriate cortisol regulation of the local cytokine response, there would be an exaggerated expression of the signs of URI, which are generated by the proinflammatory response. In study 1, stress—defined as a recent stressful life experience associated with long-term threat—predicted an increased risk of developing a cold following exposure to a rhinovirus (also reported in ref. 15). New analyses indicate that this same stress measure was also associated with GCR, with stressed persons showing less sensitivity of lymphocyte and neutrophil counts to distributional changes associated with greater circulating levels of cortisol. In turn, GCR was prospectively associated with increased risk of developing a common cold following experimental inoculation with a cold virus. These data are consistent with a model wherein stress leads to GCR, which in turn results in greater risk for developing a cold. In study 2, GCR predicted how much local proinflammatory cytokine was produced in response to infection. Because the analyses in both studies were prospective, we can eliminate reverse causation (colds did not cause stress, colds did not cause GCR, and cytokine release did not cause GCR) as an alternative explanation. The use of multiple control variables also eliminates many potential spurious explanations.
The GCR measure used in study 1 is indirect. However, experimental studies have shown that glucocorticoid-induced leukocyte redistribution is specifically attributable to glucocorticoid receptor signaling (25), and that the indirect assessment used here correlates with blunting of lymphocyte redistribution in response to dexamethasone injection (24). Even so, it is possible that the action of another hormone or mechanism both highly correlated with cortisol and having the same impact on leukocyte trafficking as cortisol could account for these effects. Likely alternative explanations include that the associations are attributable to subject health (e.g., infections) or to receptor sensitivity to E and/or NE. Health is not an issue here, because subjects are carefully screened for excellent health. Further, we found that neither E nor NE were correlated with leukocyte counts under any of the relevant conditions (similar results in ref. 5). We also found consistent evidence across studies 1 and 2, even though study 2 used a standard direct assessment of GCR.
We found no effects of cortisol levels on disease risk (e.g., refs. 19, 20), GCR, or inflammation. This apparent lack of a role for circulating cortisol levels is consistent with the possibility that impaired target tissue response to the regulatory effects of this hormone may overshadow any modulatory influences that might result from changes in circulating concentrations of cortisol itself.
In the case of the common cold, a disease for which expression of the signs/symptoms of illness is driven by the inflammatory response, the failure of the HPA to regulate the production of local proinflammatory cytokines contributes to the risk for clinical illness. Because inflammation plays a role in progression of multiple diseases, this model not only provides an explanation for the increased risk for URI under stress, but might provide a more general explanation for why prolonged stress would play a role in other inflammatory diseases as well. Moreover, although the focus here has been on GCR in circulating leukocytes and neutrophils, stress could also be associated with illness expression through its effect on the glucocorticoid sensitivity of other cells involved in immune defenses (26). For example, glucocorticoid receptors (GR) are expressed by cells involved with antigen presentation, such as dendrocytes and macrophages, not only in circulation but also at specific sites of infection and in draining lymph nodes.
Parodoxically, proinflammatory cytokines are thought to both up-regulate immunity to the virus and produce cold symptoms (13). However, here stress and GCR, conditions associated with increased levels of local proinflammatory cytokine, predicted a greater risk of clinical illness. These data are consistent with those of other viral-challenge studies that found a positive association between proinflammatory cytokine levels and symptom expression (16).
An unexplained inconsistency in the data from study 2 is that local IL-6 and TNF-α, but not IL-1β, were correlated with GCR measured by the whole blood ex vivo assay. It is possible that this discrepancy is due to the relative insensitivity of the IL-1β assay. Alternatively, it may reflect a differential sensitivity of these cytokines to glucocorticoid suppression (27).
Finally, future research on GCR would benefit from quantification of GR subtypes, whose relative abundance might underlie the findings observed here. Chronic stress does not appear to affect expression of GRα, the active isoform of the receptor (11). However, there is evidence linking stress and cytokines to higher levels of GRβ and a lower GRα/GRβ ratio (28). This could prove important because GRβ is a dominant negative receptor for cortisol, which can suppress GRα activity and thereby contribute to GCR (28).
Methods
Study 1.
Subjects and design.
The subjects were 125 men and 151 women, mean age 29.13 y (SD = 9.09 y) who responded to newspaper advertisements and were judged to be in good health after a medical examination. Subjects were paid $800 for their participation.
After we assessed life stress, complete blood counts, cortisol, demographics, anthropomorphics, and virus-specific antibody levels, subjects were quarantined in separate rooms, exposed to one of two RVs and followed for 5 d with nasal washes for viral isolation and assessment of symptoms and signs of a common cold.
Experimental plan.
At the medical screening, age, education, race, sex, body weight, and height were also assessed and used as baseline data for those deemed eligible. Eligible subjects returned to the hospital 4 and 5 wk later (1–2 wk before viral challenge) for two blood samples for determination of complete blood counts, antibody to the challenge virus, and plasma cortisol and for the stressful life event interview.
After completing the baseline assessments, subjects entered quarantine. During the first 24 h of quarantine (before viral challenge), they had a nasal examination (including a nasal wash culture for RV) and were excluded if there was any indication of recent or current URI or illness. An update stressful life event interview was administered at this time to identify events occurring between the initial interview and quarantine. Baseline respiratory signs (nasal mucociliary clearance and nasal mucus production) and symptoms were assessed. At the end of the first 24 h of quarantine, subjects were given nasal drops containing a low infectious dose of either RV21 (N = 129) or RV39 (N = 147). Quarantine continued for 5 d after challenge. Nasal secretion samples for virus culture were collected on each of the 5 d. Subjects were also tested on each day for respiratory symptoms, nasal mucociliary clearance, and nasal mucus production. Approximately 28 d postchallenge, a blood sample was collected for assaying convalescent antibodies to the challenge virus. All investigators were blinded to subjects’ status on all baseline assessments.
Stressful life events.
Life events were assessed by a standardized semistructured interview, the Bedford College Life Events and Difficulties Schedule (LEDS) (29), and conducted by trained interviewers. We considered only those events that occurred during the last 12 mo. Ratings of events were performed in an independent laboratory by consensus groups consisting of at least four trained interviewers. The interview staff was blind to other study measures. Raters were also blind to individuals’ subjective responses to the stressful events and used “dictionaries” of precedent examples to rate each stressful experience reported during the interview on long-term threat (i.e., threat that is sustained beyond the immediate experience of the event). Dictionary ratings are based on the likely response of an average person to a stressor occurring in the context of the subject's particular set of biographical circumstances. It is the long-term threat of an experience that is thought to determine its implications for health. Here, we define stress as a life experience, irrespective of duration (including both acute events and chronic difficulties in LEDS parlance), that was rated as having high moderate or marked long-term threat. We excluded stress resulting from physical illness to avoid confounding a chronic health condition with susceptibility and stressful events in which the subject was not a major focus.
GCR.
We conducted a complete blood count and assayed for cortisol in each of two blood samples collected 1 and 2 wk prechallenge. Correlations between cell counts from the two blood draws ranged from 0.61 to 0.76, P < 0.001. Plasma cortisol was measured using a double-antibody competitive RIA. The correlation between cortisol in the two samples was 0.70, P < 0.001. Because we were concerned with stable differences in cortisol and in cell distributions that occur in response to prolonged stress, we averaged the two values in both cases.
We found no associations between monocyte percentage and cortisol in the entire sample or in sample subsets split by stress or colds. Thus, we could not use whether cortisol was associated with monocytes as a marker of GCR. Our data are therefore based on the associations of cortisol with percentages of lymphocytes and neutrophils. We also use the ratio of neutrophils to lymphocytes (log10) as an overall marker of the trafficking of these cells (5, 24).
Epinephrine and Norepinephrine.
Urine was obtained from 24-h collections that began on day 0 of quarantine. E and NE were measured using high performance liquid chromatography with electrochemical detection. Values are expressed in micrograms per milligram of creatinine (log10).
Infection.
Nasal washes were performed daily during quarantine to provide samples of nasal secretions for virus culture (30). Neutralizing antibodies to the challenge virus were tested in prechallenge and 28-d postchallenge serum samples (30).
Signs of illness.
On each day of quarantine, we collected two objective signs of disease—mucus weights and mucociliary clearance function. Mucus weights were determined by collecting used tissues in sealed plastic bags (31). The bags were weighed and the weight of the tissues and bags subtracted. To adjust for baseline, mucus weight on the day before challenge (mode = 0) was subtracted from each daily mucus weight after challenge. Adjusted weights with negative values were scored as 0. The adjusted postchallenge weights were summed to create an adjusted total mucus weight.
Nasal mucociliary clearance function refers to the effectiveness of nasal cilia in clearing mucus from the nasal passage toward the nasopharnyx. Clearance function was assessed as the time required for a dye administered into the nose to reach the nasopharnyx (31). Each daily time was adjusted (by subtracting) for baseline (negative values were scored as 0) and the adjusted average time in minutes was calculated across the postchallenge days of the trial.
Clinical illness.
Subjects were considered to have a cold if they were both infected and met objective illness criteria. They were classified as infected if the challenge virus was isolated on any of the 5 postchallenge study days or if there was a fourfold or greater rise in virus-specific serum neutralizing antibody titer between prechallenge and 28-d postchallenge. Illness criterion was based on objective indicators of illness—a total adjusted mucus weight of ≥10 g or an adjusted average mucociliary nasal clearance time of ≥7 min (21).
Statistical analyses.
We conducted two sets of regression analyses, each predicting the percentage of neutrophils, lymphocytes, and the N/L ratio. The first set included the standard covariates, a dichotomous variable representing life stress (1 = stress, 0 = no stress), continuous plasma cortisol, and the interaction of life stress and cortisol. We predicted an interaction with leukocyte counts increasing (neutrophils and N/L ratio) or decreasing (lymphocytes) with increasing cortisol levels for those without a major stressful life event but not for those with an event. The second set included standard covariates, dichotomous variables for life stress, and cold status (1 = cold, 0 = no cold) and the interaction of life stress and cold status. We again predicted an interaction with cortisol associated with leukocyte counts among those without colds but not among those with colds. We also calculated the simple slopes for each group in all analyses and tested whether they were significantly different from a slope of 0.
Study 2.
Subjects and design.
The subjects were 39 men and 43 women (mean age = 36.9 y, SD = 9.2 y) who responded to advertisements and were judged to be in good health. They were paid $800 for their participation. Three subjects were excluded due to incomplete nasal cytokine data.
After we assessed GCR and the standard control variables, subjects were quarantined in separate rooms, exposed to a rhinovirus and followed for 5 d with nasal washes to assess viral shedding and local proinflammatory cytokines.
Experimental plan.
At the medical screening we also collected specific serum antibody titer to the challenge virus, demographics, weight, and height. Because we were interested in inflammatory response to infection, we maximized the rate of infection by including only subjects with prechallenge viral-specific antibody titers ≤4.
During the first 24 h of quarantine (before viral challenge) nasal mucociliary clearance and nasal mucus production were assessed. Volunteers were excluded if they had signs or symptoms of a cold and excluded from analysis if a viral pathogen was isolated from the nasal lavage obtained at that time.
Subjects were then given nasal drops containing an infectious dose of RV39. On each day of quarantine nasal lavage samples were collected for virus culture and assessment of local proinflammatory cytokines. Approximately 28 d postchallenge, blood was collected for serological testing. The investigators were blinded to all baseline measures.
Infection.
Infection was assessed with the same procedures used in study 1.
GCR.
We used a standard in vitro assay to measure GCR that assessed the ability of dexamethasone (0, 10, 50, and 250 nM) to suppress lipopolysaccharide (30 ng/mL) stimulated lymphocyte production of IL-1β, IL-6, and TNF-α. The remaining procedures were the same as those used in Miller et al. (6). Area under the dexamethasone curve (adjusted for an unstimulated control) was used to represent GCR.
Proinflammatory cytokines.
We measured IL-1β, IL-6, and TNF-α in nasal secretions. Nasal wash fluid was assayed for cytokine protein using the BioSource Ten-plex bead immunoassay and methods provided by the manufacturer (BioSource International). Assays were performed in duplicate using a Luminex 100 bead-based immunoassay system. To control for basal nasal cytokine levels, prechallenge cytokine values were subtracted from the values obtained on each of the 5 postchallenge days. Nasal cytokine area under the curve (AUC) was derived from these baseline-adjusted values and computed using the trapezoid formula.
Cortisol.
Subjects provided seven saliva samples (collected at wake-up and at 1, 2, 4, 7, 9, and 14 h after wake-up) on the day preceding viral challenge by chewing on cotton rolls until saturated and then storing the rolls in plastic salivettes. Code numbers provided by hand-held computers were used to guarantee timely collection of samples. Saliva cortisol level was determined by ELISA. Log10 AUC adjusted for wake-up time was used to represent cortisol level.
Acknowledgments
Preparation of this paper was supported by Grant AT006694 from the National Center for Complementary and Alternative Medicine; conducting the studies, by Grants MH47234 and MH50430 from the National Institute of Mental Health and Grants HL65111 and HL65112 from the National Heart, Lung, and Blood Institute; and the assay of glucocorticoid resistance in study 2, by the MacArthur Foundation Research Network on Socioeconomic Status and Health.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 3.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 4.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 5.Cole SW. Social regulation of leukocyte homeostasis: The role of glucocorticoid sensitivity. Brain Behav Immun. 2008;22:1049–1055. doi: 10.1016/j.bbi.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meagher MW, Johnson RR, Good E, Welsh TH. In: Psychoneuroimmunology. 4th Ed. Ader R, Felton D, Cohen N, editors. Vol II. New York: Academic; 2006. pp. 1107–1124. [Google Scholar]
- 7.Marques AH, Silverman MN, Sternberg EM. Glucocorticoid dysregulations and their clinical correlates. From receptors to therapeutics. Ann N Y Acad Sci. 2009;1179:1–18. doi: 10.1111/j.1749-6632.2009.04987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey M, Engler H, Hunzeker J, Sheridan JF. The hypothalamic-pituitary-adrenal axis and viral infection. Viral Immunol. 2003;16:141–157. doi: 10.1089/088282403322017884. [DOI] [PubMed] [Google Scholar]
- 9.Stark JL, et al. Social stress induces glucocorticoid resistance in macrophages. Am J Physiol. 2001;280:1799–1805. doi: 10.1152/ajpregu.2001.280.6.R1799. [DOI] [PubMed] [Google Scholar]
- 10.Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychol. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- 11.Miller GE, et al. A functional genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 13.Hendley JO. The host response, not the virus, causes the symptoms of the common cold. Clin Infect Dis. 1998;26:847–848. doi: 10.1086/513921. [DOI] [PubMed] [Google Scholar]
- 14.Cohen S, Tyrrell DAJ, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 15.Cohen S, et al. Types of stressors that increase susceptibility to the common cold in healthy adults. Health Psychol. 1998;17:214–223. doi: 10.1037//0278-6133.17.3.214. [DOI] [PubMed] [Google Scholar]
- 16.Cohen S, Doyle WJ, Skoner DP. Psychological stress, cytokine production, and severity of upper respiratory illness. Psychosom Med. 1999;61:175–180. doi: 10.1097/00006842-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Stone AA, et al. Development of common cold symptoms following experimental rhinovirus infection is related to prior stressful life events. Behav Med. 1992;18:115–120. doi: 10.1080/08964289.1992.9936961. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen A, Zachariae R, Bovbjerg DH. Influence of psychological stress on upper respiratory infection—a meta-analysis of prospective studies. Psychosom Med. 2010;72:823–832. doi: 10.1097/PSY.0b013e3181f1d003. [DOI] [PubMed] [Google Scholar]
- 19.Cohen S, et al. Objective and subjective socioeconomic status and susceptibility to the common cold. Health Psychol. 2008;27:268–274. doi: 10.1037/0278-6133.27.2.268. [DOI] [PubMed] [Google Scholar]
- 20.Edwards S, Hucklebridge F, Clow A, Evans P. Components of the diurnal cortisol cycle in relation to upper respiratory symptoms and perceived stress. Psychosom Med. 2003;65:320–327. doi: 10.1097/01.psy.0000033123.70631.8e. [DOI] [PubMed] [Google Scholar]
- 21.Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr Social ties and susceptibility to the common cold. JAMA. 1997;277:1940–1944. [PubMed] [Google Scholar]
- 22.Fauci AS, Dale DC, Balow JE. Glucocorticosteroid therapy: Mechanisms of action and clinical considerations. Ann Intern Med. 1976;84:304–315. doi: 10.7326/0003-4819-84-3-304. [DOI] [PubMed] [Google Scholar]
- 23.Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Stress-induced changes in blood leukocyte distribution. Role of adrenal steroid hormones. J Immunol. 1996;157:1638–1644. [PubMed] [Google Scholar]
- 24.Cole SW, Mendoza SP, Capitanio JP. Social stress desensitizes lymphocytes to regulation by endogenous glucocorticoids: Insights from in vivo cell trafficking dynamics in rhesus macaques. Psychosom Med. 2009;71:591–597. doi: 10.1097/PSY.0b013e3181aa95a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller AH, et al. Effects of selective type I and II adrenal steroid agonists on immune cell distribution. Endocrinology. 1994;135:1934–1944. doi: 10.1210/endo.135.5.7956914. [DOI] [PubMed] [Google Scholar]
- 26.Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- 27.DeRijk R, et al. Exercise and circadian rhythm-induced variations in plasma cortisol differentially regulate interleukin-1 β (IL-1 β), IL-6, and tumor necrosis factor-α (TNF α) production in humans: High sensitivity of TNF α and resistance of IL-6. J Clin Endocrinol Metab. 1997;82:2182–2191. doi: 10.1210/jcem.82.7.4041. [DOI] [PubMed] [Google Scholar]
- 28.Derijk RH, et al. A human glucocorticoid receptor gene variant that increases the stability of the glucocorticoid receptor beta-isoform mRNA is associated with rheumatoid arthritis. J Rheumatol. 2001;28:2383–2388. [PubMed] [Google Scholar]
- 29.Brown GW, Harris TO. Life Events and Illness. New York: Guilford; 1989. [Google Scholar]
- 30.Gwaltney JM, Jr, Colonno RJ, Hamparian VV, Turner RB. In: Diagnostic Procedures for Viral, Rickettsial and Chlamydial Infections. 6th Ed. Schmidt NJ, Emmons RW, editors. Washington, DC: American Public Health Association; 1989. pp. 579–614. [Google Scholar]
- 31.Doyle WJ, McBride TP, Swarts JD, Hayden FG, Gwaltney JM., Jr The response of the nasal airway, middle ear, and Eustachian tube to provocative rhinovirus challenge. Am J Rhinol. 1988;2:149–154. doi: 10.1097/00006454-198803000-00033. [DOI] [PubMed] [Google Scholar]