Abstract
The determination of structural models of the various stable states of an ion channel is a key step toward the characterization of its conformational dynamics. In the case of nicotinic-type receptors, different structures have been solved but, thus far, these different models have been obtained from different members of the superfamily. In the case of the bacterial member ELIC, a cysteamine-gated channel from Erwinia chrisanthemi, a structural model of the protein in the absence of activating ligand (and thus, conceivably corresponding to the closed state of this channel) has been previously generated. In this article, electrophysiological characterization of ELIC mutants allowed us to identify pore mutations that slow down the time course of desensitization to the extent that the channel seems not to desensitize at all for the duration of the agonist applications (>20 min). Thus, it seems reasonable to conclude that the probability of ELIC occupying the closed state is much lower for the ligand-bound mutants than for the unliganded wild-type channel. To gain insight into the conformation adopted by ELIC under these conditions, we solved the crystal structures of two of these mutants in the presence of a concentration of cysteamine that elicits an intracluster open probability of >0.9. Curiously, the obtained structural models turned out to be nearly indistinguishable from the model of the wild-type channel in the absence of bound agonist. Overall, our findings bring to light the limited power of functional studies in intact membranes when it comes to inferring the functional state of a channel in a crystal, at least in the case of the nicotinic-receptor superfamily.
Keywords: electrophysiology, structure-function
The use of bacterial and archaeal ion channels as models of the structure and function of their eukaryotic counterparts has become one of the mainstays of ion-channel biophysics. It seems to us that this practice is particularly well justified whenever the use of noneukaryotic channels facilitates experiments that are otherwise too difficult or too cumbersome to perform. A clear case in point is the determination of structural models of these membrane proteins using X-ray crystallography.
One of the main goals of direct structural approaches as applied to ion channels is to determine what these proteins look like in their different functional states. Assuming that all of these conformations can form well-diffracting crystals, the problem is reduced to finding the conditions under which the occupancy of each particular state is maximized. In general, for well-characterized ion channels, the experimental maneuvers that are needed to favor or disfavor at least some of these different conformations are known. For example, in the case of (wild-type) neurotransmitter-gated channels, the binding of the natural neurotransmitter strongly stabilizes the desensitized state (e.g., ref. 1), whereas the absence of neurotransmitter or the binding of a competitive antagonist keeps the channel mostly closed (2, 3). Similarly, mutations can also affect the conformational free-energy landscape of ion channels in such a way that the occupancy of a particular conformation is markedly favored or disfavored.
Thus far, in the case of the bacterial members of the nicotinic-receptor superfamily, the use of ligands to select the crystallized conformation of the channel has yielded results that are difficult to interpret. For example, although GLIC (a proton-gated channel from Gloeobacter violaceus) was crystallized at pH values that strongly favor the desensitized state (pH ∼4.5) (4), the properties of the pore domain inferred from the obtained structural models have been interpreted to suggest that the channel was crystallized, actually, in its open, ion-conductive conformation (5, 6; but see ref. 7). In addition, the structural models of GLIC bound to desflurane and propofol at pH 4.0 fail to reveal differences at the level of the pore domain compared with the structure of the channel at the same pH but not bound to these general anesthetics, even when electrophysiological recordings show that the binding of these ligands favors a nonconductive conformation (8). In the case of ELIC (a cysteamine-gated channel from Erwinia chrisanthemi), the situation is not very different. Indeed, the structural models of wild-type ELIC with and without bound agonist seem indistinguishable from each other, even though, in the membrane, the unliganded channel is mostly closed and the fully-liganded channel is largely desensitized (9, 10). (We would like to emphasize here that, throughout this article, the term “unliganded” is used to denote the lack of molecules with agonist properties in the solutions bathing the channel, but we cannot rule out the possibility that some component of the solutions, such as proton-buffer molecules, can bind to the agonist-binding sites).
As mentioned above, mutagenesis provides an alternative means to tilt the conformational free-energy landscape of proteins, but whether this approach facilitates the crystallization of nicotinic-type channels in their different functional states has yet to be explored. In this article, we present the X-ray crystal structures of two pore-mutant variants of ELIC in the presence of a high concentration of activating ligand. The engineered mutations (L240A + F247L and L240S + F247L, with the 240 and 247 residues corresponding to the 9′ and 16′ positions of the M2 transmembrane segment, respectively) slow down the time course of entry into desensitization of the ligand-bound channel to the extent that no net desensitization could be observed for the duration of our electrophysiological recordings. However, aside from the mutated side chains, no difference could be appreciated between the structural models of these open-state stabilizing mutants and that of the wild-type ELIC channel in the absence of activating ligand (9). Several scenarios that may account for this puzzling finding are discussed.
Results
Two main structural models of bacterial nicotinic-type receptors have been inferred from X-ray crystallographic data, thus far: that of the unliganded ELIC channel (PDB ID code: 2VL0), which was deemed to represent the closed-channel conformation (9), and that of the proton-gated GLIC channel in the presence of a desensitizing concentration of protons (PDB ID codes: 3EAM and 3EHZ), which was deemed to represent the open state (5, 6). These models have been interpreted to represent the end states of the closed ⇋ open conformational transition (5, 6), and thus, form the basis of recently proposed channel-gating mechanisms. However, it may be argued that it would be desirable to obtain crystal structures of the same channel in the different functional states. Because favoring the crystallization of specific conformations of these channels using ligands has so far proved ineffective, we set out to test the effect of mutations. Here, we present data on ELIC.
Because the 2VL0 model of ELIC was obtained from crystals grown in the absence of activating ligands, it seems reasonable to assume that the crystallized conformation represents the closed state, at least, of this particular channel. Hence, we decided to engineer mutations to ELIC that would tilt the conformational free-energy landscape away from the closed state. In the absence of much data on the effect of mutations on ELIC function, it also seemed reasonable to take advantage of the wealth of information that is available for the eukaryotic members of the superfamily. In these channels (to our knowledge, without exception; e.g., see refs. 11–24), mutation of the aliphatic residues that line the extracellular half of the transmembrane pore (between positions 9′ and 20′ of M2) to residues bearing shorter or more polar side chains has been found to cause a dramatic increase in the closed ⇋ open equilibrium constant; the effect on channel desensitization has been less well documented. It is this increased gating equilibrium constant that leads to the well-known lower EC50 values, the much higher frequency of unliganded openings, and the prolongation of the mean duration of bursts of single-channel openings (or, in macroscopic terms, the slowing of the deactivation time course) that are characteristic of these pore mutants.
Remarkably, when engineered in all five subunits of ELIC, the leucine-to-alanine mutation at position 9′ of the M2 α-helix, leads to a faster—not slower—deactivation time course upon cysteamine (that is, the agonist) washout (Fig. 1 A and B). This finding is highly surprising because even the deactivation time course of the bacterial GLIC channel is prolonged by an isoleucine-to-alanine mutation at this position (Fig. S2A). As for desensitization, we found that when this mutation is engineered in ELIC, no current decay at all seems to occur upon continued exposure to a high concentration of cysteamine (Fig. 1 C and D). In contrast, under these conditions, wild-type ELIC desensitizes with a time constant of 12.6 ± 2.0 s (mean ± SE; n = 5 patches). This effect of the leucine-to-alanine mutation at position 9′ on the kinetics of desensitization is unsurprising inasmuch as similar findings have been reported for other members of the nicotinic-receptor superfamily; here, we show that this is also the case for GLIC (Fig. S2B).
Fig. 1.
Effect of canonical gain-of-function mutations on ELIC. The kinetics of deactivation and desensitization of ELIC wild-type and pore mutants were studied in fast-perfused outside-out patches of membrane (solution-exchange time10–90%<150 μs) excised from Xenopus oocytes. The pH on both sides of the membrane was 7.4. According to the prime-numbering system, residues 240 and 247 correspond to positions 9′ and 16′ of M2, respectively (Fig. S1 and SI Materials and Methods). (A and B) Kinetics of deactivation. The displayed time courses (shown normalized) were fitted with monoexponential functions, and the corresponding time constants (± SEs) are shown. (C) Normalized time courses of currents elicited by the application of cysteamine for 5 s. (D) Extent of desensitization. Because the macroscopic-current responses of the mutants decay negligibly upon exposure to high concentrations of cysteamine, their time courses could not be fitted to exponential-decay functions; instead, extent-of-desensitization values (mean ± SE) were calculated. (E and F) Time courses of currents elicited by the application of cysteamine for 1 min. (G) Extent-of-desensitization values (mean ± SE) corresponding to the macroscopic traces in E and F. (H) Time course of the current elicited by the application of cysteamine for 20 min. To prolong the lifetime of the patch of membrane, the potential (−80 mV) was applied only briefly during the 20-min application of agonist, as indicated above the current trace. For all other panels, the potential was held constant at −80 mV. The color code is the same for all panels.
Incidentally, we noticed that both wild-type and L9′A mutant ELIC channels, heterologously expressed in Xenopus oocytes, activate extremely slowly in response to cysteamine-concentration jumps from 0 to 10 mM (Fig. 2 A and B): the time constants of monoexponential fits to the rising phase of the macroscopic currents are 84.6 ± 7.0 ms (n = 15) and 58.6 ± 13.1 (n = 9), respectively. These values are much slower than those of the most-slowly activating eukaryotic members of the superfamily, such as the α3β4 ACh receptor (3.1 ± 0.2 ms; n = 5; jumps in ACh concentration from 0 to 1 mM) and the serotonin type 3A receptor (7.3 ms; jumps in serotonin concentration from 0 to 100 μM) (25), upon exposure to comparably fast agonist applications. To make sure that 10 mM cysteamine was high enough a concentration, we increased the concentration of agonist to 100 mM (Fig. 2 C and D), and no changes in the activation time course were observed [the values of cysteamine concentration given here correspond to those estimated colorimetrically by determining the amount of 5-thio-2-nitrobenzoate released upon reaction with 5,5′-dithio-bis(2-nitrobenzoate) at pH ∼7.3] (26). To rule out a specific effect of the Xenopus-oocyte expression system, we also expressed ELIC in HEK-293 cells; the recorded activation time courses turned out to be nearly indistinguishable from those obtained in oocytes (Fig. 2 C and D). To eliminate the possibility that problems with the perfusion system slowed down the time course of the recorded responses, we transfected HEK-293 cells with cDNA coding the (adult-type) muscle AChR, and estimated the kinetics of activation. As expected for this fast synaptic channel, we found that activation proceeds very rapidly upon stepping the concentration of ACh from 0 to 100 μM (Fig. 2 C and D), to the extent that its time course is likely to be limited by the kinetics of the solution exchange at the tip of the patch pipette. Finally, for comparison, we analyzed the activation time course of GLIC upon stepping the extracellular pH from 7.4 to 4.5 (Fig. 2 C and D) and found that the time constant of a monoexponential fit is 18.9 ± 5.6 ms (n = 11). We conclude that the slow activation of ELIC is an intrinsic property of this channel.
Fig. 2.
ELIC currents activate slowly. (A–D) Kinetics of activation studied in fast-perfused outside-out patches of membrane at −80 mV. The pH on both sides of the membrane was 7.4. The time courses (shown normalized) were fitted with monoexponential functions, and the corresponding time constants (± SEs) are shown.
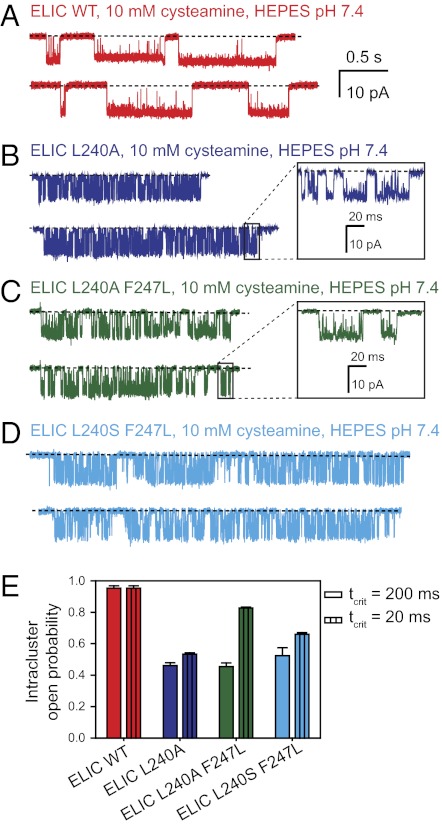
In an attempt to better understand the “nondesensitizing” phenotype of the L9′A mutant, we turned to single-channel recordings (Fig. 3). At the single-channel level, the activity of wild-type ELIC in the presence of 10 mM cysteamine occurs as series of several openings in quick succession interrupted by long shuttings. This grouping of consecutive openings from individual channels in the presence of high concentrations of agonist (“clusters”) is highly reminiscent of the single-channel behavior of the eukaryotic members of the superfamily (27), and thus, it is tempting to identify the longer shut intervals as sojourns in desensitized conformations. With this in mind, we examined single-channel recordings from the L9′A mutant of ELIC and found that openings still occur as clusters. On average, these clusters are longer than those recorded from wild-type ELIC, albeit not long enough for this lengthening, alone, to account for the sustained response to prolonged agonist applications (Fig. 1C). Hence, it seems as though the mutant channel desensitizes and that the nonzero steady level attained by the macroscopic currents represents a balance between channels that enter and channels that recover from desensitized conformations. Our limited understanding of this channel's gating and desensitization kinetics in terms of mechanisms, however, precludes us from estimating how many of the channels in the patch are closed, how many are open, and how many are desensitized. This limitation notwithstanding, it seems prudent to suggest that the probability of the mutant channel being closed in the presence of 10 mM cysteamine is much lower than that of the wild-type ELIC in the absence of agonist.
Fig. 3.
Single-channel behavior. (A–D) Stretches of single-channel currents recorded in the outside-out configuration (Xenopus oocyte membranes) at −80 mV, in the continued presence of cysteamine. The pH on both sides of the membrane was 7.4. Channel openings are downward deflections. Display fc ∼5 kHz. The calibration bars are the same for all traces (with the exception of those in the Insets). (E) Intracluster open probabilities (mean ± SE). In the absence of a more detailed understanding of the single-channel behavior of these constructs, clusters were defined (somewhat arbitrarily) as series of openings separated by shuttings shorter than 200 ms. Our conclusions would not change if we defined clusters using a critical time of 20 ms, instead.
The lining of the extracellular half of ELIC is unique in that it contains a full ring of phenylalanines at the pore-facing position 16′ (4) (Fig. S1A). Certainly, among the known eukaryotic members of the superfamily, a full ring of phenylalanines is present at this position only in a few channels from nematodes whose functional properties remain unexplored. Engineering such a ring at position 16′ of the mouse-muscle AChR causes the channel to adopt a highly stable refractory state that seems to be distinct from the well-known desensitized conformation (4). We considered, then, the possibility that the presence of these aromatic side chains in ELIC could compensate for the effect of shortening the leucines at 9′, thus preventing the leucine-to-alanine mutation from increasing the gating equilibrium constant of this channel. To test this idea, we mutated the phenylalanine at position 16′ of ELIC to leucine, one of the most common amino acids at this position in the cation-selective subset of eukaryotic nicotinic-type receptors (Fig. S1A). Electrophysiological characterization of this double mutant revealed little, if any, additional effect of the mutation at 16′ (Figs. 1–3): most importantly, deactivation remains faster than the wild-type's and desensitization remains remarkably slow, with little (if any) decay of the current response on application of 10 mM cysteamine for 20 min (Fig. 1H). The negligible effect of this mutation on the kinetics of deactivation is unexpected too, because, for example, mutating the ring of four leucines and one phenylalanine at position 16′ of the mouse-muscle AChR to a full ring of alanines slows down channel deactivation by a factor of ∼18 (Fig. S3). Furthermore, in the zebrafish-muscle AChR, mutating (only) the two α1-subunit leucines at this position to proline slows down deactivation by a factor of ∼4 (28).
Other substitutions at position 9′ of ELIC give rise to, essentially, the same results. Indeed, substituting a serine at this position (in the background of the phenylalanine-to-leucine mutation at position 16′) (Figs. 1–3) yields a mutant that, like the alanine-substituted channel, deactivates faster than the wild-type. In stark contrast, a leucine-to-serine mutation at position 9′ of the mouse-muscle AChR causes a dramatic increase in the gating equilibrium constant, leading to a much-prolonged mean duration of bursts of single-channel openings (e.g., ref. 13). It is worth emphasizing that the muscle AChR is merely used here as an example: mutation of the 9′ aliphatic side chain to a shorter or polar one has been shown to lead to a “gain-of-function” phenotype in all eukaryotic members of the superfamily in which the effect of this type of mutation has been tested.
Peculiarities of the bacterial channels aside, the results above led us to identify a combination of mutations that, in the continued presence of a high concentration of cysteamine, greatly prolong the occupancy of the open-channel conformation. Of course, we cannot rule out the possibility that these mutants desensitize completely upon even longer incubations with agonist of the sort that is needed for protein crystals to grow. Whatever the case might be, though, it seems sensible to conclude that the probability of ELIC occupying the closed-channel state is much lower for the ligand-bound mutants than it is for the unliganded wild-type channel. Because we wanted to obtain structural information about states other than the closed-channel conformation, we set out to crystallize the two double-mutant variants of ELIC (L9′A + F16′L and L9′S + F16′L) in the presence of cysteamine.
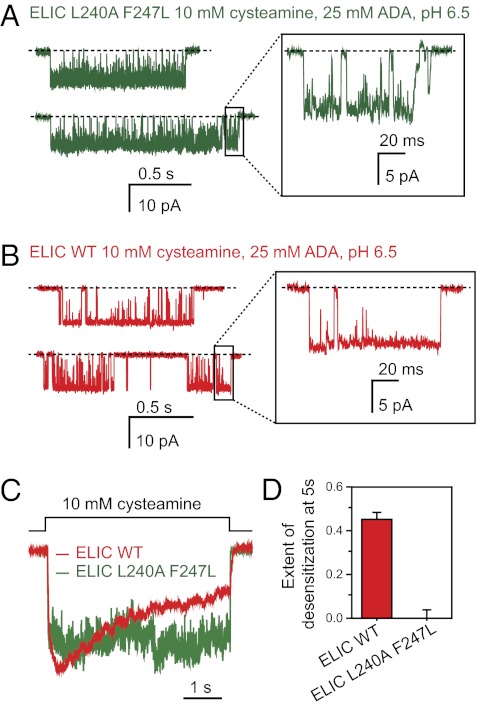
The best-diffracting crystals were those grown in the presence of 10 mM cysteamine at pH ∼6.5–6.9. To assess the effect of this lower pH on channel function (note that the electrophysiological characterization presented above was carried out at pH 7.4), we recorded currents from the L9′A + F16′L double mutant in the presence of an external solution of pH 6.5 containing the same proton buffer as that used in the reservoir solution [that is, N-(2-acetamido)iminodiacetic acid; see SI Materials and Methods]. Interestingly, we found that the change of external pH from 7.4 to 6.5 (in the presence of 10 mM cysteamine) increases the intracluster open probability of the double mutant from ∼0.44 to ∼0.93 (Figs. 3 C and E and 4 A and B); the desensitization time course remains unaffected (Fig. 4 C and D). This effect of pH on channel-gating is highly advantageous in the context of this work because it increases the contribution of the open-channel state to the mixture of interconverting conformations. At pH 7.4, on the other hand, even a concentration of cysteamine as high as 50 mM only elicits an open probability of ∼0.75 (Fig. S4). A more detailed analysis of the effect of protons (and, perhaps, of the proton buffer itself) on the cysteamine-gated activity of ELIC is needed. However, with cysteamine pKa1 and pKa2 values of ∼8.60 and ∼10.75, it is clear that the effect of low pH on the L9′A + F16′L double mutant cannot be ascribed to the increased concentration of the fully protonated form of this ligand. Certainly, the latter is expected to account for almost all of the cysteamine present in solution already at pH 7.4 (e.g., ∼9.4 mM for a total concentration of 10 mM).
Fig. 4.
Effect of pH. (A and B) Stretches of single-channel currents recorded in the outside-out configuration (Xenopus oocyte membranes) at −80 mV, in the continued presence of cysteamine. The pH of the patch-pipette solution was 7.4. The pH of the external solution was adjusted to 6.5 using 25 mM N-(2-acetamido)iminodiacetic acid (ADA) as the proton buffer in an attempt to mimic the conditions in the crystallization buffer (SI Materials and Methods). Channel openings are downward deflections. Display fc ∼5 kHz. Clusters were defined as series of openings separated by shuttings shorter than 200 ms. The intracluster open probability of the L9′A + F16′L double mutant increases from ∼0.44 at external pH 7.4 (Fig. 3E) to ∼0.93 at external pH 6.5. (C) Normalized time courses of currents elicited by the application of cysteamine for 5 s. The pH of the two external solutions flowing from the θ-type tubing was adjusted to 6.5 using 25 mM ADA as the proton buffer. The pH of the patch-pipette solution was 7.4. (D) Extent of desensitization (mean ± SE).
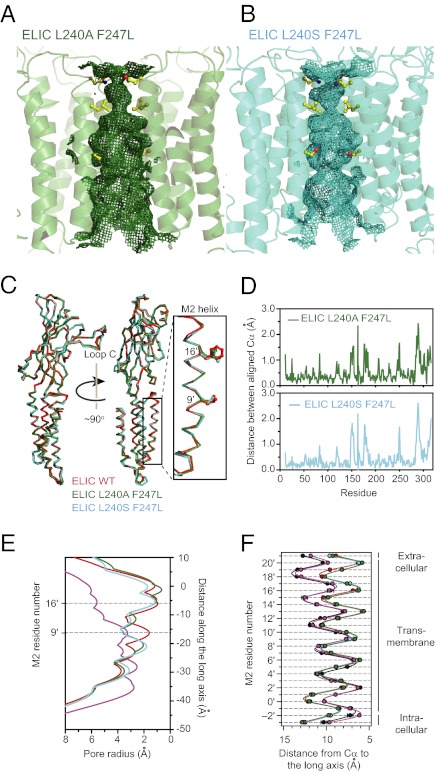
The crystals from the two double mutants diffracted to 3.8 Å (L9′S + F16′L) and 4.2 Å (L9′A + F16′L), a resolution that, albeit low, is comparable to that of other X-ray crystal structures of agonist-bound (10) and full-length (29, 30) bacterial channels. Evidently, this resolution is too low to allow the determination of the conformation of individual side chains or to detect the rearrangement of loop regions unambiguously, but it is enough to detect large changes in the orientation of the transmembrane α-helices of the kind that would make the pore of ELIC look like that of GLIC. A comparison of the structural models of the mutants with that of unliganded wild-type ELIC, however, failed to reveal such changes (Fig. 5 and Fig. S5A). Similar results were obtained when the structural models of the two double mutants in the presence of 10 mM cysteamine were compared with the model of the unliganded F16′L single mutant (Fig. S5 B–D), the structure of which we solved at 3.5 Å resolution. Thus, in the absence of more detailed information about the conformation of side chains, we are compelled to conclude that the pore structure of the two double mutants of ELIC in the presence of a high concentration of cysteamine is essentially the same as that of the unliganded wild-type channel, at least in the crystalline state. This result is quite puzzling because our electrophysiological characterization of the two double mutants led us to expect, perhaps naïvely, that they would be crystallized in the open or, eventually, in some desensitized conformation. However, there are other similar examples of structural models having a tenuous connection to electrophysiological data. For example, Fig. 5F shows that the distances between the Cα atoms of the M2 segments and the long axis of the pore in the GLIC open-channel model are indistinguishable from those in the muscle-type AChR model from Torpedo (PDB ID code: 2BG9), even though the latter was built from data collected in the complete absence of cholinergic ligands (32), and hence, is expected to represent the closed-channel conformation.
Fig. 5.
X-ray crystal structures of two mutants of ELIC in the presence of 10 mM cysteamine. (A and B) Ribbon representation of the transmembrane region of the indicated structural models. The mesh shows the solvent-accessible surface. The side chains corresponding to pore-facing positions 9′, 16′, and 20′ (that is, the side chains forming the three narrowest constrictions of the pore in unliganded wild-type ELIC) are shown in ball-and-stick representation. The front subunit was removed, for clarity. (Upper) Extracellular. (Lower) Intracellular. The corresponding representation of unliganded wild-type ELIC is shown in Fig. S5A. (C) Aligned Cα traces. Only one subunit per construct is shown. (D) Distance between aligned Cα atoms. The Cα atoms of each subunit in the structural models of the mutants L9′A + F16′L and L9′S + F16′L in the presence of 10 mM cysteamine were aligned with those of the unliganded wild-type ELIC (mutant chain A with wild-type chain A, mutant chain B with wild-type chain B, and so on, for all 10 subunits in the asymmetric unit), and the distances between Cα atoms were calculated and averaged. (E) HOLE profiles (31) of ELIC wild-type and the two pore mutants. The value of zero along the distance axis corresponds to position 20′. The color code is the same as that in C. The profile of GLIC in the presence of a desensitizing concentration of protons (PDB ID code: 3EAM; in purple) is also included. (F) Distances from the Cα atoms to the long axis of the pore (mean ± SE of all subunits) for residues in and flanking the transmembrane segments M2 of the four constructs compared in E. The color code is the same as that in E. The distance profile of the AChR from Torpedo in the absence of activating ligands (PDB ID code: 2BG9; in black) is also included; this profile is nearly indistinguishable from that of GLIC. The three profiles of ELIC (corresponding to the unliganded wild-type and the two double mutants in the presence of 10 mM cysteamine at pH ∼6.5) are also indistinguishable from each other.
Further analysis of the X-ray experimental data revealed additional electron density within the transmembrane pore at the level of the ring formed by the five asparagines at position 20′. Tentatively, we modeled this signal (in the F–J pentamer of the L9′A + F16′L double mutant and in both pentamers in the asymmetric unit of the F16′L single mutant) as arising from a Na+ ion, but we cannot rule out the possibility that this density actually corresponds to NH4+, the other cation present at a high concentration in the crystallization milieu. In the case of the crystals grown in the presence of cysteamine, additional electron density can also be observed at some of the five subunit–subunit interfaces in the region expected to harbor the agonist-binding sites (Fig. S6). However, because of the limited resolution of the data, no attempt was made to model cysteamine molecules at these locations.
Discussion
By no means should our results be taken to imply that mutations, in general, are ineffective at favoring the crystallization of specific conformations of nicotinic-type receptors. Rather, to the extent that many aspects of membrane-protein crystallization remain a matter of trial and error, we suggest that other closed-channel destabilizing mutations should be tested as well. However, it is likely that approaches other than point mutations or ligand binding will be needed to coax ELIC to crystallize in its different functional states. Perhaps, the lack of a phospholipid membrane, the presence of osmolytes and precipitants, and the limitations imposed by the periodic packing of the protein in the crystal lattice alter the energetics of ELIC's conformational equilibria to the extent that the effect of mutations on the occupancy probabilities in the crystallization milieu cannot be predicted from electrophysiological recordings. In fact, these factors have been shown to affect the conformational dynamics of BtuB (the Escherichia coli outer-membrane transporter for vitamin B12) (33–35) and the kinetics of the photocycle in bacteriorhodopsin (36), for example. In addition, of particular relevance to this article, the effect of membrane lipids on the relative occupancies of the closed, open and desensitized states of the AChR from Torpedo's electric organ has been well documented (3, 37, 38). Alternatively, it may well be that only one conformation of ELIC crystallizes under the tested conditions, in which case all efforts to crystallize specific functional states of the channel by appropriately tilting the free-energy landscape would be futile.
As for the kinetic properties of ELIC, we think that its uncharacteristically slow activation time course and the noncanonical effect of pore mutations on some of its functional properties should be regarded as intriguing features (rather than as disappointing findings) that may help unlock some of the secrets that this superfamily of ion channels still holds. The high single-channel conductance, the tractable kinetics, and the relative ease with which crystals form justify the use of this bacterial channel for further studies.
Materials and Methods
Current recordings from ELIC, GLIC, and the mouse-muscle AChR (adult type) heterologously expressed in Xenopus laevis oocytes (ELIC) or HEK-293 cells (ELIC, GLIC, and AChR) were performed at ∼22 °C using the outside-out or the cell-attached patch-clamp configuration, as indicated. In the outside-out configuration, agonist was applied to the excised patches of membrane as rapid jumps (solution-exchange time10–90%<150 μs) achieved by the switching of two solutions (differing only in the presence or absence of agonist) flowing from either barrel of a piece of θ-type capillary glass mounted on a piezo-electric device (Burleigh-LSS-3100; Thorlabs). The patch-pipette solution consisted of: 110 mM KF, 40 mM KCl, 1.0 mM CaCl2, 11 mM EGTA, and 10 mM Hepes/KOH, pH 7.4, whereas the agonist-free solution flowing through one of the barrels of the theta-type tubing consisted of: 142 mM KCl, 5.4 mM NaCl, 1.8 mM CaCl2, 1.7 mM MgCl2, and 10 mM Hepes/KOH, pH 7.4. The concentration of agonist in the second-barrel solution was 100 μM ACh for recordings from the AChR and 10–100 mM cysteamine for recordings from ELIC. For some of the latter, the pH of both θ-tube solutions was adjusted to 6.5 with KOH using 25 mM N-(2-acetamido)iminodiacetic acid (ADA), instead of Hepes, as the proton buffer; the agonist-containing solution also contained 10 mM cysteamine. For recordings from GLIC, the pH values of the two θ-tube solutions differed; these were adjusted to 4.5, 7.4, or 9.0 with KOH using 10 mM acetic acid, Hepes or N-Tris[hydroxymethyl]methyl-4-aminobutane-sulfonic acid, respectively, as the proton buffer. Because the thiol group of cysteamine is prone to oxidation, cysteamine solutions used for electrophysiology experiments were prepared daily from frozen (−80 °C) stock solutions. To estimate the extent to which the concentration of cysteamine decreases during the several day-long incubations at 4 °C that were required for crystals to grow, we measured its time course at 4 °C in 10-mM solutions by determining the amount of 5-thio-2-nitrobenzoate released upon reaction with 5,5′-dithio-bis(2-nitrobenzoate) at pH ∼7.3 (26). Our cysteamine-calibration curves yielded an extinction coefficient for the released 5-thio-2-nitrobenzoate at 412 nm of ∼14,183 M−1⋅cm−1, which is in reasonable agreement with the reported value of 14,150 M−1⋅cm−1 (26). From these measurements, we estimate that the half-life of reduced cysteamine at 4 °C is ∼20 d (Fig. S7). ELIC overexpression, purification, crystallization, and structure determination were performed essentially as described by Hilf and Dutzler (9) with minor modifications (39). Because of the limited resolution of the X-ray data, no attempt was made to force the side chains in our models to adopt conformations that correspond to rotamers or that maximize the number of hydrogen bonds formed. Detailed descriptions are provided in SI Materials and Methods. Crystal parameters, data-collection parameters, and refinement statistics are indicated in Table S1.
Supplementary Material
Acknowledgments
We thank Luis Cuello, Vivian González-Pérez, and Emad Tajkhorshid for expert advice; Steven Sine for wild-type muscle AChR subunit cDNA; Keith Brister, Joseph Brunzelle, David Smith, and Zdzislaw Wawrzak at the Life Sciences Collaborative Access Team (21 ID–F/G at Argonne National Laboratory, Advanced Photon Source) for technical assistance during crystallographic data collection; and M. Pasquini and A. Holmstrom for assistance with cell culturing. This work was supported by National Institutes of Health Grants R01 GM095600 (to S.K.N.) and R01 NS042169 and corresponding American Recovery and Reinvestment Act of 2009 supplement (to C.G.); and National Institutes of Health Training Grant T32GM008276 (to D.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The crystallography, atomic coordinates, and structure factors have been deposited in the Protein Data Bank, www.pdb.org [PDB ID codes: 3UQ4 (ELIC F247L), 3UQ5, (ELIC L240A + F247L in the presence of 10 mM cysteamine) and 3UQ7 (ELIC L240S + F247L in the presence of 10 mM cysteamine)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119268109/-/DCSupplemental.
References
- 1.Papke D, Gonzalez-Gutierrez G, Grosman C. Desensitization of neurotransmitter-gated ion channels during high-frequency stimulation: A comparative study of Cys-loop, AMPA and purinergic receptors. J Physiol. 2011;589:1571–1585. doi: 10.1113/jphysiol.2010.203315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd ND, Cohen JB. Kinetics of binding of [3H]acetylcholine and [3H]carbamoylcholine to Torpedo postsynaptic membranes: slow conformational transitions of the cholinergic receptor. Biochemistry. 1980;19:5344–5353. doi: 10.1021/bi00564a031. [DOI] [PubMed] [Google Scholar]
- 3.Martinez KL, et al. Allosteric transitions of Torpedo acetylcholine receptor in lipids, detergent and amphipols: molecular interactions vs. physical constraints. FEBS Lett. 2002;528:251–256. doi: 10.1016/s0014-5793(02)03306-9. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Gutierrez G, Grosman C. Bridging the gap between structural models of nicotinic receptor superfamily ion channels and their corresponding functional states. J Mol Biol. 2010;403:693–705. doi: 10.1016/j.jmb.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bocquet N, et al. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009;457:111–114. doi: 10.1038/nature07462. [DOI] [PubMed] [Google Scholar]
- 6.Hilf RJ, Dutzler R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature. 2009;457:115–118. doi: 10.1038/nature07461. [DOI] [PubMed] [Google Scholar]
- 7.Parikh RB, Bali M, Akabas MH. Structure of the M2 transmembrane segment of GLIC, a prokaryotic Cys loop receptor homologue from Gloeobacter violaceus, probed by substituted cysteine accessibility. J Biol Chem. 2011;286:14098–14109. doi: 10.1074/jbc.M111.221895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nury H, et al. X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature. 2011;469:428–431. doi: 10.1038/nature09647. [DOI] [PubMed] [Google Scholar]
- 9.Hilf RJ, Dutzler R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature. 2008;452:375–379. doi: 10.1038/nature06717. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann I, Dutzler R. Ligand activation of the prokaryotic pentameric ligand-gated ion channel ELIC. PLoS Biol. 2011;9:e1001101. doi: 10.1371/journal.pbio.1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Revah F, et al. Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor. Nature. 1991;353:846–849. doi: 10.1038/353846a0. [DOI] [PubMed] [Google Scholar]
- 12.Filatov GN, White MM. The role of conserved leucines in the M2 domain of the acetylcholine receptor in channel gating. Mol Pharmacol. 1995;48:379–384. [PubMed] [Google Scholar]
- 13.Labarca C, et al. Channel gating governed symmetrically by conserved leucine residues in the M2 domain of nicotinic receptors. Nature. 1995;376:514–516. doi: 10.1038/376514a0. [DOI] [PubMed] [Google Scholar]
- 14.Tierney ML, et al. Effects of mutating leucine to threonine in the M2 segment of α1 and β1 subunits of GABAA α1β1 receptors. J Membr Biol. 1996;154:11–21. doi: 10.1007/s002329900128. [DOI] [PubMed] [Google Scholar]
- 15.Chang Y, Weiss DS. Substitutions of the highly conserved M2 leucine create spontaneously opening ρ1 γ-aminobutyric acid receptors. Mol Pharmacol. 1998;53:511–523. doi: 10.1124/mol.53.3.511. [DOI] [PubMed] [Google Scholar]
- 16.Grosman C, Zhou M, Auerbach A. Mapping the conformational wave of acetylcholine receptor channel gating. Nature. 2000;403:773–776. doi: 10.1038/35001586. [DOI] [PubMed] [Google Scholar]
- 17.Kosolapov AV, Filatov GN, White MM. Acetylcholine receptor gating is influenced by the polarity of amino acids at position 9′ in the M2 domain. J Membr Biol. 2000;174:191–197. doi: 10.1007/s002320001043. [DOI] [PubMed] [Google Scholar]
- 18.Bianchi MT, Macdonald RL. Mutation of the 9′ leucine in the GABAA receptor γ2L subunit produces an apparent decrease in desensitization by stabilizing open states without altering desensitized states. Neuropharmacol. 2001;41:737–744. doi: 10.1016/s0028-3908(01)00132-0. [DOI] [PubMed] [Google Scholar]
- 19.Cymes GD, Grosman C, Auerbach A. Structure of the transition state of gating in the acetylcholine receptor channel pore: A phi-value analysis. Biochemistry. 2002;41:5548–5555. doi: 10.1021/bi011864f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panicker S, Cruz H, Arrabit C, Slesinger PA. Evidence for a centrally located gate in the pore of a serotonin-gated ion channel. J Neurosci. 2002;22:1629–1639. doi: 10.1523/JNEUROSCI.22-05-01629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shan Q, Nevin ST, Haddrill JL, Lynch JW. Asymmetric contribution of α and β subunits to the activation of alphabeta heteromeric glycine receptors. J Neurochem. 2003;86:498–507. doi: 10.1046/j.1471-4159.2003.01872.x. [DOI] [PubMed] [Google Scholar]
- 22.Plazas PV, et al. Key roles of hydrophobic rings of TM2 in gating of the α9α10 nicotinic cholinergic receptor. Br J Pharmacol. 2005;145:963–974. doi: 10.1038/sj.bjp.0706224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purohit P, Auerbach A. Unliganded gating of acetylcholine receptor channels. Proc Natl Acad Sci USA. 2009;106:115–120. doi: 10.1073/pnas.0809272106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taranda J, et al. A point mutation in the hair cell nicotinic cholinergic receptor prolongs cochlear inhibition and enhances noise protection. PLoS Biol. 2009;7:e18. doi: 10.1371/journal.pbio.1000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mott DD, Erreger K, Banke TG, Traynelis SF. Open probability of homomeric murine 5-HT3A serotonin receptors depends on subunit occupancy. J Physiol. 2001;535:427–443. doi: 10.1111/j.1469-7793.2001.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riddles PW, Blakeley RL, Zerner B. Ellman's reagent: 5,5′-dithiobis(2-nitrobenzoic acid)—A reexamination. Anal Biochem. 1979;94:75–81. doi: 10.1016/0003-2697(79)90792-9. [DOI] [PubMed] [Google Scholar]
- 27.Sakmann B, Patlak J, Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980;286:71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- 28.Lefebvre JL, et al. Increased neuromuscular activity causes axonal defects and muscular degeneration. Development. 2004;131:2605–2618. doi: 10.1242/dev.01123. [DOI] [PubMed] [Google Scholar]
- 29.Uysal S, et al. Crystal structure of full-length KcsA in its closed conformation. Proc Natl Acad Sci USA. 2009;106:6644–6649. doi: 10.1073/pnas.0810663106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uysal S, et al. Mechanism of activation gating in the full-length KcsA K+ channel. Proc Natl Acad Sci USA. 2011;108:11896–11899. doi: 10.1073/pnas.1105112108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smart OS, Neduvelil JG, Wang X, Wallace BA, Sansom MS. HOLE: A program for the analysis of the pore dimensions of ion channel structural models. J Mol Graph. 1996;14:354–360, 376. doi: 10.1016/s0263-7855(97)00009-x. [DOI] [PubMed] [Google Scholar]
- 32.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 33.Fanucci GE, Lee JY, Cafiso DS. Membrane mimetic environments alter the conformation of the outer membrane protein BtuB. J Am Chem Soc. 2003;125:13932–13933. doi: 10.1021/ja0376442. [DOI] [PubMed] [Google Scholar]
- 34.Fanucci GE, Lee JY, Cafiso DS. Spectroscopic evidence that osmolytes used in crystallization buffers inhibit a conformation change in a membrane protein. Biochemistry. 2003;42:13106–13112. doi: 10.1021/bi035439t. [DOI] [PubMed] [Google Scholar]
- 35.Freed DM, Horanyi PS, Wiener MC, Cafiso DS. Conformational exchange in a membrane transport protein is altered in protein crystals. Biophys J. 2010;99:1604–1610. doi: 10.1016/j.bpj.2010.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Efremov R, Gordeliy VI, Heberle J, Büldt G. Time-resolved microspectroscopy on a single crystal of bacteriorhodopsin reveals lattice-induced differences in the photocycle kinetics. Biophys J. 2006;91:1441–1451. doi: 10.1529/biophysj.106.083345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamouda AK, Sanghvi M, Sauls D, Machu TK, Blanton MP. Assessing the lipid requirements of the Torpedo californica nicotinic acetylcholine receptor. Biochemistry. 2006;45:4327–4337. doi: 10.1021/bi052281z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.daCosta CJB, et al. Anionic lipids allosterically modulate multiple nicotinic acetylcholine receptor conformational equilibria. J Biol Chem. 2009;284:33841–33849. doi: 10.1074/jbc.M109.048280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newby ZE, et al. A general protocol for the crystallization of membrane proteins for X-ray structural investigation. Nat Protoc. 2009;4:619–637. doi: 10.1038/nprot.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.