Schizophrenia (SZ) is a complex disorder that results from the interplay of genetic and environmental risk factors. It is characterized by the presence of delusions and hallucinations (positive symptoms), apathy and social withdrawal (negative symptoms), and stable impairments in specific domains of cognitive function. Over the past decade, significant progress in understanding SZ has been made by establishing consortia that conduct very large genome-wide association studies (GWASs). The GWAS design provides high-throughput SNP genotyping, following an “agnostic” approach that lacks subjective assumptions concerning gene candidacy. The report by Quednow et al. (1) in PNAS focuses on SZ risk polymorphisms in the transcription factor 4 (TCF4) gene, a locus that has been identified as a significant genetic risk factor for SZ in recent GWASs (2, 3). The authors examined whether any of the TCF4 risk alleles affect sensory gating, as measured by the P50 suppression of the auditory evoked potential. As initially hypothesized, P50 suppression was significantly decreased in carriers of four TCF4 risk alleles.
Interestingly, the impact of one of the TCF4 polymorphisms (rs9960767) on the prepulse inhibition (PPI) of the acoustic startle response, a measurement of sensorimotor gating, was recently examined by the same investigators in two independent cohorts. Similar to the current findings, the TCF4 risk allele significantly reduced PPI (4). Although it is widely accepted that suppression of both PPI and P50 examines different gating measurements and is partially regulated by distinct neuronal mechanisms (5), there is growing evidence supporting the notion that both paradigms share some common genetic pathways (4, 6, 7). In addition, suppression of both PPI and P50 is significantly heritable and is disrupted in both patients with SZ and their healthy first-degree relatives, consistent with the endophenotype criteria (5, 8). Thus, both paradigms are considered to be highly informative SZ-related intermediate phenotypes, and they support the role of the TCF4 gene as an important determinant of the sensory and sensorimotor gating.
What is the importance of such sensory and sensorimotor gating mechanisms? The nervous system is constantly receiving incoming sensory information, and it is necessary to filter out distracting sensory information during the early stages of processing so that attention can be focused on more salient features of the environment. The initial screening during the preattentive stages of information processing occurs by gating of the redundant stimuli. Patients with SZ demonstrate significant abnormalities in gating mechanisms as tested by certain paradigms, such as P50 suppression and PPI, and these
Quednow et al. examine whether an interaction between SNPs and smoking may affect the suppression of P50.
deficits are stable traits that are independent of medication status or clinical state (5). It has been speculated that failure to filter out overwhelming sensory information in early stages of processing leads to sensory overload and a low signal-to-noise ratio, resulting in psychotic symptoms and cognitive deficits.
According to the neurodevelopmental hypothesis, SZ is associated with abnormalities of early brain development that are caused by a combination of genetic and early environmental factors. These factors eventually interact with normal maturational processes of the brain, thus increasing the risk for the subsequent emergence of clinical symptoms. The product of the TCF4 gene has a critical role during development, which is supported by findings in animal and human studies. The Tcf4 KO mice demonstrate significant abnormalities in the pontine nucleus development and die prematurely (9). Transgenic mice that overexpress Tcf4 displayed profound deficits in fear conditioning and sensorimotor gating (10). In humans, TCF4 haploinsufficiency leads to neurodevelopmental disorders, such as the Pitt–Hopkins syndrome, which is characterized by severe cognitive deficit, microcephaly, disrupted motor development, and hyperventilation (11, 12). The above findings in both animals and humans provide compelling evidence for the significance of TCF4 during early development, and raise the possibility that TCF4 genetic perturbations may affect early brain development and increase the risk for SZ during adulthood.
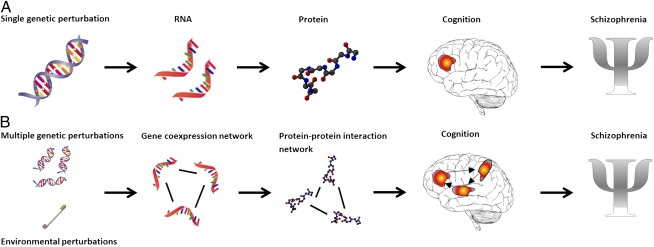
The report by Quednow et al. (1) addresses a very important challenge that we have to deal with in the post-GWAS era: How do we move from a statistically significant association of a genetic polymorphism to understanding of the mechanism and etiopathogenesis of SZ? Molecular and cognitive traits controlled by genetic loci associated with disease can be treated as intermediate phenotypes of disease (Fig. 1A). This can aid in the interpretation of GWAS data by identifying how the genetic variability affects intermediate phenotypes at different levels (e.g., molecular, cognitive), leading to the development of disease, as defined based on the clinical criteria. At a cognitive level, intermediate phenotypes, such as PPI and P50 suppression, carry the potential to provide a way forward for parsing out complex, heterogeneous disease phenotypes like SZ. It has been suggested that SZ-related intermediate phenotypes are more proximal to the genetic variability driving the disease effects compared with the current nosological framework represented by the Diagnostic and Statistical Manual of Mental Disorders, fourth edition and International Classification of Diseases, 10th revision (8). Thus, integrating multiple intermediate phenotypes will increase our understanding of the mechanism by which genetic variation leads to the disease.
Fig. 1.
Importance of intermediate phenotypes in SZ research. (A) Single genetic perturbation approach: SZ risk genetic variants lead to changes in molecular traits that affect higher order cognitive functions, and subsequently increase the risk for developing the disease. (B) Multiple perturbations approach: Constellations of multiple genetic and environmental perturbations affect molecular states of gene coexpression and protein-protein interaction networks that, in turn, affect higher order cognitive functions and disease risk.
Another critical challenge of the post-GWAS era is to understand how multiple, modestly associated genes interact with other genes (epistatic interactions) or nongenetic factors (environmental interactions) to influence a phenotype. Very limited progress has been made in this direction. Based on the higher prevalence of smoking in patients who have SZ as well as its effect on sensory gating, Quednow et al. (1) examine whether an interaction between SNPs and smoking may affect the suppression of P50. The TCF4 gene effect on P50 suppression was more prominent in the heavy smokers compared with light smokers or those who never smoked, indicating a possible gene-by-environment interaction. Although such interaction is plausible, a definite conclusion about the influence of smoking behavior cannot be reached with high confidence. Another possible explanation is that the TCF4 genetic variation interacts with other genetic or unknown environmental factors, leading to significant deficits in sensory gating, and that increased smoking behavior acts as a compensatory mechanism to restore such deficits. In that case, a significant gene-by-environment interaction would be observed; however, the environmental factor would be an epiphenomenon rather than a true causal factor that drives the disease.
Because of genetic and phenotypic heterogeneity, epistatic interactions, and environmental and epigenetic factors, reductionist studies of genetic variants and specific molecular pathways in SZ will only provide limited insight into the pathophysiology of the disease. One of the major challenges that we have to encounter in the post-GWAS era is how to integrate multiple layers of data on a genome-wide scale using a holistic approach. The large-scale application of genomic, transcriptomic, and proteomic high-throughput technologies has led to the accumulation of molecular intermediate phenotypes on a genome-wide scale (“omics” data). Integration of molecular intermediate phenotypes controlled by genetic loci associated with SZ will further help us to interpret the GWAS data, as well as to infer causal relationships between molecular traits and disease states (13). This will provide an excellent opportunity to uncover molecular drivers of SZ. However, to understand the behavior of any single gene in the context of complex disorders like SZ, individual genes must be understood in the context of molecular networks that define the disease states. Network analysis provides a systems level understanding of how genetic and environmental perturbations affect the transcriptomic and proteomic organization that leads to alterations in the higher order cognitive functions, which, in turn, affect disease risk (Fig. 1B). Importantly, integration of molecular intermediate phenotypes with cognitive, neuroimaging, and clinical data will help us to distinguish the biological pathways and environmental risk factors that are upstream and primary causes of SZ from those that are downstream or homeostatic consequences of the disease. The identification of upstream networks and driver genes has the potential to lead to previously undescribed targets for development of unique treatments.
Footnotes
The author declares no conflict of interest.
See companion article on page 6271.
References
- 1.Quednow BB, et al. Schizophrenia risk polymorphisms in the TCF4 gene interact with smoking in the modulation of auditory sensory gating. Proc Natl Acad Sci USA. 2012;109:6271–6276. doi: 10.1073/pnas.1118051109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ripke S, et al. Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stefansson H, et al. Genetic Risk and Outcome in Psychosis (GROUP) Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quednow BB, et al. The schizophrenia risk allele C of the TCF4 rs9960767 polymorphism disrupts sensorimotor gating in schizophrenia spectrum and healthy volunteers. J Neurosci. 2011;31:6684–6691. doi: 10.1523/JNEUROSCI.0526-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thaker GK. Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophr Bull. 2008;34:760–773. doi: 10.1093/schbul/sbn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu BY, et al. Effect of catechol O-methyltransferase val(158)met polymorphism on the p50 gating endophenotype in schizophrenia. Biol Psychiatry. 2007;62:822–825. doi: 10.1016/j.biopsych.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 7.Roussos P, et al. Prepulse inhibition of the startle reflex depends on the catechol O-methyltransferase Val158Met gene polymorphism. Psychol Med. 2008;38:1651–1658. doi: 10.1017/S0033291708002912. [DOI] [PubMed] [Google Scholar]
- 8.Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 9.Flora A, Garcia JJ, Thaller C, Zoghbi HY. The E-protein Tcf4 interacts with Math1 to regulate differentiation of a specific subset of neuronal progenitors. Proc Natl Acad Sci USA. 2007;104:15382–15387. doi: 10.1073/pnas.0707456104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brzózka MM, Radyushkin K, Wichert SP, Ehrenreich H, Rossner MJ. Cognitive and sensorimotor gating impairments in transgenic mice overexpressing the schizophrenia susceptibility gene Tcf4 in the brain. Biol Psychiatry. 2010;68:33–40. doi: 10.1016/j.biopsych.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Amiel J, et al. Mutations in TCF4, encoding a class I basic helix-loop-helix transcription factor, are responsible for Pitt-Hopkins syndrome, a severe epileptic encephalopathy associated with autonomic dysfunction. Am J Hum Genet. 2007;80:988–993. doi: 10.1086/515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zweier C, et al. Haploinsufficiency of TCF4 causes syndromal mental retardation with intermittent hyperventilation (Pitt-Hopkins syndrome) Am J Hum Genet. 2007;80:994–1001. doi: 10.1086/515583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schadt EE. Molecular networks as sensors and drivers of common human diseases. Nature. 2009;461:218–223. doi: 10.1038/nature08454. [DOI] [PubMed] [Google Scholar]