Abstract
IL-15 has potential as an immunotherapeutic agent for cancer treatment because of its ability to effectively stimulate CD8 T cell, natural killer T cell, and natural killer cell immunity. However, its effectiveness may be limited by negative immunological checkpoints that attenuate immune responses. Recently a clinical trial of IL-15 in cancer immunotherapy was initiated. Finding strategies to conquer negative regulators and enhance efficacy of IL-15 is critical and meaningful for such clinical trials. In a preclinical study, we evaluated IL-15 combined with antibodies to block negative immune regulator cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed death ligand 1 (PD-L1) in an established murine transgenic adenocarcinoma of mouse prostate (TRAMP)-C2 prostate tumor model. IL-15 treatment resulted in a significant prolongation of survival in tumor-bearing animals. Coadministration of anti-PD-L1 or anti-CTLA-4 singly with IL-15 did not improve animal survival over that of IL-15 alone. However, simultaneous administration of IL-15 with anti-CTLA-4 and anti-PD-L1 was associated with increased numbers of tumor antigen-specific tetramer-positive CD8 T cells, increased CD8 T-cell tumor lytic activity, augmented antigen-specific IFN-γ release, decreased rates of tumor growth, and improved animal survival compared with IL-15 alone. Furthermore, triple combination therapy was associated with inhibition of suppressive functions of CD4+CD25+ regulatory T cells and CD8+CD122+ regulatory T cells. Thus, simultaneous blockade of CTLA-4 and PD-L1 protected CD4 and/or CD8 T-cell activity from these regulatory T cells. Combining the immune stimulatory properties of IL-15 with simultaneous removal of two critical immune inhibitory checkpoints, we showed enhancement of immune responses, leading to increased antitumor activity.
IL-15 is critically important for development and homeostasis of memory CD8 T cells, natural killer (NK) cells, NK T cells, and intraepithelial lymphocytes (1–3). Compared with IL-2, IL-15 favors survival of NK and memory phenotype CD8 T cells without side effects of IL-2, such as expansion of regulatory T cells (Tregs) or induction of activation-induced cell death (1, 4–6). In light of these differences, a phase I dose-escalation trial of recombinant human IL-15 in patients with metastatic malignant melanoma and renal cell cancer was initiated. Although IL-15 may ultimately show efficacy in treatment of patients with metastatic malignancy, it may not be optimal when used as a single agent.
There are multiple inhibitory mechanisms that “brake” or attenuate immune responses. These negative feedback systems include binding of ligands expressed by antigen-presenting cells (APCs) to inhibitory receptors on T cells [e.g., cytotoxic T lymphocyte antigen 4 (CTLA-4) (7) and programmed death 1(PD1) (8)], secreted circulating protein inhibitors [e.g., IL-10 (9) and TGF-β (10)], and inhibitory cells [e.g., Tregs (11), myeloid-derived suppressor cells (12), and a subset of CD8+CD122+ cells (13)].
PD1 is a member of the CD28/CTLA-4 family (8, 14). Interaction of PD-L1 with PD1 and B7-1 initiates an inhibitory signal to activated T cells (15). Tumors may exploit this to inhibit antitumor immune responses. CTLA-4 is recognized as another critical negative regulator (7). CTLA-4 ligation by B7-1 and B7-2 was shown to inhibit IL-2 production, generation of cyclins, cytokine-dependent kinases, and other components of the machinery needed for cell-cycle progression.
Regulatory T-cells including CD4+CD25+FoxP3+ Tregs and a subset of CD8+CD122+ T cells are also critical to maintain peripheral self-tolerance and avoid autoimmunity (11, 13). However, it has been noted that tumors take advantage of Tregs to help them evade immune attacks. Increased numbers of Tregs were found in peripheral blood and especially in tumor microenvironments of patients with malignancies (16–18). It is likely that Tregs contribute to decreasing immunity during tumor development and progression, leading to poor outcomes in cancer patients.
Recent studies have shown a naturally occurring subset of CD8+CD122+ T cells involved in maintaining T-cell homeostasis and suppressing T-cell responses (13). CD8+CD122+ regulatory cells suppressed proliferation and IFN-γ secretion by effector CD8 T cells. Therefore, CD8+CD122+ regulatory cells may play an inhibitory role in antitumor immunity and thus are rational targets for immunotherapy.
In our previous study, administration of mouse IL-15 (mIL-15) alone significantly prolonged CT26 tumor-bearing animal survival. Moreover, combining mIL-15 with anti-CTLA-4 and anti-PD-L1 provided more protection than IL-15 alone or its combination with either agent singly (19). In the present study, with an established transgenic adenocarcinoma of mouse prostate (TRAMP)-C2 murine prostate cancer model, we further explored simultaneous inhibition of two specific regulatory T-cell subsets using anti-CTLA-4 plus anti-PD-L1 and demonstrated that the combination enhanced IL-15 therapeutic efficacy. We demonstrated that combining IL-15 with multiple negative checkpoint blockade involving anti-CTLA-4 and anti-PD-L1 not only enhanced CD8+ T cell cytotoxic activity but also inhibited the suppressive functions of CD4+CD25+ Tregs and CD8+CD122+ regulatory T-cells.
Results
IL-15 Plus Simultaneous CTLA-4 and PD-L1 Blockade Significantly Reduced Tumor Growth Rate in Vivo and Resulted in Prolonged Survival of Tumor-Bearing Mice.
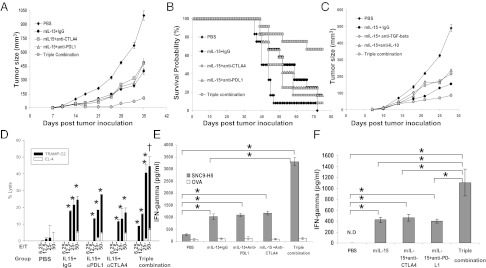
Our studies were directed toward defining effects of IL-15 treatment combinations against established TRAMP-C2 tumors (20). IL-15+IgG significantly inhibited tumor growth compared with mice receiving PBS (Fig. 1A; on day 35, P = 0.028). Coadministration of anti-CTLA-4 or anti-PD-L1 singly with mIL-15 did not significantly improve the inhibition compared with mIL-15–treated mice (on day 35, P = 0.357 and P = 0.442). However, combination of anti-CTLA-4 and anti-PD-L1 with mIL-15 (triple combination) was significantly more effective than other mIL-15–containing groups at inhibiting tumor growth (P < 0.05 at multiple time points).
Fig. 1.
IL-15 and simultaneous blockade of CTLA-4 and PD-L1 protected animals against tumor growth. (A) Growth curves illustrate in vivo growth rates of TRAMP-C2 tumors associated with diverse treatments. Tumor sizes shown represent means ± SEM, n = 10. (B) Kaplan-Meier survival curves illustrate survivals of mice with treatments. (C) mIL-15 combined with anti-IL-10/anti-TGF-β antibodies did not inhibit tumor growth beyond that achieved with IL-15 treatment alone. n = 10. (D) Tumor lysis activity was tested on day 21; TRAMP-C2 cells were used as the target cells. EL4 cells were used as control. Tumor-specific CD8 T-cell target cell killing was detected. *P < 0.05, lysis activity against TRAMP-C2 compared with PBS; †P < 0.05, lysis activity against TRAMP-C2 compared with other mIL-15 involved groups. (E) Splenic CD8 T cells were stimulated with SNC9-H8 or OVA peptide and cultured with irradiated splenocytes as APCs. IFN-γ secretion was measured by ELISA. Data represent five independent experiments. *P < 0.05. (F) Tumor-infiltrating cells were separated and stimulated with SNC9-H8 peptide. IFN-γ secretion was detected by ELISA. Data represent two experiments. *P < 0.05.
Animals receiving IL-15+IgG showed prolonged survival times compared with PBS-treated mice (P = 0.037; Fig. 1B). Single antibody treatment with anti-CTLA-4 or anti-PD-L1 did not reduce tumor growth rates compared with the PBS group, or improve animal survival (P = 0.261 and P = 0.117 respectively; Fig. S1 A and B). Animals receiving Anti-CTLA-4+Anti-PD-L1 demonstrated reduced tumor growth and increased survival compared with the PBS group; however, the combination was significantly less effective than mIL-15+IgG treatment (P = 0.033; Fig. S1 A and B). Even though the two blockade antibodies have different isotypes, we showed that the different IgG isotypes did not influence tumor growth (Fig. S1C). Combination of IL-15 with either antibody alone did not prolong survival compared with the mIL-15+IgG group (P > 0.05; Fig. 1B). However, the triple combination group exhibited a significant survival advantage compared with mIL-15 alone (P = 0.022) or groups receiving mIL-15 plus anti-PD-L1 or anti-CTLA-4–alone treatment (P = 0.042, P = 0.027). Moreover, more than 60% of triple combination-treated mice remained tumor free at study termination (Fig. 1B).
Combining IL-15 with Anti-IL-10 or Anti-TGF-β Failed to Enhance Protective Effects of IL-15.
Previously, with a murine CT26 tumor model, we showed that administration of IL-15 was associated with increased secretion of IL-10 by CD8 T cells that decreased therapeutic effects (19). Other studies reported that TRAMP-C2 cells express high levels of TGF-β to escape immune attacks (21). In the present study we provided mIL-15 with anti-IL-10 or anti-TGF-β to examine whether these combinations would increase therapeutic efficacy mediated by IL-15. IL-15 plus anti-CTLA-4 and anti-PD-L1 was used as the positive control (triple combination; Fig. 1C). Compared with the IL-15–alone group, no significant improvement was observed by adding anti-IL-10 or anti-TGF-β to IL-15. On day 28, no statistically significant effect on tumor size was observed with the mIL-15+anti-IL-10 or mIL-15+anti-TGF-β groups, compared with mIL-15+IgG, whereas the triple combination group demonstrated much smaller tumor sizes (P < 0.05 compared with other groups). Attempting to achieve a better effect, we provided anti-TGF-β with triple combination, but no further benefit was observed on day 35 with this regimen according to tumor growth curve (P = 0.069 compared with triple combination) (Fig. S2).
IL-15 Induced Increased CD8+ T-Cell Tumor Lytic Activity and Antigen-Specific IFN-γ Release, Which Was Enhanced with Anti-CTLA-4 and Anti-PD-L1 Blockade.
After stimulation with tumor antigens, CD8+ T cells isolated from PBS-treated mice demonstrated little lytic activity against TRAMP-C2 cells or IFN-γ secretion (Fig. 1 D and E), whereas CD8+ T cells isolated from mIL-15+IgG–treated animals alone or in combination with anti-CTLA-4 and/or anti-PD-L1 showed increased lytic activity and IFN-γ secretion. Addition of either anti-CTLA-4 or anti-PD-L1 to IL-15 did not significantly increase TRAMP-C2 cell lysis or IFN-γ accumulation compared with that observed with the mIL-15+IgG group. However, when both antibodies were combined with IL-15, there was a significantly greater tumor lysis and IFN-γ secretion (P < 0.05 compared with that observed with other IL-15 administration groups). Meanwhile, statistically significantly higher levels of tumor-specific IFN-γ secretion by TRAMP-C2 tumor-infiltrating cells were detected with the triple combination group, compared with other groups (Fig. 1F). There was no significant lytic activity against EL4 cells (Fig. 1D) observed in any groups, nor was high-level IFN-γ secretion detected by CD8 T cells upon ovalbumin(OVA) peptide stimulation (Fig. 1E).
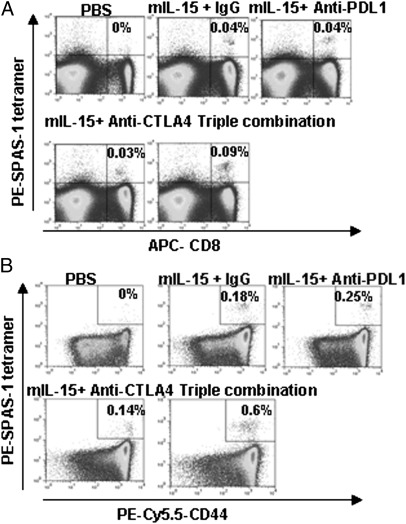
IL-15 Induced Increased Numbers of TRAMP-C2 Antigen-Specific CD8+ T Cells.
Tumor-specific CD8+ T cells were detected by using SPAS-1 (22) tetramer staining. Splenocytes from PBS-treated mice did not contain tetramer+ CD8 T cells (Fig. 2A). Animals treated with mIL-15 alone or in single combination with anti-CTLA-4 or anti-PD-L1 had increased numbers of tetramer+ CD8 T cells. There were no significant differences among these groups in terms of percentages (Fig. 2A) or absolute numbers of tetramer+ CD8 cells (Fig. S3A) (P > 0.05). However, when IL-15 was combined with both anti-CTLA-4 and anti-PD-L1, significantly greater numbers of tetramer+ CD8 T cells were detected compared with other treatment groups (P < 0.05). This trend continued when tetramer+CD8+CD44high memory phenotype populations were examined: significantly greater numbers of tetramer+ cells were detected after administration of triple combination compared with all other groups (P < 0.05) (Fig. 2B). Most of the CD8+tetramer+ T cells in triple combination treated animals were included within the CD44high population (Fig. 2B and S3B).
Fig. 2.
IL-15 treatment with simultaneous blockade of CTLA-4 and PD-L1 was associated with an increase of both numbers and function of tumor-specific CD8 T cells. On day 21, splenocytes derived from treated tumor-bearing mice were stained with Phycoerythrin (PE)-conjugated SPAS-1-tetramer, APC-anti-CD8, and PE-Cy5.5-CD44 antibodies. (A) The value presented represents the percentage of tetramer+CD8+ cells in the total cells. (B) Expression of CD44 on tetramer+ CD8 cells. Data represent five independent experiments.
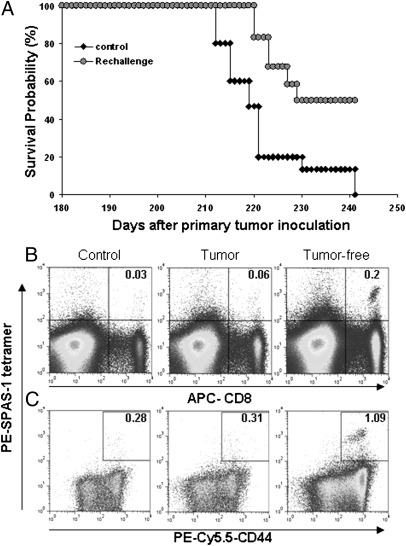
Antigen-Specific CD8+ T Cells Were Involved in Protection of Tumor-Free Animals Against Rechallenge.
In a rechallenge study, only mice treated with triple combination survived the initial challenge with tumor and were able to be rechallenged. The numbers of mice surviving after IL-15 alone or combination with single antibody treatment were not sufficient to perform a rechallenge study (Fig. 1B). Triple combination-treated tumor-free animals were injected with TRAMP-C2 cells without any treatment 180 d after first tumor inoculation. Half of the animals (50%) remained tumor free more than 60 d after the second inoculation (Fig. 3A). At termination, a large population of tetramer+ splenic CD8 T cells was detected in tumor-free mice (Fig. 3 B and C).
Fig. 3.
TRAMP-C2 antigen-specific CD8+ T cells were involved in protection of tumor-free animals on rechallenge. Mice that received triple combination initially that became tumor-free were injected s.c. with the same dose of TRAMP-C2 cells on the opposite flank 180 d after primary challenge. Without any treatment, tumor reoccurrence and animal survival were recorded (n = 12). Age-matched naïve C57BL/6 mice were used as controls (n = 15). (B) At the end point, splenocytes were stained with PE-conjugated SPAS-tetramer, APC-anti-CD8, and PE-Cy5.5-CD44 antibodies. The value represents the percentage of tetramer+ CD8 cells in the total cells. (C) The percentage of CD44 expression on tetramer+ CD8 cells.
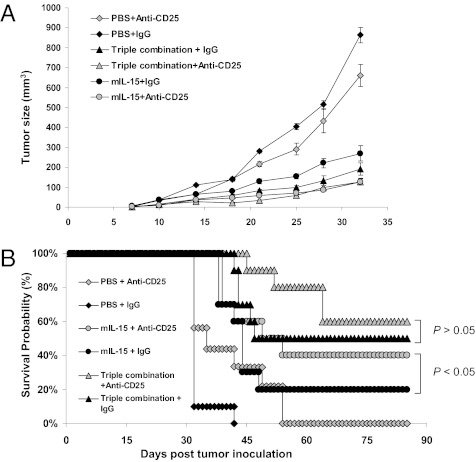
Depletion of CD25+ Cells Reduced Tumor Growth Rates and Prolonged Survival of IL-15–Treated Tumor-Bearing Mice.
To examine the effect of Tregs on tumor growth, we depleted CD25+ cells by injecting PC61 (i.p.) 4 d before tumor inoculation. Animals treated with PBS and PC61 had significantly smaller tumors than mice not receiving anti-CD25 (on day 32, P = 0.044) (Fig. 4A). This translated into improved survival (P < 0.05) (Fig. 4B). Similarly, addition of PC61 to IL-15–treated animals resulted in comparative tumor growth inhibition and prolonged survival compared with IL-15 alone. However, mice treated with IL-15+anti-PD-L1+anti-CTLA-4+anti-CD25 did not demonstrate decreased tumor growth (on day 32, P = 0.072) or a statistically significant survival advantage over those receiving triple combination alone (P = 0.068).
Fig. 4.
PC61-mediated removal of CD25+ cells augmented antitumor responses against TRAMP-C2 mediated by IL-15 alone but not those modulated by triple combination. Four days before tumor inoculation, selected animals received 400 μg/mouse i.p. of isotype IgG, or PC61 to deplete CD25+ cells. We then followed the same procedure to inoculate tumor and start treatments. (A) Tumor growth curves are shown by means ± SEM, n = 10. (B). Survivals of tumor-bearing mice are shown. Data represent two independent experiments.
Simultaneous Blockade of CTLA-4 and PD-L1 Combined with mIL-15 Decreased the Percentage but Did Not Affect Absolute Numbers of CD4+CD25+ Tregs.
To determine whether triple treatment had an effect on numbers of Tregs, we examined splenocytes from treated mice 21 d after tumor implantation (Fig. S4). IL-15 treatment did not alter the percentage of CD4+CD25+ cells (Fig. S4A) or CD4+CD25+FoxP3+ cells (Fig. S4B). This phenomenon was also observed on absolute numbers of Tregs, with no differences detected compared with the PBS group (Fig. S4 C and D). Treatment with IL-15+anti-PD-L1 and/or anti-CTLA-4 decreased the percentages of both CD4+CD25+ and CD4+CD25+FoxP3+ cells. However, because the treatments also increased the number of splenocytes, it did not translate into a decrease in absolute numbers of Tregs. A similar tendency was observed in the rechallenge study: no meaningful difference in either the percentage or absolute numbers of Tregs among tumor-free, tumor-reoccurred, or age-matched control mice was detected (Fig. S5).
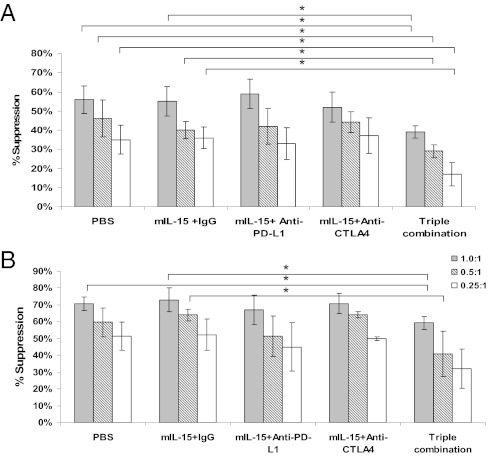
To examine whether the biological activity of Tregs was altered after treatment, CD4+CD25+ T-cell suppressor function was performed ex vivo (Fig. 5A). Tregs from mice treated with IL-15 alone or combined singly with anti-PD-L1 or anti-CTLA-4 suppressed proliferation of donor CD4+CD25− cells to the same extent as Tregs isolated from PBS-treated mice. In contrast, Tregs isolated from triple combination-treated mice had significantly less Treg suppressor activity compared with those isolated from PBS- and IL-15+IgG–treated animals (P < 0.05).
Fig. 5.
IL-15 with simultaneous blockade of CTLA-4 and PD-L1 reduced CD4+CD25+ Treg suppressive function. Splenic CD4+CD25+/CD4+CD25− cells were separated from treated tumor-bearing animals. (A) CD4+CD25+ T cells cultured with CD4+CD25− T cells at various ratios upon plate-bound anti-CD3 antibody stimulation for 72 h. 3H-TdR was added to cultures during the last 6 h. %suppression = 100% × [1-cpm (CD4+CD25++CD4+CD25−)/cpm (CD4+CD25−)].(B). Naïve CD8+ T cells were cultured with CD4+CD25+ T cells derived from treated tumor-bearing animals and stimulated with plate-bound anti-CD3 for 72 h. Proliferation was assessed by 3H-TdR incorporation. %suppression = 100% × [1-cpm (CD4+CD25++CD8+)/cpm CD8+].Data represent three independent experiments. n = 8–10 mice. *P < 0.05.
This trend continued when looking at Tregs suppressing CD8 T-cell proliferation (Fig. 5B). Tregs isolated from mice treated with IL-15 alone or IL-15+anti-PD-L1 or IL-15+anti-CTLA-4 did not affect suppressor activity of Tregs compared with those from PBS-treated animals. However, Tregs from triple combination-treated animals had significantly less CD8 suppressor activity than those isolated from PBS and IL-15+IgG groups at certain ratios (P < 0.05).
Simultaneous Blockade of CTLA-4 and PD-L1 Combined with mIL-15 Decreased the Suppressor Action of CD8+CD122+ T Cells in Tumor-Bearing Animals.
We examined a subset of CD8+CD122+ T cells with suppressor activity and defined the effects on numbers of CD8+CD122+ cells by diverse treatments. IL-15–treated mice had a greater percentage of CD8+CD122+ cells in spleens than those not receiving IL-15 (Fig. S6A), as well as a significantly greater absolute number of these cells (Fig. S6B). There was no significant difference in numbers of CD8+CD122+ cells among IL-15–treated groups. In the rechallenge study, no meaningful difference in either the percentage or absolute numbers of CD8+CD122+ T cells among tumor-free animals, tumor-reoccurred animals, or control mice was observed (Fig. S7).
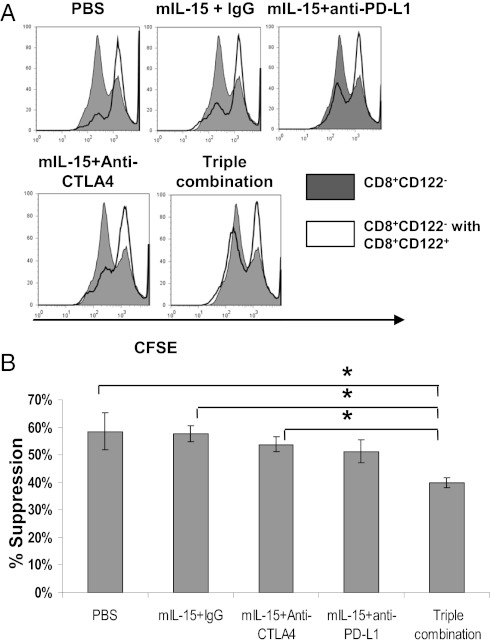
To determine the suppressive function of CD8+CD122+ cells, we measured the effects on proliferation of naïve Ly5.1 CD8+CD122− cells upon anti-CD3 and anti-CD28 stimulation. By Carboxyfluorescein succinimidyl ester (CFSE) staining, there was no significant difference between the mIL-15+IgG group and the PBS, mIL-15+anti-CTLA4, or mIL-15+anti-PD-L1 groups (Fig. 6 A and B). In contrast, CD8+CD122+ cells isolated from triple combination-treated mice had significantly less suppressor activity than those isolated from mIL-15+IgG–treated mice (Fig. 6 A and B).
Fig. 6.
IL-15 combined with blockade of CTLA-4 and PD-L1 decreased the suppressor function on a per-cell basis. (A) CD8+CD122+ T cells derived from treated tumor-bearing mice were cultured with CFSE-labeled naïve Ly-5.1+ CD8+CD122− T cells and stimulated with plate-bound anti-CD3 plus soluble anti-CD28 for 48 h. Cells were gated on Ly5.1+ T cells to detect CFSE dilution. CD8+CD122− T cells cultured alone were used as control. (B) Percentage suppression was enumerated. Data represent four independent experiments. n = 8–10 mice. *P < 0.05.
Discussion
IL-15 is a pivotal cytokine in the development and homeostasis of CD8, NK, and NK T cells. Here we showed that IL-15 treatment increased the absolute numbers of SPAS-1-tetramer+ CD8 T cells, up-regulated IFN-γ secretion and tumor lytic activity of CD8 T cells, significantly reduced tumor growth rate, and resulted in prolonged long-term survival of TRAMP-C2–bearing animals. Although IL-15 expanded the numbers of NK1.1+ cells (Fig. S8), anti-asialo-GM1 injected to deplete these cells did not produce a significant effect on tumor growth. In contrast, CD8 depletion abrogated antitumor effects of both IL-15 alone and triple combination treatments (Fig. S9 A and B). Further we showed that TRAMP-C2 cells express high levels of MHC class I (Fig. S9 C and D), which may limit the efficacy of NK killing of this tumor.
Nevertheless, the effectiveness of IL-15 immunotherapy as a single agent is potentially limited by its up-regulation of negative checkpoints that induce increased expression of PD1 on CD8 T cells, including CD8+CD44hi memory phenotype T cells and increased production of IL-10 (19). To address the impaired tumor efficacy mediated by negative feedback systems, we used monoclonal antibodies to neutralize multiple inhibitory pathways individually and in combination. Simultaneous blockade of two specific negative checkpoints, CTLA-4 and PD-L1, combined with IL-15 was associated with enhanced antitumor activity. Our results in the TRAMP-C2 model support our previous observation that simultaneous blockade with anti-CTLA-4 and anti-PD-L1 enhanced antitumor activity mediated by IL-15 in the CT26 model. In addition, Curran et al. (23) demonstrated that combination blockade of PD1 and CTLA-4 expanded the number of infiltrating effector T cells and reduced numbers of Tregs and myeloid cells within B16 melanoma tumors, thereby augmenting antitumor efficacy. Combination of checkpoint inhibition of both CTLA-4 and PD-L1 seems to be exceptionally propitious because further additional elimination of other potential checkpoints provided little benefit. In particular, combining IL-15 with anti-IL-10 or anti-TGF-β failed to improve the efficacy mediated by IL-15 in this model. Furthermore, although depletion of CD25+ cells with PC61 reduced tumor growth rate and prolonged survival times of animal treated with IL-15, mice treated with IL-15+anti-CTLA-4+anti-PD-L1+anti-CD25 did not have a further decrease of tumor growth rates or statistically significant survival advantage over those receiving triple combination.
It is of value to define the mode of action of the anti-CTLA-4 and anti-PD-L1 combination. Blockade of PD-L1 and CTLA-4 checkpoints may act directly on CD8 T cells to restore their responsiveness and also indirectly by inhibiting actions of such negative regulatory cells as CD4+CD25+ Tregs and CD8+CD122+ regulatory T-cells. In terms of direct effects on CD8 effector cells, both PD1 and CTLA-4 ligation inhibit Akt activation and block T-cell responses by targeting distinct signaling molecules (24). PD1 engagement inhibits an upstream proximal step, blocking phosphatidylinositol-3-kinase (PI3K) activation. In contrast, signaling mediated by CTLA-4 preserves PI3K activity; instead it functions through binding to the phosphatase PP2A, leading to inhibition of Akt phosphorylation and ultimately inhibition of T-cell activation (24). By blocking both PD-L1 and CTLA-4 we were able to target two pathways leading to Akt activation—the result being a likely synergistic response allowing effective T-cell activation and ultimately an increased therapeutic effect.
The efficacy of IL-15 combined with anti-PD-L1 and anti-CTLA-4 may also be an indirect effect through inhibition of negative regulatory cytokines and cells that act on CD8 effector T-cell responders. Blockade of CTLA-4 was shown to inhibit IL-10 secretion (25). Furthermore, mice treated with the combination of IL-15 with anti-PD-L1 also had CD8+ T cells that secreted lower amounts of IL-10. In addition, triple combination therapy reduced surface PD1 expression on CD8 T cells and was associated with a decrease in IL-10 secretion (19).
Regulatory T cells in particular CD4+CD25+Foxp3+ Tregs play a critical role in immune dysfunction and induction of tolerance, leading to immune escape and tumor progression. Here we showed that eliminating Tregs by PC61 significantly inhibited tumor growth when used alone. However, addition of anti-CD25 to triple combination did not improve the activity. Itoh et al. (26) suggested that suppressor activity of Tregs was cell-contact dependent and mediated by molecules including CTLA-4, whereas others reported that IL-10 (27, 28) and TGF-β (29) were critical for suppressive effects of Tregs. We did not observe additional benefit by addition of either anti-IL-10 or anti-TGF-β with IL-15. It was reported that CTLA-4 and PD1/PD-L1 systems were important mediators of development and function of naïve and induced Tregs (30–33). In our model the effect of IL-15+anti-CTLA-4+anti-PD-L1 did not seem to be related to overall changes in the numbers of CD4+CD25+FoxP3+ Tregs but rather a decrease in their suppressor function. Studies have suggested that CTLA-4 blockade interferes with Treg function and inhibits their ability to suppress T-cell proliferation (32, 33). Similarly, blockade of the PD-L1 pathway using anti-PD-L1 inhibited the ability of Tregs to suppress and restored T-cell proliferation in vitro (31). Curran et al. (23) reported that combining CTLA-4 and PD1/PD-L1 blockade had effects on the local tumor microenvironment that resulted in an increase in the tumor-infiltrating ratio of T-effector cells to Tregs. This action was able to augment the efficacy of a Flt3 ligand-expressing tumor vaccine. In our study we found that Tregs isolated from mice treated with IL-15+anti-PD-L1 or IL-15+anti-CTLA-4 suppressed CD4+CD25− and CD8+ T-cell proliferation at levels comparable to those of Tregs isolated from IL-15–treated mice. In contrast, Tregs isolated from triple combination-treated animals exhibited significantly reduced suppressor function when cultured with either CD4+CD25− or naïve CD8+ T cells. Furthermore, we suggest that triple combination treatment tipped the balance between Tregs and effector T cells toward the dominance of the latter to provoke tumor immunity, without causing side-effects of autoimmunity that might be unacceptable if complete removal of suppressor Treg function was achieved.
In addition to CD4+CD25+FoxP3+ Tregs, a subset of CD8+CD122+ cells has been identified as having a suppressor function in mice (13). We showed that IL-15 either alone or in combination with PD-L1 and CTLA-4 blockade led to significant increases in the numbers of CD8+CD122+ T cells. When examining the capacity of such CD8+CD122+ T cells to suppress proliferation of naïve CD8+ CD122− T cells in the presence of anti-CD3, we demonstrated that those cells isolated from IL-15–treated mice retained their suppressor activity. In contrast, CD8+CD122+ T cells isolated from triple combination-treated mice exhibited significantly less suppressor activity, allowing naïve cells to proliferate. Recently it was reported that CD8+CD122+ suppressor cells express PD1 on their surfaces, and this expression distinguishes them from memory T cells with the same phenotype (34). Furthermore, it was shown that PD1 expression was necessary for IL-10 production and suppressor function (34). We previously reported that combination of IL-15 with anti-CTLA-4 and anti-PD-L1 significantly reduced PD1 expression on CD8+ T cells and inhibited IL-10 secretion. The combination of anticheckpoint antibodies reduced this action to a greater extent than either antibody alone (19).
In summary, generation of an effective immune response leading to meaningful antitumor effects requires not only an increase in immune activation but also removal of suppressor or inhibitory elements of the immune system. Here we demonstrate that the simultaneous combination blockade of anti-CTLA-4 and anti-PD-L1 along with administration of IL-15 inhibited the suppressor function of both CD4+CD25+ and CD8+CD122+ regulatory T-cells while retaining CD8 effector function, ultimately leading to significant and long-term increases in both antitumor effects and tumor-bearing animal survival in a prostate cancer model. These studies provide the scientific basis for a clinical trial that would involve simultaneous CTLA-4 and PD-L1 blockade in association with IL-15 administration in the therapy of patients with solid tumors.
Materials and Methods
Animals, Cell Lines, and Peptides.
See SI Materials and Methods. TRAMP-C2 (20) and EL4 cell lines were used in the study.
Tumor Challenge and Treatment Experiments.
See SI Materials and Methods. Treatment was initiated only in mice with palpable s.c. tumors. mIL-15, anti-CTLA4, anti-PDL1, anti-IL-10, and anti-mouse TGF-β1, 2, 3 mAb were given to selected animals.
Flow Cytometry Analysis.
SPAS-1 (22) tetramer was obtained from James Allison (Memorial Sloan-Kettering Cancer Center, New York). Analyses for cell expression of CD8, CD44, CD25, CD4, Ly5.1 and FoxP3 were performed with antibodies from eBioscience.
IFN-γ Secretion and Cytotoxicity Assays.
IFN-γ secretion by splenic cells and tumor infiltrating cells was measured by ELISA. For additional details, see SI Materials and Methods.
Regulatory T Cells Suppressor Assays.
Suppressor assays of CD4+CD25+ and CD8+CD122+ cells were performed. For additional details, see SI Materials and Methods.
Statistical Analysis.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research (P.Y., J.C.S., M.Z., J.C.M., and T.A.W.). R.W., M.F., and J.P.A. were funded by the Howard Hughes Medical Institute, Ludwig Center of Cancer Immunotherapy, Prostate Cancer Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203479109/-/DCSupplemental.
References
- 1.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: Implications for immunotherapy. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- 2.Kennedy MK, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lodolce JP, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 4.Waldmann TA. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 5.Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8(+) T cells. J Exp Med. 2002;196:935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 7.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1 deficient mice: Implication of PD-1 as a negative regulator for B cell responses. Int Immunol. 1998;10:1563–1572. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S, et al. T cell-derived IL-10 promotes lung cancer growth by suppressing both T cell and APC function. J Immunol. 1999;163:5020–5028. [PubMed] [Google Scholar]
- 10.Chen WJ, Jin W, Wahl SM. Engagement of CTLA-4 induces TGF-β production by murine CD4+ T cells. J Exp Med. 1998;188:1849–1857. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakaguchi S, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: Their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 12.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–645. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 13.Endharti AT, et al. Cutting edge: CD8+CD122+ regulatory T cells produce IL-10 to suppress IFN-gamma production and proliferation of CD8+ T cells. J Immunol. 2005;175:7093–7097. doi: 10.4049/jimmunol.175.11.7093. [DOI] [PubMed] [Google Scholar]
- 14.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrausch U, et al. Cancer immunotherapy: The role regulatory T cells play and what can be done to overcome their inhibitory effects. Curr Mol Med. 2009;9:673–682. doi: 10.2174/156652409788970670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Attenuation of CD8(+) T-cell function by CD4(+)CD25(+) regulatory T cells in B-cell non-Hodgkin's lymphoma. Cancer Res. 2006;66:10145–10152. doi: 10.1158/0008-5472.CAN-06-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woo EY, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 19.Yu P, Steel JC, Zhang M, Morris JC, Waldmann TA. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res. 2010;16:6019–6028. doi: 10.1158/1078-0432.CCR-10-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325–3330. [PubMed] [Google Scholar]
- 21.Baron V, et al. Inhibition of Egr-1 expression reverses transformation of prostate cancer cells in vitro and in vivo. Oncogene. 2003;22:4194–4204. doi: 10.1038/sj.onc.1206560. [DOI] [PubMed] [Google Scholar]
- 22.Fassò M, et al. SPAS-1 (stimulator of prostatic adenocarcinoma-specific T cells)/SH3GLB2: A prostate tumor antigen identified by CTLA-4 blockade. Proc Natl Acad Sci USA. 2008;105:3509–3514. doi: 10.1073/pnas.0712269105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parry RV, et al. CTLA4 and PD1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9545–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jovasevic VM, Gorelik L, Bluestone JA, Mokyr MB. Importance of IL-10 for CTLA-4-mediated inhibition of tumor-eradicating immunity. J Immunol. 2004;172:1449–1454. doi: 10.4049/jimmunol.172.3.1449. [DOI] [PubMed] [Google Scholar]
- 26.Itoh M, et al. Thymus and autoimmunity: Production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 27.Pontoux C, Banz A, Papiernik M. Natural CD4 CD25(+) regulatory T cells control the burst of superantigen-induced cytokine production: the role of IL-10. Int Immunol. 2002;14:233–239. doi: 10.1093/intimm/14.2.233. [DOI] [PubMed] [Google Scholar]
- 28.Annacker O, et al. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol. 2001;166:3008–3018. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor β. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Francisco LM, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ni L, et al. PD-1 modulates regulatory T cells and suppresses T-cell responses in HCV-associated lymphoma. Immunol Cell Biol. 2011;89:535–539. doi: 10.1038/icb.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 33.Friedline RH, et al. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J Exp Med. 2009;206:421–434. doi: 10.1084/jem.20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai H, et al. Cutting edge: Programmed death-1 defines CD8+CD122+ T cells as regulatory versus memory T cells. J Immunol. 2010;185:803–807. doi: 10.4049/jimmunol.1000661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.