Abstract
Judging the presence or absence of a stimulus is likely the most basic perceptual decision. A fundamental difference of detection tasks in contrast to discrimination tasks is that only the stimulus presence decision can be inferred from sensory evidence, whereas the alternative decision about stimulus absence lacks sensory evidence by definition. Detection decisions have been studied in an intentional, action-based framework, in which decisions were regarded as intentions to pursue particular actions. These studies have found that only stimulus-present decisions are actively encoded by neurons, whereas the decision about the absence of a stimulus does not affect default neuronal responses. We tested whether this processing mechanism also holds for abstract detection decisions that are dissociated from motor preparation. We recorded single-neuron activity from the prefrontal cortex (PFC) of monkeys performing a visual detection task that forced a report-independent decision. We not only found neurons that actively encoded the subjective decision of monkeys about the presence of a stimulus, but also cells responding actively for the decision about the absence of stimuli. These results suggest that abstract detection decisions are processed in a different way compared with the previously reported action-based decisions. In a report-independent framework, neuronal networks seem to generate a second set of neurons actively encoding the absence of sensory stimulation, thus translating decisions into abstract categories. This mechanism may allow the brain to “buffer” a decision in a nonmovement-related framework.
Keywords: perceptual detection, abstract decision, single-cell recordings, rhesus monkey
Perceptual decisions are choices among alternatives based on sensory information. To arrive at distinct choices, sensory input has to be classified into behaviorally meaningful categories. The detection of a stimulus (decision about its presence or absence) is the most basic form of a perceptual decision. The peculiarity of detection decisions is that only one choice alternative can be based on sensory evidence, whereas the alternative decision about stimulus absence lacks sensory information. How does the brain arrive at categorical stimulus-absent and stimulus-present decisions when only one response category (stimulus-present) can rely on sensory input?
The neuronal underpinnings of perceptual decisions have been studied extensively in an intentional, action-based framework (1–3). Here, decisions are regarded as intensions to choose among actions associated with the stimuli (1, 4). Decision-related neurons showed activity encoding the process of converting sensory information (5–7) or cognitive cues (8–10) into choices. In agreement with the view that detections are discriminations of a stimulus from noise (11), elegant studies by Romo and coworkers (12, 13) reported neurons actively encoding the decision about the stimulus presence. The decision about stimulus absence, however, was represented as a default (baseline) neuronal response (12–16) for action-based detection decisions.
When dissociated from action preparation or studied in a report-independent framework, decisions can be seen as distinct processes that are encoded as abstract categories (17–19). In a dot-motion discrimination task, neurons in the lateral intraparietal area were shown to encode the abstract decision about motion directions independently from how they signaled the associated motor response (20). In such discrimination and categorization tasks, subjects decide based on sensory stimuli represented for both alternatives. Thus, two separate neuronal populations encode the respective choice categories.
We investigated how abstract detection decisions, not linked to motor actions, are implemented by single neurons in the prefrontal cortex (PFC) of rhesus monkeys. To ensure report-independent decisions, we designed a rule-based visual detection task that allowed a clear dissociation of a decision about the stimulus from motor preparation. In such a protocol, also the stimulus absent decision is not just “noise” but a discrete category.
Results
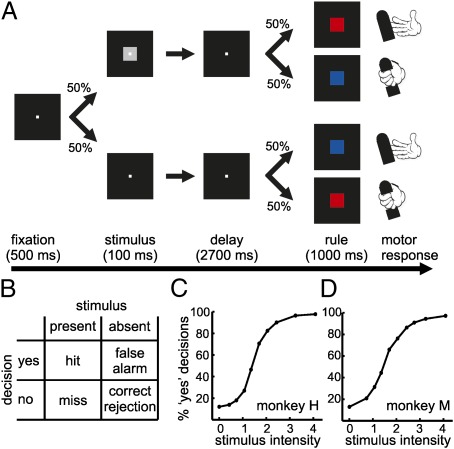
We trained two rhesus monkeys to report the presence or absence of a visual stimulus in a rule-based delayed detection task that allowed a clear dissociation of the decision about the stimulus from motor preparation (Fig. 1A). The visual stimulus was presented at different intensity values centered around perceptual threshold. For stimuli of identical intensities, the internal status of the monkey determined whether it had (“yes” decision) or had not (“no” decision) seen the stimulus. Three different visual objects were used to ensure that the monkeys relied on the mere presence of the stimuli, whereas ignoring low-level object properties. Because a rule cue informed the animal about the appropriate motor action of how to report the decision, the monkey could not prepare any motor response during the delay period. The possible decision outcomes were classified according to signal detection theory (Fig. 1B). The proportion of “yes” decisions for each stimulus intensity was used to create psychometric detection curves for both monkeys (Fig. 1 C and D). The monkeys were only rewarded in correct trials (hits and correct rejections). No reinforcement was given in trials in which the monkeys failed to detect stimuli (misses), even if they were presented below the perceptual threshold. This reward contingency leads to a small bias of the monkeys to erroneously report the presence of a stimulus in some of the stimulus absent trials (false alarms) (Fig. 1 C and D).
Fig. 1.
Visual detection protocol and behavioral performance. (A) The monkeys initiated each experimental trial by grasping a lever and fixating a central fixation target. After 500 ms, a stimulus was displayed for 100 ms in 50% of the trials (intensity varied in nine levels, centered around the perceptual threshold). In the other 50% of the trials, no stimulus was shown. Both types of trials appeared randomly. After the delay period (2,700 ms), a color cue appeared to indicate the rule of how to respond to a particular decision. If a stimulus was presented, a red square cue required the monkey to release the lever within 1,000 ms to receive a fluid reward, whereas a blue cue demanded the monkey to keep holding the lever for another 1,200 ms. The rule applied in the inverse way if no stimulus was presented. Thus, movement preparation was excluded during the delay period. (B) Signal detection theory classifies an observer's behavioral options (hit, miss, correct rejection, and false alarms) at detection threshold, given two stimulus conditions (stimulus present or absent) and two possible decisions (“yes, stimulus present” and “no, stimulus absent”). (C and D) Psychometric detection curve for monkey H (C) and monkey M (D). Stimulus intensity is represented as % visual contrast; visual contrast of 0 indicates absence of stimulus. [Error bars (SEM) are so small that they are hidden behind the markers].
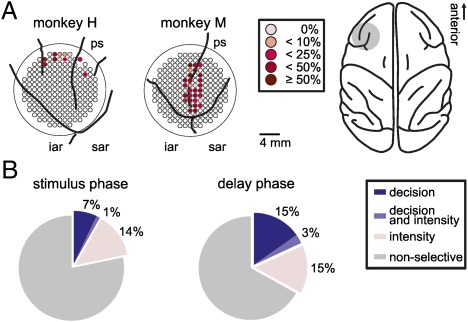
While the monkeys performed the detection task, we recorded the activity of 708 randomly selected neurons from the PFC (Fig. 2A). We analyzed neuronal activity during the stimulus phase (immediately after stimulus presentation) to investigate the very early stage of decision formation, and the late-delay phase during which motor preparation was still excluded. We applied stepwise linear regression (SLR) analysis to study the conjoint contributions of stimulus intensity and the subjective “yes” and “no” decisions on the discharge rates of the neurons. During both analysis phases, we found a proportion of neurons significantly coding the subjective judgments of monkeys about the stimulus presence or absence [Fig. 2B; 8% (58/708) during the stimulus phase and 18% (128/708) during the delay phase, P < 0.05, SLR analysis]. A proportion of 14% of the cells (96/708) during the stimulus phase and 15% of the neurons (106/708) during the delay phase only coded the intensity of the stimulus (P < 0.05, SLR analysis). Only 1% and 3% of the recorded neurons were modulated by both the factors stimulus intensity and the subjective decision during the stimulus and delay phase, respectively (Fig. 2B). Neurons significantly covarying with the monkey's choices were termed “decision neurons.” Overall, we found a significantly higher proportion of decision neurons in the delay phase compared with the stimulus phase, (P < 0.01; χ2 test).
Fig. 2.
Recording sites and proportion of selective cells. (A) Right shows a top view of a monkey brain. The gray area marks the chamber location. The circular panels on Left show the precise recording sites inside each recording chamber in the lateral PFC for both monkeys. The proportion of decision neurons at individual recording sites is color-coded. iar, inferior arcuate sulcus; ps, principal sulcus; sar, superior arcuate sulcus. (B) Proportions of neurons coding stimulus intensity and decision in both phases.
Receiver operating characteristics (ROC) analysis was used to quantify the probability with which the decision of the monkey could be predicted from the neuronal responses. Choice probability indices were calculated for “yes” decisions in clearly visible, salient stimulus trials (hits) versus “no” decisions in stimulus-absent trials (correct rejections), as well as for “yes” (hits) versus “no” (misses) decisions in threshold trials when stimuli were presented close to the perceptual threshold.
Neuronal selectivity of a given neuron is usually determined by the experimental condition that elicits the highest discharge rate. This approach ignores that suppressive effects (decreases in firing rates relative to baseline discharge) are sometimes the dominant influences of a particular stimulus. Thus, we subdivided and classified decision neurons according to their active modulation of neuronal activity (modulation strength) during “yes” or “no” decisions rather than highest discharge rate. Neurons modulating (increasing or decreasing) their firing rates more strongly for “yes” decisions were termed “yes” neurons, cells modulating their discharges more strongly to “no” decision were called “no” neurons.
“Yes” Neurons Actively Encode Decisions During the Stimulus Phase.
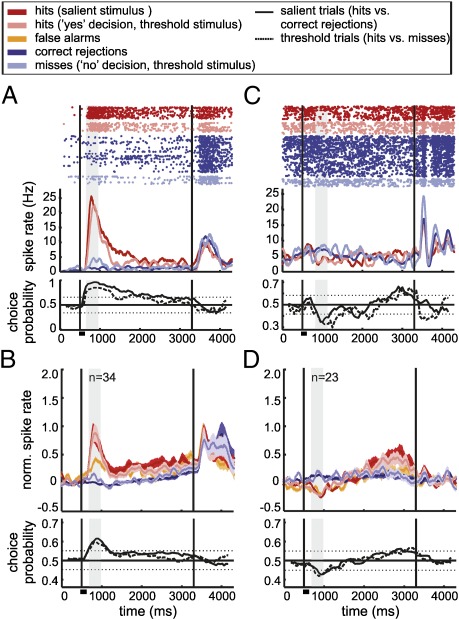
During the stimulus phase, virtually all decision neurons (98%) modulated their discharge rates only for “yes” judgments (merely one neuron was classified as a “no” cell; summary in Table S1). Fig. 3A shows an exemplary “yes” cell that increased its discharge rates for hits in salient stimulus trials, whereas the firing rates for correct rejections in stimulus-absent trials remained at baseline level. Neuronal responses for threshold trials correlated significantly with the judgment of the monkey: for “yes” decisions, neurons increased their activity, mirroring the firing rate in salient stimulus trials. For erroneous “no” decisions (misses), activity remained at baseline level, just as in stimulus absent trials. The choice probability indices for salient and threshold trials are depicted as a function of time in Fig. 3A, Lower. Indices significantly above chance level indicate that these discharges of neurons reliably predict the decision of the monkey (P < 0.05; ROC analysis, bootstrapping). This effect was also present on the neuronal population level (34 cells; Fig. 3B). Several cells showed transient suppression of the firing rate for “yes” decisions (Fig. 3C); the neuronal population data (23 units) are depicted in Fig. 3D. The population analysis includes the neuronal responses during false alarms. Interestingly, decision neurons increased (Fig. 3B) or decreased (Fig. 3D) the firing rates for this erroneous “yes” decisions in a similar way as during hit trials, already during this early decision phase. The average peak latencies of the neuronal responses for false alarms and hits were comparable (neurons increasing the firing rate: hit latency = 242 ms, false alarms latency = 289 ms; neurons decreasing firing rates: hit latency = 346 ms, false alarms latency = 341 ms). Overall, during the stimulus phase, PFC neurons represented “yes” decisions by either increasing or decreasing their responses, whereas “no” decisions were represented by default discharge rates.
Fig. 3.
Decision coding by “yes” neurons during the stimulus phase. (A and C) Responses of two example neurons coding the “yes” decision by increasing (A) or decreasing (C) their firing rates during the stimulus phase (analysis window highlighted by the gray shaded area). Top depict dot raster plots; Middle represent the corresponding spike density histograms averaged and smoothed with a Gaussian kernel for illustration. The vertical black lines indicate the presentation of the stimulus (at 500 ms) and the rule cue (at 3,300 ms). Stimulus duration is marked by a small horizontal bar underneath the x axis of each plot. Bottom show the choice probability indices as a function of time. Dotted lines mark significance levels. (B and D) Averaged and normalized responses (SI Materials and Methods) and choice probability indices of decision neurons grouped by response type. Shaded regions indicate SEM; n, number of neurons.
“Yes” and “No” Neurons Actively Encode Decisions During the Delay Phase.
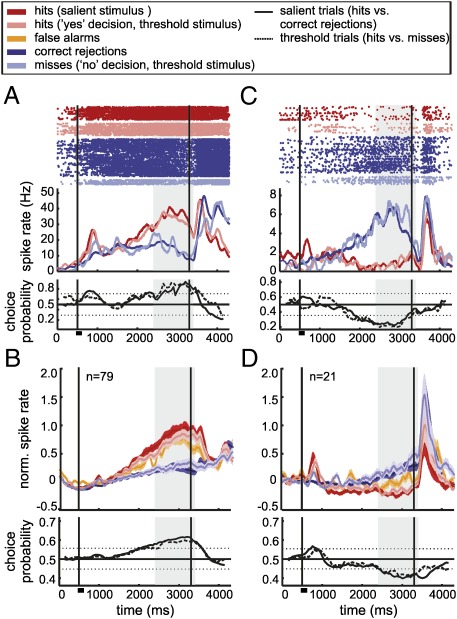
In striking contrast to the findings in the sample phase, the processing of decisions in the delay phase was based on “yes” (Fig. 4 A and B) as well as active “no” responses (Fig. 4 C and D) (summary in Table S1). Just as in the stimulus phase, we found neurons increasing (79 neurons; Fig. 4B) or decreasing (25 units) their firing rates for “yes” decisions (hits and false alarms). In addition, however, a new class of decision cells, “no” neurons, exhibited significantly increased discharge rates whenever the monkey decided to report the absence of a stimulus (correct rejections and misses) (Fig. 4C; average responses of 21 neurons in Fig. 4D). Three cells were classified as decreasing their firing rate for “no” responses. These active “no” neurons represent an abstract category that is neither generated by a specific input nor linked to a preparation of a motor response.
Fig. 4.
Decision coding by “yes” and “no” neurons during the delay phase. (A and C) Raster plots, spike density functions, and choice probability indices for neurons increasing their activity for “yes” decisions (A) or for “no” decisions (C) during the delay phase. (B and D) Normalized averaged responses (SI Materials and Methods) of the corresponding groups. Active “no” decision neurons newly emerged during the delay phase. Same layout as in Fig. 3.
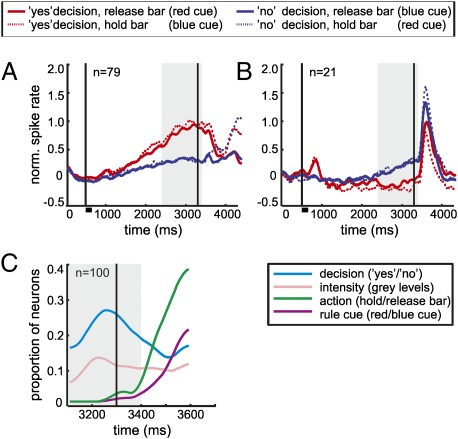
Finally, we also investigated the neuronal selectivity of delay phase decision neurons to the rule cue and the instructed motor action during the rule cue phase. For both “yes” and “no” decision neurons, decision activity remained the dominant factor well into the rule cue phase (Fig. 5 A and B). To identify the proportions of decision neurons responding to decision, stimulus intensity, rule cue, and motor action during the cue presentation, we next performed a sliding SLR analysis by using these factors (Fig. 5C). Selectivity for the color cue or the motor action was absent during the delay analysis period. Only after a latency of ≈100 ms after rule cue onset, decision cells started to encode the color of the rule cue and, most dominantly, the instructed motor action.
Fig. 5.
Selectivity of decision cells during rule cue presentation. (A and B) Averaged neuronal activity of “yes” (A) and “no” delay phase decision neurons (B) is shown throughout the trial during “yes” and “no” decisions separated according to the rule cue (requiring a particular motor action). The figure has the same layout as in Fig. 3. (C) Proportion of all “yes” and “no” delay phase decision neurons significantly selective for the factors decision, stimulus intensity, motor action, and rule cue during the cue phase. The vertical black line at 3,300 ms depicts the onset of the rule cue. On average, the monkeys performed a motor action 300 ms after the rule cue onset in release trials. The gray area highlights the analysis window of the delay phase. No selectivity for the rule cue or the motor action is present during the delay phase analysis window.
Discussion
We found single neurons in the PFC that encode abstract “yes” and “no” decisions during a visual detection task. In this perceptual decision task that dissociated the decision both from low-level sensory processing and preparatory motor activity, the neuronal activity covaried with the subjective reports of monkeys about the percepts. A very small proportion of decision neurons showed an additional significant effect of the intensity of the visual stimulus; thus, we report a predominantly categorical, binary activation pattern of “yes” or “no” decision coding. During the stimulus phase, decision neurons exclusively either increased or decreased their firing rates for “yes” decisions, whereas “no” responses were represented by baseline discharge rates. During the delay period, however, neurons also actively encoded “no” decisions. We propose that the coding of abstract, report-independent decisions is fundamentally similar to the representation of abstract categories (21), even if one choice alternative is devoid of sensory evidence. Our data thus extend previous findings about the representation of perceptual decisions in detection tasks (12).
Behavioral Relevance of the Perceptual Decision Encoding.
Decision neurons modulated their firing rates according to the sensory percept of monkeys: Activity signaling categorical “yes” decisions (hits and false alarms) was different from neuronal responses to correct rejections and misses (as shown by SLR analysis). The behavioral relevance of these responses becomes clear in the analysis of error trials. Miss trials clearly mimicked the responses to correct rejections during stimulus and delay phase. Similarly, a population analysis revealed that false alarm responses resembled the neuronal representations of hit trials. (The small number of false alarm trials precluded an analysis for individual cells). The slightly lower response amplitude of average false alarm responses compared with hit activity most likely reflects the different causes for such erroneous “yes” decisions that can appear during the course of a trial.
“No” Neurons.
The presence of active “no” neurons—in addition to active “yes” neurons—is an important finding of our study. During the delayed decision process, “no” cells encoded the decision actively by modulating their activity more strongly for “no” decisions, even in the absence of sensory evidence. One possible explanation for our finding is that decisions irrespective of motor preparation require additional neuronal representations compared with decisions in previous task designs (12, 13, 22, 23). Deco and coworkers used the term “type ‘no’ neurons” for cells that showed a transient peak activity during stimulus presentation and suppressed activity during the delay period whenever the monkey reported stimulus presence (24). Importantly, these neurons were reported to maintain baseline activity if no stimulus was presented. According to the definition we use in our study, these neurons would most likely correspond to “yes” neurons increasing their firing rate in the stimulus phase and decreasing the firing rate during the delay phase for “stimulus present” decisions.
Two Processing Steps of Abstract Decisions.
Our physiological results argue for two discrete processing steps involved in abstract decisions in detection tasks, implemented by “yes” cells in the stimulus phase and by “yes” and “no” cells during the delay phase. During the stimulus phase, the responses might reflect the subjective experience of the stimulus, based on the accumulation of sensory information (12). The emergence of “no” neurons in the delay phase likely constitutes a second active decision-processing step transforming the subjective experience to abstract categories in rule-based detection tasks.
In an abstract, report-independent decision protocol, in which the appropriate motor response is instructed later, a default motor action cannot easily be defined. From the computational perspective, “buffering” of the decision in a nonmovement-related framework (21) and applying two sets of active decision neurons (“yes” and “no” cells) would constitute an advantage. The principle of using two sets of active neurons is also implemented in (delayed) report-dependent (6, 7, 25–27) and report-independent (20) coding and models of decisions in discrimination tasks. In such comparison tasks, decision can be based on the evaluation of sensory evidence (e.g., “rightward” versus “leftward” motion), so the information of two sets of neurons actively coding the two alternative categories can be “translated” into a movement-related framework. With the applied detection task, we show that this coding scheme is a fundamental principle in representing abstract decisions. Our data show that, if sensory evidence is not available in the absence of sensory stimulation, the second set of active coding neurons is purposefully generated in neuronal networks.
The question of how the second active decision-processing step and the emergence of “no” neurons in abstract decisions is generated requires further investigation. One speculation might be that information during the stimulus phase is transferred and further processed throughout the delay phase. Another possibility might be that this late decision processing is triggered by midbrain dopamine (DA) neurons. A recent study reports high levels of DA activity for high uncertainty, which arises internally because of the evaluation of a sensory stimulus (28). Because stimulus-absent events carry a high level of uncertainty, this DA activity might account for the active “no” decision responses we measured in the PFC.
To guide behavior, the accuracy of neuronal decision signals should improve if information is combined across neurons. Sensory-related decisions have been found to rely on neurons with increasing and decreasing response profiles (13, 25). We find that both “yes” and “no” decision categories are encoded by facilitation and suppression. However, combining both apparently opposing information streams by a simple linear summation or averaging pooling rule would diminish/cancel out the information. A mechanistically similar situation occurs during the discrimination of opposite directions. Here, computational models suggest pooling profiles that specify how each neuron (tuned to its preferred direction) contributes to the decision (29). These pooling profiles result in opposite weighting of the contribution of neurons tuned to opposite directions. The difference of the weighted responses is used to determine the decision. Cells found in our study that encode the same decision category based on increasing and decreasing activity (but not opposing decisions) could exploit the same pooling principle within a decision category. To take full advantage of both information streams, pooling might rely on the difference between averaged subpopulations of neurons increasing and neurons decreasing their responses. This pooling-rule might be achieved if a subpopulation of increased-discharge decision neurons excites a downstream neuron, whereas a subpopulation of suppressed neurons inhibits this neuron.
Intentional and Report-Independent Frameworks.
Our data suggest that the best-suited neuronal representations of decision may be implemented depending on the nature of the behavioral task (intentional or report-independent). Decisions that can be formed as intensions to pursue a particular action may not require an abstract decision; thus, a direct link between stimulus activity (sensory input) and premotor activity (motor output) might be established. Therefore, the abstract decision may not even be represented as a discrete processing step at all (21, 30).
Our data indicate a complementary mechanism of decision processing, one that is deployed by the monkey brain when an abstract decision is forced. According to this hypothesis, deciding does not inevitably mean to plan a motor response (31). Rather, if required, decisions can be represented in an abstract processing step separated from motor effector systems, thus permitting complex operations between decision and action. Our results suggest that, if a rule cue were to be introduced in action-based detection tasks, the same kind of mechanism as observed in the current detection study would presumably emerge.
Brain Areas Encoding Abstract Decisions.
Although early sensory brain areas reflect the physical properties of a stimulus (12, 32), the correlation between neuronal discharges and interpretation (subjective experience) of a stimulus progressively increase across higher cortical hirarchy and result in a choice of an appropriate behavior (13, 33). We selected the PFC, a classical association area known to operate at the apex of the cortical hierarchy, as a candidate structure. PFC neurons have been shown to be engaged in highly abstract processes (34) including evaluation of sensory information (19, 35), decision-related processes (25, 36, 37), and abstract behavioral planning (38–40). Moreover, human fMRI suggested this area as an abstract decision-making module that is functionally separate from the motor systems (31, 41). We show that neurons in the PFC are strongly involved in the processing of abstract decisions.
However, other highly associative brain areas might also be strong candidates for the processing of abstract decision information. The medial premotor cortex (MPC) has been reported to be crucial for linking sensory information to action investigated from a motor perspective (37, 42, 43). The anterior cingulate cortex (ACC) has also been shown to reflect the intention for a particular action based on sensory (44) or reward information (45). It would be interesting to investigate whether and how these areas encode abstract decisions.
Materials and Methods
Behavioral Protocol.
Two rhesus monkeys (Macaca mulatta) were trained to report the presence or absence of a visual stimulus (Fig. 1A). The stimulus consisted of a gray object (4° of visual angle) presented at nine levels of contrast close to the perception threshold (monkey H: 4.1%, 3.2%, 2.4%, 2.0%, 1.7%, 1.4%, 1.1%, 0.7%, 0.4%; monkey M: 4.1%, 3.2%, 2.4%, 2.8%, 2.0%, 1.7%, 1.4%, 1.1%, 0.7%), measured with a J16 Digital Photometer (Tektronix). The shape of the object was chosen randomly from a set of three objects: square, circle, and hexagon for monkey H; cross, triangle, and rhomboid for monkey M. The area of the object was kept constant to maintain the same visual contrast of the stimulus across different shapes.
Monkeys kept their gaze within 1.75° of visual angle of the fixation target during stimulus and delay period. Eye movements were monitored with an infrared eye-tracking system (ISCAN). CORTEX program (National Institute of Mental Health) was used for experimental control and behavioral data acquisition. For the behavioral analysis, we gathered the proportion of “yes” decisions for stimulus present (hits) and stimulus absent (false alarms) trials (15021 and 14830 trials for monkey H; 14207 and 14326 trials for monkey M). For each stimulus intensity and type of decision, we pooled trials requiring lever release and holding trials from all recording sessions (Fig. 1 B and C).
Neurophysiological Recordings.
Extracellular single-cell activity was recorded by using arrays of four to eight glass-coated tungsten microelectrodes of 1 MΩ impedance (Alpha Omega) (SI Materials and Methods). All of the surgery procedures were carried out under aseptic conditions and under general anesthesia in accordance with the guidelines for animal experimentation approved by the local authorities, the Regierungspräsidium Tübingen, Germany.
Data Analysis.
Data analysis was performed by using MATLAB (MathWorks). We studied all well-isolated neurons and focused our analysis on two decision periods: a 300-ms interval after stimulus onset shifted by the individual response latency of the cell (stimulus phase) and a 1,000-ms window starting 1,900 ms after stimulus onset (delay phase).
Excluding Nonabstract, Object Feature-Selective Neurons.
To ensure that the studied neurons encoded abstract object properties irrespective of low-level visual features, we only analyzed cells, whose responses generalized over all three presented objects. We performed a Kruskal–Wallis test to analyze the selectivity of neurons for the three types of objects. For this test, hit trials of all intensities were grouped by object type. Only few neurons showed significantly different discharge rates for at least one of the three object types: (5% during the stimulus and delay phase). These cells were excluded from further analysis.
SLR Analysis.
The SLR analysis (46) was used to investigate the relationship of firing rate with stimulus intensity and firing rate with monkey's choice (43, 46, 47). We fitted the neuronal activity during stimulus and delay analysis phases to an arbitrary linear function of both factors: intensity (all tested values) and decision (“yes” decision: hits and false alarms vs. “no” decision: misses and correct rejections). The firing rate (FR) can be formulated as FR = a0 + aint × INT + ad × D, where aint and ad are the coefficients that quantify the firing rate dependence on intensity (INT) and decision (D), respectively. For the analysis of the rule cue phase (Fig. 5C), a sliding SLR analysis was calculated. Here, the dependence of firing rates on intensity (INT), decision (D), action (A), and rule cue (R) was assessed according to the equation FR = a0 + aint × INT + ad × D + aa × A + ar × R (SI Materials and Methods). We chose a significance level of 5% to determine which factors had a significant effect on the firing rates. Coefficients were included in the model if the P value for a predictor was below this level. Mulicollinearity did not affect the calculations (SI Materials and Methods).
Classification of Decision Cells into “Yes” and “No” Neurons.
Neurons showing a significant effect of decision (SLR analysis) were classified according to the modulation strength of their firing rates during “yes” and “no” decisions. As a measure of the modulation strength (M), we used the mean absolute change of the firing rate (FR) in intervals of t = 100 ms, which were shifted in 10-ms steps 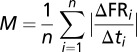 . The starting point of the modulation analysis (i = 1) for both phases was advanced from the defined phase onset to a time point at which the firing rates for the “yes” and “no” decisions started to diverge significantly (see ROC analysis); the analysis ended (i = n) at the defined offset of the respective phase. The firing rate was convolved with a Gaussian kernel (bin width 150 ms; step 1 ms). If the modulation strength (M) was larger during “yes” decisions compared with the modulation strength during “no” decisions, the neuron was classified as a “yes” neuron. For stronger modulation during “no” decisions, the neuron was assigned to the “no” neuron class.
. The starting point of the modulation analysis (i = 1) for both phases was advanced from the defined phase onset to a time point at which the firing rates for the “yes” and “no” decisions started to diverge significantly (see ROC analysis); the analysis ended (i = n) at the defined offset of the respective phase. The firing rate was convolved with a Gaussian kernel (bin width 150 ms; step 1 ms). If the modulation strength (M) was larger during “yes” decisions compared with the modulation strength during “no” decisions, the neuron was classified as a “yes” neuron. For stronger modulation during “no” decisions, the neuron was assigned to the “no” neuron class.
ROC Analysis.
To analyze the representation of the abstract decision across time, we compared the discharge rates of salient hit trials to correct rejections and activity in hit trials to misses of threshold trials. Sliding ROC analysis was used to calculate choice probability indices, which estimated the strength of decision coding (SI Materials and Methods).
Response Latency.
Latency calculations of neuronal responses were based on the sliding ROC analysis. No significant latency difference was found between cells coding only stimulus intensity (239 ms) and neurons coding the decision (194 ms) (P > 0.05, Wilcoxon test).
Supplementary Material
Acknowledgments
We thank Simon Jacob for comments on this manuscript and members of the A.N. laboratory for discussions. Support was provided by the Boehringer Ingelheim Fonds (to K.M.), the Leibniz Graduate School for Primate Neurobiology, and research group Grant C11/SFB 550 from the German Research Foundation (to A.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121084109/-/DCSupplemental.
References
- 1.Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- 2.Wang X-J. Decision making in recurrent neuronal circuits. Neuron. 2008;60:215–234. doi: 10.1016/j.neuron.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schall JD. Neural basis of deciding, choosing and acting. Nat Rev Neurosci. 2001;2:33–42. doi: 10.1038/35049054. [DOI] [PubMed] [Google Scholar]
- 4.Shadlen M, Kiani R, Hanks T, Churchland A. In: Better Than Conscious? Decision Making, the Human Mind, and Implications For Institutions. Engel C, Wolf S, editors. Cambridge, MA: MIT Press; 2008. [Google Scholar]
- 5.Horwitz GD, Newsome WT. Separate signals for target selection and movement specification in the superior colliculus. Science. 1999;284:1158–1161. doi: 10.1126/science.284.5417.1158. [DOI] [PubMed] [Google Scholar]
- 6.Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol. 2001;86:1916–1936. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- 7.Roitman JD, Shadlen MN. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci. 2002;22:9475–9489. doi: 10.1523/JNEUROSCI.22-21-09475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400:233–238. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- 9.Yang T, Shadlen MN. Probabilistic reasoning by neurons. Nature. 2007;447:1075–1080. doi: 10.1038/nature05852. [DOI] [PubMed] [Google Scholar]
- 10.Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley; 1966. [Google Scholar]
- 12.de Lafuente V, Romo R. Neuronal correlates of subjective sensory experience. Nat Neurosci. 2005;8:1698–1703. doi: 10.1038/nn1587. [DOI] [PubMed] [Google Scholar]
- 13.de Lafuente V, Romo R. Neural correlate of subjective sensory experience gradually builds up across cortical areas. Proc Natl Acad Sci USA. 2006;103:14266–14271. doi: 10.1073/pnas.0605826103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ress D, Heeger DJ. Neuronal correlates of perception in early visual cortex. Nat Neurosci. 2003;6:414–420. doi: 10.1038/nn1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shulman GL, Ollinger JM, Linenweber M, Petersen SE, Corbetta M. Multiple neural correlates of detection in the human brain. Proc Natl Acad Sci USA. 2001;98:313–318. doi: 10.1073/pnas.021381198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson KG, Schall JD. The detection of visual signals by macaque frontal eye field during masking. Nat Neurosci. 1999;2:283–288. doi: 10.1038/6398. [DOI] [PubMed] [Google Scholar]
- 17.Freedman DJ, Assad JA. Experience-dependent representation of visual categories in parietal cortex. Nature. 2006;443:85–88. doi: 10.1038/nature05078. [DOI] [PubMed] [Google Scholar]
- 18.Freedman DJ, Riesenhuber M, Poggio T, Miller EK. A comparison of primate prefrontal and inferior temporal cortices during visual categorization. J Neurosci. 2003;23:5235–5246. doi: 10.1523/JNEUROSCI.23-12-05235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Categorical representation of visual stimuli in the primate prefrontal cortex. Science. 2001;291:312–316. doi: 10.1126/science.291.5502.312. [DOI] [PubMed] [Google Scholar]
- 20.Bennur S, Gold JI. Distinct representations of a perceptual decision and the associated oculomotor plan in the monkey lateral intraparietal area. J Neurosci. 2011;31:913–921. doi: 10.1523/JNEUROSCI.4417-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedman DJ, Assad JA. A proposed common neural mechanism for categorization and perceptual decisions. Nat Neurosci. 2011;14:143–146. doi: 10.1038/nn.2740. [DOI] [PubMed] [Google Scholar]
- 22.Romo R, Brody CD, Hernández A, Lemus L. Neuronal correlates of parametric working memory in the prefrontal cortex. Nature. 1999;399:470–473. doi: 10.1038/20939. [DOI] [PubMed] [Google Scholar]
- 23.Lemus L, Hernández A, Romo R. Neural encoding of auditory discrimination in ventral premotor cortex. Proc Natl Acad Sci USA. 2009;106:14640–14645. doi: 10.1073/pnas.0907505106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deco G, Pérez-Sanagustín M, de Lafuente V, Romo R. Perceptual detection as a dynamical bistability phenomenon: A neurocomputational correlate of sensation. Proc Natl Acad Sci USA. 2007;104:20073–20077. doi: 10.1073/pnas.0709794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JN, Shadlen MN. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat Neurosci. 1999;2:176–185. doi: 10.1038/5739. [DOI] [PubMed] [Google Scholar]
- 26.Shadlen MN, Newsome WT. Motion perception: Seeing and deciding. Proc Natl Acad Sci USA. 1996;93:628–633. doi: 10.1073/pnas.93.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newsome WT, Britten KH, Movshon JA. Neuronal correlates of a perceptual decision. Nature. 1989;341:52–54. doi: 10.1038/341052a0. [DOI] [PubMed] [Google Scholar]
- 28.de Lafuente V, Romo R. Dopamine neurons code subjective sensory experience and uncertainty of perceptual decisions. Proc Natl Acad Sci USA. 2011;108:19767–19771. doi: 10.1073/pnas.1117636108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jazayeri M, Movshon JA. Optimal representation of sensory information by neural populations. Nat Neurosci. 2006;9:690–696. doi: 10.1038/nn1691. [DOI] [PubMed] [Google Scholar]
- 30.Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci. 2010;33:269–298. doi: 10.1146/annurev.neuro.051508.135409. [DOI] [PubMed] [Google Scholar]
- 31.Rorie AE, Newsome WT. A general mechanism for decision-making in the human brain? Trends Cogn Sci. 2005;9:41–43. doi: 10.1016/j.tics.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Mountcastle VB, Talbot WH, Sakata H, Hyvärinen J. Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol. 1969;32:452–484. doi: 10.1152/jn.1969.32.3.452. [DOI] [PubMed] [Google Scholar]
- 33.Hernández A, et al. Decoding a perceptual decision process across cortex. Neuron. 2010;66:300–314. doi: 10.1016/j.neuron.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 34.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 35.Asaad WF, Rainer G, Miller EK. Neural activity in the primate prefrontal cortex during associative learning. Neuron. 1998;21:1399–1407. doi: 10.1016/s0896-6273(00)80658-3. [DOI] [PubMed] [Google Scholar]
- 36.Barraclough DJ, Conroy ML, Lee D. Prefrontal cortex and decision making in a mixed-strategy game. Nat Neurosci. 2004;7:404–410. doi: 10.1038/nn1209. [DOI] [PubMed] [Google Scholar]
- 37.Romo R, Salinas E. Flutter discrimination: Neural codes, perception, memory and decision making. Nat Rev Neurosci. 2003;4:203–218. doi: 10.1038/nrn1058. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe M, Sakagami M. Integration of cognitive and motivational context information in the primate prefrontal cortex. Cereb Cortex. 2007;17(Suppl 1):i101–i109. doi: 10.1093/cercor/bhm067. [DOI] [PubMed] [Google Scholar]
- 39.Wallis JD, Anderson KC, Miller EK. Single neurons in prefrontal cortex encode abstract rules. Nature. 2001;411:953–956. doi: 10.1038/35082081. [DOI] [PubMed] [Google Scholar]
- 40.Bongard S, Nieder A. Basic mathematical rules are encoded by primate prefrontal cortex neurons. Proc Natl Acad Sci USA. 2010;107:2277–2282. doi: 10.1073/pnas.0909180107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heekeren HR, Marrett S, Bandettini PA, Ungerleider LG. A general mechanism for perceptual decision-making in the human brain. Nature. 2004;431:859–862. doi: 10.1038/nature02966. [DOI] [PubMed] [Google Scholar]
- 42.Hoshi E, Tanji J. Differential involvement of neurons in the dorsal and ventral premotor cortex during processing of visual signals for action planning. J Neurophysiol. 2006;95:3596–3616. doi: 10.1152/jn.01126.2005. [DOI] [PubMed] [Google Scholar]
- 43.Hernández A, Zainos A, Romo R. Temporal evolution of a decision-making process in medial premotor cortex. Neuron. 2002;33:959–972. doi: 10.1016/s0896-6273(02)00613-x. [DOI] [PubMed] [Google Scholar]
- 44.Hoshi E, Sawamura H, Tanji J. Neurons in the rostral cingulate motor area monitor multiple phases of visuomotor behavior with modest parametric selectivity. J Neurophysiol. 2005;94:640–656. doi: 10.1152/jn.01201.2004. [DOI] [PubMed] [Google Scholar]
- 45.Procyk E, Tanaka YL, Joseph JP. Anterior cingulate activity during routine and non-routine sequential behaviors in macaques. Nat Neurosci. 2000;3:502–508. doi: 10.1038/74880. [DOI] [PubMed] [Google Scholar]
- 46.Draper NR, Smith H. Applied Regression Analysis. New York: Wiley; 1966. [Google Scholar]
- 47.Romo R, Hernández A, Zainos A. Neuronal correlates of a perceptual decision in ventral premotor cortex. Neuron. 2004;41:165–173. doi: 10.1016/s0896-6273(03)00817-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.