Abstract
Autosomal recessive loss-of-function mutations within the PARK2 gene functionally inactivate the E3 ubiquitin ligase parkin, resulting in neurodegeneration of catecholaminergic neurons and a familial form of Parkinson disease. Current evidence suggests both a mitochondrial function for parkin and a neuroprotective role, which may in fact be interrelated. The antiapoptotic effects of parkin have been widely reported, and may involve fundamental changes in the threshold for apoptotic cytochrome c release, but the substrate(s) involved in parkin dependent protection had not been identified. Here, we demonstrate the parkin-dependent ubiquitination of endogenous Bax comparing primary cultured neurons from WT and parkin KO mice and using multiple parkin-overexpressing cell culture systems. The direct ubiquitination of purified Bax was also observed in vitro following incubation with recombinant parkin. We found that parkin prevented basal and apoptotic stress-induced translocation of Bax to the mitochondria. Moreover, an engineered ubiquitination-resistant form of Bax retained its apoptotic function, but Bax KO cells complemented with lysine-mutant Bax did not manifest the antiapoptotic effects of parkin that were observed in cells expressing WT Bax. These data suggest that Bax is the primary substrate responsible for the antiapoptotic effects of parkin, and provide mechanistic insight into at least a subset of the mitochondrial effects of parkin.
Keywords: Parkinson's disease, apoptosis, neuroprotection, mitophagy
Parkinson disease (PD) is a neurodegenerative disorder that affects 1–3% of the population over the age of 65 years (1). The symptoms include tremor, rigidity, bradykinesia, and postural instability. These physical characteristics are caused by the progressive degeneration of dopaminergic neurons of the substantia nigra pars compacta and, to a lesser extent, the catecholaminergic neurons of the locus coeruleus. Although most cases of PD are sporadic in nature, a small number of genes are responsible for the rare familial forms of PD (2). Loss-of-function mutations within the PARK2 locus, which encodes the protein parkin, are the most common cause of autosomal recessive PD (3).
Parkin is a 465-amino acid protein that is expressed in multiple tissues and functions as an E3 ubiquitin ligase (4). Ubiquitination of substrates is a tightly regulated process, requiring the combined activity of three enzymes: an E1 ubiquitin-activating enzyme, an E2 ubiquitin conjugating enzyme, and an E3 ubiquitin ligase (5). E3 ubiquitin ligases are responsible for substrate recognition, and as such contribute the specificity of a ubiquitin reaction. Defects in parkin-mediated ubiquitination may result in the failure to target specific substrates for degradation, leading to accumulation of potentially toxic proteins and consequent cell death (6). Parkin is widely neuroprotective (7); however, many of the putative parkin substrates reported to date are not thought to directly mediate toxicity in such a simple fashion [reviewed elsewhere (8)]; therefore, parkin deficiency may limit cell survival via more complex means. For example, under specific conditions of mitochondrial depolarization, parkin is recruited to damaged mitochondria and mediates their autophagic degradation (9). Despite these recent observations, additional mechanistic insights into parkin function and its role in inhibiting apoptosis are necessary, as well as further elucidation of the targets of parkin-dependent ubiquitination.
We recently showed that parkin expression induces fundamental changes in the mitochondrial response to apoptotic stressors (10). Although these effects could be observed from isolated mitochondria, several lines of evidence have suggested that cytosolic parkin was primarily responsible. This model would also be consistent with another recently identified parkin substrate, PARIS, which is acted upon by cytosolic parkin but which influences mitochondrial biology (11). Therefore, we hypothesized that the protective effects of parkin were due to its ability to regulate the function of a protein or series of proteins within the cytosol that influence apoptosis, such as members of the B-cell lymphoma 2 (Bcl-2) protein family. We report here that parkin expression reduces the mitochondrial accumulation of Bax under basal conditions, prevents the acute stress-induced translocation of Bax to the mitochondria, and directly ubiquitinates Bax. Furthermore, the expression of a ubiquitination-resistant form of Bax renders cells unresponsive to the well-described antiapoptotic function of parkin. These data provide evidence of a specific ubiquitin E3 ligase that may inactivate Bax to promote cell survival. Furthermore, these findings provide mechanistic insight into the antiapoptotic effects of parkin and the various mitochondrial phenotypes in parkin-deficient models, and may be relevant to the neuronal degeneration in PD.
Results
Parkin Prevents Apoptosis and Cell Death.
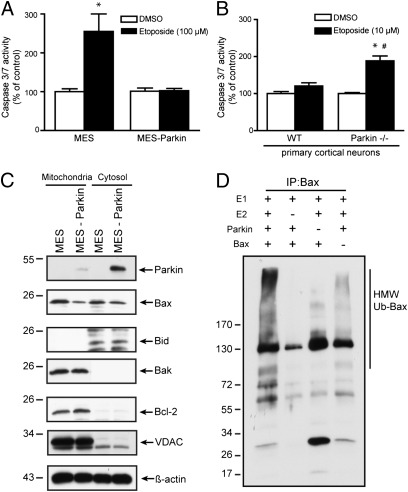
Parkin expression protects against caspase activation and cell death induced by stressors such as staurosporine, C2 ceramide, rotenone, and 6-OHDA (10, 12–14), and we extended these observations to include another stressor, etoposide. Neuronal dopaminergic MES and MES cells stably overexpressing human parkin (MES-Parkin) were treated with vehicle (DMSO) or etoposide (100 μM), and caspase 3/7 activity was measured at 18 h. Parental MES cells demonstrated a 100% increase in caspase 3/7 activity that was prevented by the stable over-expression of parkin (Fig. 1A). To determine the impact of parkin deficiency in a more physiological context, primary neurons cultured from WT and parkin KO (parkin−/−) mice (Fig. S1A) were treated with a subtoxic dose of etoposide (10 μM). The parkin-deficient neurons were significantly more sensitive to apoptosis, showing a 74% increase in caspase 3/7 activity (Fig. 1B), confirming a role for endogenous neuronal parkin in regulating the apoptotic response of the neuron. Parallel experiments examining multiple cell lines (Fig. S1B) confirmed that ectopic parkin expression blocked the induction of apoptotic markers (Fig. S1 C and D) and prevented cell death at later time points under a variety of well-published conditions (Fig. S1E).
Fig. 1.
Parkin protects against apoptosis, reduces Bax at the mitochondria, and mediates the ubiquitination of Bax. Caspase 3/7 activity was measured from (A) MES and MES-Parkin cell lines 18 h after treatment with vehicle (DMSO) or 100 μM etoposide (mean ± SEM, n = 16; *P < 0.05 from WT DMSO) or (B) WT and parkin−/− mouse primary cortical neurons 18 h after treatment with DMSO or 10 μM etoposide (mean ± SEM, n = 24; *P < 0.05 from WT DMSO, #P < 0.05 from parkin−/− DMSO). (C) Soluble (cytoplasmic) and mitochondrial fractions from MES and MES-Parkin cells were analyzed by Western blot. (D) Cell-free in vitro ubiquitination assay of Bax by parkin. FLAG-Ubiquitin, ubiquitin enzymes E1 and E2, and ATP were incubated with recombinant parkin and purified Bax as indicated. Reactions were then subjected to a Bax/FLAG-Ub IP–Western blot.
Numerous approaches have demonstrated the influence of parkin on mitochondrial biology and apoptosis, with evidence that this effect was mediated within the cytosol (10). Bax and Bak are each sufficient for the apoptotic release of cytochrome c (15), but only Bax is found both in the cytosol and at the mitochondria. Thus, a primary effect of parkin on regulating mitochondrial Bax could account for both the increased cell viability demonstrated here and the altered threshold for mitochondrial cytochrome c release previously reported (10). Cytosol and mitochondrial extracts from MES and MES-Parkin cells were analyzed by Western blot (WB) for the levels of several endogenous Bcl-2 family proteins. The mitochondrial fraction from MES-Parkin cells consistently showed decreased levels of Bax, but not Bid, Bak, or Bcl-2 (Fig. 1C), whereas only modest changes in cytosolic Bax were observed (Fig. 1C). Stable expression of the PD-linked parkin mutants R275W and W453X had no effect on Bax levels (Fig. S2A), indicating that functional parkin was required. We have previously demonstrated that 48 h of parkin expression was required to confer maximal protection from apoptosis (10), and a similar time-dependent change in Bax was observed in WT but not mutant parkin-expressing cells (Fig. S2B), temporally linking changes in Bax with parkin E3 ligase activity and parkin-dependent cell survival.
To examine direct ubiquitination of Bax by parkin, we purified Bax to homogeneity from Escherichia coli (Fig. S3A), and then performed a cell-free in vitro ubiquitin assay using recombinant parkin, E1, and E2, and Flag-Ubiquitin. Bax protein was immunoprecipitated from the reaction mixtures (to separate ubiquitinated Bax from auto-ubiquitinated parkin), and the Western blot was probed for Flag-Ubiquitin. Data showed a robust increase in high-molecular-weight, poly-ubiquitinated Bax in the presence, but not the absence, of parkin (Fig. 1D). Parkin was unable to ubiquitinate Bax in the absence of E2, consistent with the requirement of the combined activity each enzyme to carry out the biological function of ubiquitination (5).
Parkin Selectively Ubiquitinates Endogenous Bax.
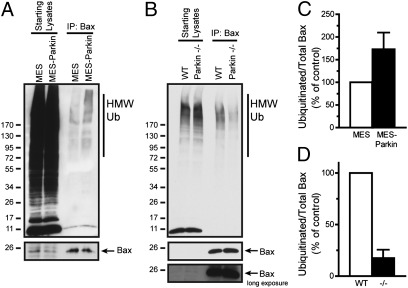
To examine whether parkin ubiquitinated Bax in intact cells, we harvested whole-cell lysates from MES and MES-Parkin cells treated with the proteasomal inhibitor MG-132 (10 μM, 6 h) and used immunoprecipitation (IP) to capture endogenous Bax and to probe for ubiquitination. Data revealed an increase in high-molecular-weight, ubiquitinated Bax in the MES-Parkin cells, compared with MES controls (Fig. 2 A and C; a shorter exposure of the starts is also provided in Fig. S3B). The specificity of this parkin-dependent ubiquitination was addressed by analyzing endogenous Bid, another proapoptotic Bcl-2 family protein, whose relative ubiquitination was not consistently altered by parkin (Fig. S3 C and D). Additional controls for the effects of parkin and the specificity of the antibodies used were conducted in WT and Bax KO MEF cells (Fig. S4A), where parkin increased the levels of ubiquitinated Bax in WT MEFs compared with non-parkin expressing cells, and no signal was observed in the Bax KO cells (Fig. S4B).
Fig. 2.
Ubiquitination of endogenous Bax by parkin. MES and MES-Parkin cells (A) or primary cortical neurons from WT and parkin−/− mice (B) were treated with proteasome inhibitor (MG-132, 10 μM) for 6 h and lysed in 1% Nonidet P-40, and Bax was immunoprecipitated, peptide eluted, and probed for ubiquitin by Western blot. A densitometric analysis of ubiquitinated Bax relative to IPed Bax from MES and MES-Parkin cells (C) and cortical neurons from WT and parkin−/− mice (D) is provided (mean ± SEM, n = 3).
It was critical to determine whether endogenous neuronal parkin mediates the ubiquitination of Bax; therefore, we examined the levels of endogenous ubiquitinated Bax in primary neurons cultured from WT and parkin−/− mice. Cortical neurons were cultured for 3 d in vitro (DIV) and then treated with MG-132 (10 μM, 6 h). Whole-cell lysates were subjected to Bax IP, followed by WB for ubiquitin. Results showed a decrease in ubiquitinated Bax in parkin−/− compared with WT neurons (Fig. 2 B and D), consistent with a preferential ubiquitination of endogenous Bax by endogenous parkin.
Although many intracellular proteins are degraded by the ubiquitin–proteasome pathway, some are degraded within lysosomes or during autophagy (16). To examine the fate of parkin-ubiquitinated Bax, Bax was immunoprecipitated from parental WT and Bax KO MEF cells with or without stable parkin expression 4 h after treatment with DMSO, the proteasome inhibitor MG-132 (10 μM), or the lysosome inhibitor ammonium chloride (NH4Cl; 20 mM). Data confirmed a specific increase in ubiquitinated Bax in parkin-expressing MEFs, as well as a further selective increase in ubiquitinated Bax by the combination of parkin expression and proteasomal inhibition (Fig. S4C), indicating that a portion of parkin-ubiquitinated Bax may be targeted for proteasomal degradation.
Parkin Physically Interacts with Bax in a Bona Fide E3 Ligase–Substrate Relationship.
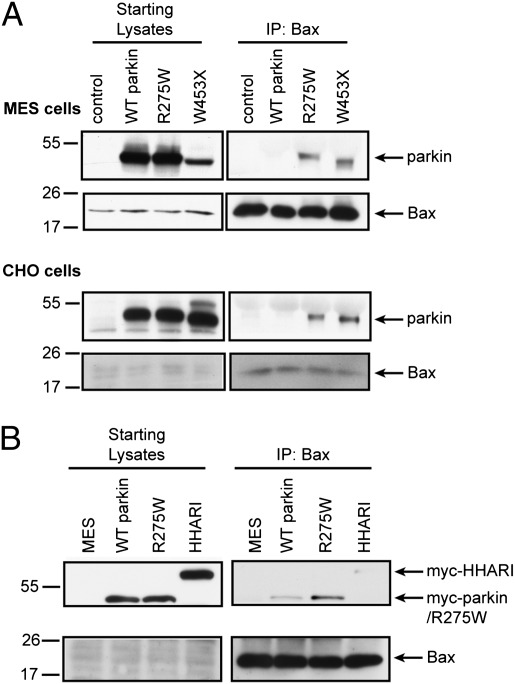
Catalytically inactive E3 ligase mutants bind their cognate substrates with greater efficiency than the active forms of the proteins (17), which has specifically been observed with respect to parkin and its pathogenic, loss-of-function mutants (18). We analyzed two distinct types of PD-linked parkin mutations, a missense mutation (R275W), and a premature termination (W453X), both presumed to result in loss of parkin function. The association of Bax with parkin was determined by immunoprecipitating endogenous Bax from parental MES and CHO cells or those stably expressing WT parkin or its PD-linked mutants, and probing for the levels of coimmunoprecipitated parkin. In both cell types, the R275W and W453X parkin mutants coimmunoprecipitated with endogenous Bax, whereas the interaction between active WT parkin and endogenous Bax was at or below our limit of detection (Fig. 3 A and B).
Fig. 3.
Bax physically interacts with parkin in cultured cells. (A) Whole-cell lysates from naive MES or CHO cells and MES and CHO cells stably expressing WT parkin (WT Parkin) or parkin mutants R275W and W453X were subjected to Bax IP. The eluates were probed for parkin to visualize a direct parkin–Bax association. (B) MES cells were transiently transfected to express myc-parkin, parkin mutant myc-R275W, or myc-HHARI, and were subjected to Bax IP and the eluates probed for myc.
To address the specificity of this interaction, we examined the protein HHARI, an E3 ligase with structural similarity to parkin. MES cells were transiently transfected with myc-tagged vectors encoding WT parkin, R275W parkin, or HHARI and their relative co-IP with endogenous Bax was compared. Results confirmed that R275W parkin interacts with endogenous Bax, whereas only trace amounts of WT parkin and HHARI were visible upon long exposures (Fig. 3B). These data are consistent with the hypothesis that Bax is selectively recognized by parkin and is a genuine substrate.
Lysine Residues in Bax Are Dispensable for Its Apoptotic Function but Are Required for the Prosurvival Function of Parkin.
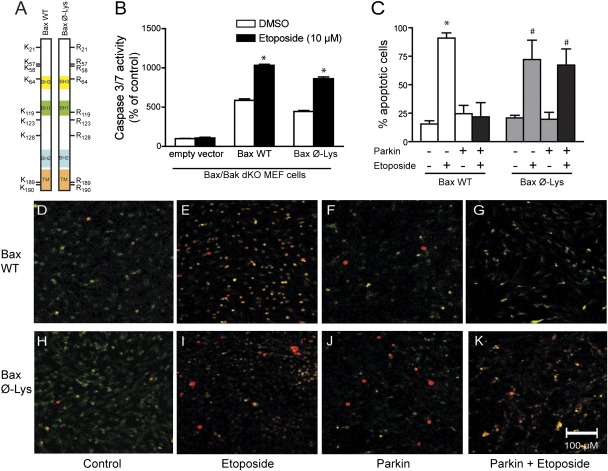
To examine the importance of ubiquitination as a regulator of Bax-dependent cell death, we engineered a mutant Bax protein in which each of its nine lysine residues were mutated to arginine (K→R; Bax Ø-Lys, Fig. 4A), thus rendering it unavailable for ubiquitination. This construct demonstrated significantly longer half-life than that of WT Bax (Fig. S4D), consistent with resistance toward ubiquitination. We evaluated the apoptotic capacity of Bax Ø-Lys in Bax/Bak double KO (dKO) MEF cells, as these cells would require ectopic Bax expression for caspase activation. The Bax/Bak dKO MEFs were transfected with empty vector, WT Bax, or Bax Ø-Lys. Twenty-four hours posttransfection, the cells were treated with DMSO or etoposide (10 μM) for 18 h, and caspase 3/7 was measured. Caspase 3/7 activity was low in the empty vector–transfected dKO MEFs treated with DMSO and was not elevated by etoposide (Fig. 4B), as expected. The overexpression of WT Bax promoted a significant fivefold elevation in caspase activity that was further elevated to a 10-fold increase by etoposide (Fig. 4B). Expression of the Bax Ø-Lys mutant resulted in a comparable fourfold increase in basal caspase activity, as well as a similar ninefold increase in etoposide-induced caspase activation, compared with those of the empty vector controls (Fig. 4B). These data indicate that the multiple lysine mutations did not disrupt the apoptotic function of Bax, making it a suitable tool for further investigation.
Fig. 4.
Bax ubiquitination is required for the antiapoptotic function of parkin. (A) Schematic representation of WT and Bax Ø-Lys constructs. Bax/Bak dKO MEFs with and without stable parkin expression were transiently transfected with empty vector, Bax WT, or Bax Ø-Lys and treated with vehicle (DMSO) or 10 μM etoposide for 18 h. (B) Caspase 3/7 activity was measured (mean ± SEM, n = 24; *P < 0.05 from DMSO). (C) Cells were fixed and immunostained for Bax (green) and cleaved caspase 3 (red). The percentage of Bax-transfected cells that contained cleaved caspase 3 was scored by a blinded observer (mean ± SEM; *P < 0.05 from WT DMSO, #P < 0.05 from Bax Ø-Lys DMSO). (D–K) Representative images of C.
Reconstituting the dKO MEFs with either Bax variant allowed us to evaluate the importance of a single protein on the protective effects of parkin. Parental dKO MEFs and those stably expressing human parkin were transiently transfected with WT Bax or Bax Ø-Lys and treated with DMSO or etoposide (10 μM) for 16 h. Because of the low transfection efficiency of the MEFs (<10%), biochemical evaluation was not feasible; thus, cells were immunocytochemically stained for the presence of Bax (green) and cleaved caspase 3 (red) and analyzed on a cell-by-cell basis by a blinded recorder (Fig. 4 D–K). Etoposide treatment resulted in 91% of the cells expressing WT Bax to appear apoptotic, compared with 16% of DMSO-treated cells, although the stable expression of human parkin prevented the etoposide-induced activation of caspase 3 in WT Bax-expressing cells (Fig. 4C). In the Bax Ø-Lys–expressing MEFs, etoposide treatment resulted in 72% of the transfected cells becoming apoptotic, similar to cells expressing WT Bax. However, the stable expression of human parkin did not reduce the percentage of cells with active caspase 3 when expressing Bax Ø-Lys (Fig. 4C), indicating that parkin does not inhibit the induction of apoptosis when Bax cannot be ubiquitinated.
Parkin Prevents Stress-Induced Translocation of Bax to Mitochondria.
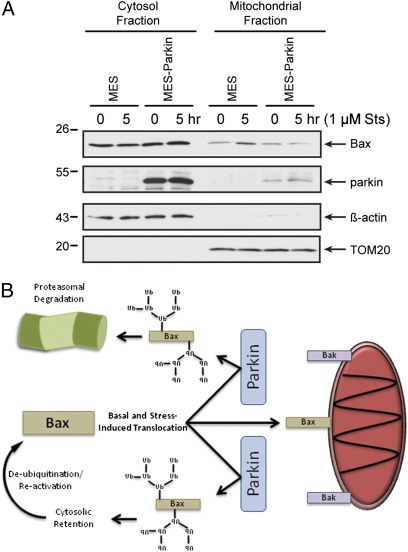
Recent data from multiple groups have shown that parkin can be rapidly recruited to the mitochondria after mitochondrial depolarization, where it participates in mitophagy (9, 19). Prior work from our group suggested that the antiapoptotic effects of parkin were mediated by cytosolic parkin, the major pool of the protein at rest. Interestingly, under apoptotic conditions but not depolarization, Bax is recruited from the cytosol to the mitochondria, where it participates in cytochrome c release (20). Thus, we asked whether the subcellular localization of Bax or parkin was altered during apoptotic stress, and whether parkin influenced the established translocation of Bax during apoptosis. MES and MES-Parkin cells were treated with DMSO or staurosporine (1 μM, 5 h), and cytosolic and mitochondrial fractions were prepared from each condition. Consistent with prior work (21), staurosporine induced the translocation of Bax from the cytosol to the mitochondria in MES cells (Fig. 5A). However, this redistribution of Bax did not occur in MES-Parkin cells (Fig. 5A). Similar data were also obtained after exposure to C2 ceramide (Fig. S5A), another stressor inhibited by parkin (10, 12). Parkin, however, did not translocate to the mitochondria after either staurosporine or C2 ceramide treatment (Fig. 5A and Fig. S5A, respectively). Conversely, CHO-Parkin and MES-Parkin cells showed robust mitochondrial translocation of parkin after mitochondrial depolarization with CCCP (Fig. S5 B and C). However, Bax localization remained largely unchanged in either the absence or presence of parkin (Fig. S5 B and C), indicating divergent effects of apoptotic and mitophagic induction on the cellular localization of Bax and parkin. Evaluation of live CHO and MES cells demonstrated staurosporine-induced but not CCCP-induced GFP-Bax translocation to mito-mCherry–labeled mitochondria (Fig. S6 A and B), which was prevented by both stable and transient parkin expression (Fig. S6 C–F), consistent with the biochemical findings. Conversely, we found that CCCP but not staurosporine induced translocation of GFP-Parkin (Fig. S7), as previously shown (9).
Fig. 5.
Parkin prevents stress-induced accumulation of Bax at the mitochondria. (A) MES and MES-Parkin cells were treated with vehicle (DMSO) or 1 μM staurosporine (Sts) for 5 h and subjected to cellular fractionation. The fractions were probed for Bax, parkin, and the cytosol and mitochondrial markers actin and TOM20, respectively. (B) Schematic representation of the effects of parkin E3 ligase activity on the apoptotic function of Bax.
Discussion
Loss-of-function mutations within the PARK2 locus, encoding the ubiquitin E3 ligase parkin, are the most common cause of autosomal recessive PD (3). Parkin expression has been associated with significant protection from a variety of toxic stressors both in cell culture and in vivo [reviewed elsewhere (7)]. Although numerous substrates have been proposed, few, if any, speak directly to such a potent prosurvival mechanism. The current study builds on the prior observation that parkin expression or deficiency induces a fundamental change in the cellular vulnerability to stressors by altering the mitochondrial response to stress. Here we show that parkin limits the mitochondrial pool of Bax at rest and during stress, which may be sufficient to account for numerous reports of parkin-dependent alterations in apoptosis observed in vivo (22, 23), at the whole cell level in vitro (12), and even in isolated mitochondria (10). The identification of a Bax E3 ligase expands the established pathways for regulating Bax function to include ubiquitination as a means of promoting cell survival and may also reconcile prior findings of the de-ubiquitinating enzyme Ku70 potentiating Bax-dependent cell death (24). Furthermore, these findings provide insight into the neuronal consequences of parkin deficiency as they relate to inherited defects in the Parkin gene or the numerous stress conditions and posttranslational modifications that disrupt normal parkin function [reviewed elsewhere (25)].
Here we demonstrate the parkin-dependent ubiquitination of endogenous Bax using parkin stable and transiently transfected dopaminergic MES cells and numerous MEF lines. In addition, primary cultured neurons were studied to confirm the influence of endogenous neuronal parkin. To validate that the ubiquitination of Bax observed in living cells (Fig. 2) was a direct recognition of Bax by the E3 ligase activity of parkin, and not a downstream effect of parkin expression, we used purified Bax and recombinant parkin, and likewise found a robust ubiquitination of Bax in vitro (Fig. 1D). In addition, we observed that WT parkin, but not its pathogenic loss-of-function PD-linked mutants, decreased the mitochondrial levels of Bax (Fig. 1C and Fig. S2A), consistent with the requirement for intact parkin E3 ligase activity to modulate Bax localization.
The physical interactions between a catalytically active E3 ligase and its substrate are transient, and therefore often difficult to detect. However, catalytically inactive E3 ligase mutants may bind their cognate substrates with a decreased off-rate, and may be more readily detected in a complex (17); which has been reported for parkin and its PD-linked mutants (18). Here, we report in multiple cell lines that two distinct pathogenic parkin mutants demonstrated greater association with endogenous Bax than WT parkin (Fig. 3A). In addition, an E3 ligase structurally related to parkin, HHARI, did not associate with Bax (Fig. 3B). Additional experiments explored the relationship between the duration of parkin expression and changes in mitochondrial levels of Bax. Temporal changes in mitochondrial Bax after WT parkin expression (Fig. S2B) mirrored the requisite duration of parkin expression necessary to alter the mitochondrial threshold for cytochrome c release (10). Several other cytoplasmic and mitochondrial proteins known to participate in the complex process of cytochrome c release in both proapoptotic and antiapoptotic roles were analyzed, but Bax was the only protein that was consistently altered in a parkin-dependent manner (Fig. 1C).
Parkin has also been suggested to mediate neuroprotection via its role in mitophagy. Indeed, numerous proteins at the outer mitochondrial membrane have been found to be ubiquitinated following the induction of mitophagy and subsequent translocation of parkin to mitochondria [reviewed elsewhere (26)]. However, it is important to note that the effects of parkin on Bax that we report here were observed both at rest and during the induction of apoptosis (Fig. 5A and Fig. S7), conditions not consistent with CCCP-induced parkin-dependent mitophagy. Furthermore, biochemical and image-based analyses in multiple cell lines did not demonstrate the translocation of parkin to the mitochondria during the induction of apoptosis, but did demonstrate parkin-dependent effects on Bax localization (Fig. 5A and Figs. S5A and S6), which differs from our findings following CCCP treatment where parkin, but not Bax, robustly translocates to mitochondria (Figs. S5 B and C, S6, and S7). These data may further segregate the discrete parkin-dependent effects on Bax reported here from the promiscuous ubiquitination of mitochondrial proteins recently reported during mitophagy (27, 28), and indicate that the antiapoptotic and mitophagic functions of parkin proceed via different mechanisms, with only mitophagy involving the mitochondrial translocation of parkin.
We speculate that the ubiquitination of Bax by parkin may depend on conformational changes in Bax that allow for its recognition by parkin. On the other hand, mitochondrial depolarization may induce changes in parkin folding (i.e., unique access to the UBL domain, phosphorylation by PINK1) that promote its mitochondrial translocation and facilitate the observed docking of the proteasome adjacent to parkin-decorated mitochondria during mitophagy (28). The hypothesis that a specific conformation or pool of Bax is recognized by parkin is supported by the observation that the cytosolic levels of Bax were not robustly affected by parkin expression and that proteomic screens of whole tissue failed to identify changes in Bax levels in parkin KO brain (29, 30). Conversely, it may also indicate that not all of the parkin-ubiquitinated Bax is destined for degradation, as some studies suggest that parkin can facilitate K63 ubiquitin linkages (31), which may alter protein trafficking and not induce degradation. Further work will be required to examine the precise biochemical fate of parkin-ubiquitinated Bax in a neuronal context.
The ubiquitination of Bax has been argued to play a role in the regulation of apoptotic cell death (24); however, the identity of a specific E3 ligase responsible was previously unknown. Therefore, the current data provide a missing link between de-ubiquitination and the proapoptotic activity of Bax and mechanisms for inhibiting Bax-dependent cell death via ubiquitination. We propose that one of the likely critical functions that parkin plays within the cell is to limit, via ubiquitination, the translocation of Bax to the mitochondria, thus dampening the apoptotic response of the cell. These data directly address the cellular resistance to apoptosis broadly observed following parkin expression in vitro and in vivo, and the intrinsic effect that parkin expression or deficiency has on isolated mitochondria. We recognize that other E3 ligases may ubiquitinate Bax (32), but these may or may not influence Bax-dependent cell death. Based on the unique vulnerability of the substantia nigra and locus coeruleus in Parkin-null patients, however, we speculate that parkin plays a specialized role in modulating Bax within these discrete neuronal subpopulations. The interaction between parkin and Bax may also play a role in the natural age-dependent degeneration of catecholaminergic neurons in the normal human brain (33–35), which may be a consequence of the age-dependent changes in parkin solubility (36) or stress-induced loss of parkin function [reviewed elsewhere (25)].
Materials and Methods
Cell Culture, Plasmids, and Transfection Methods.
All cell culture, plasmids, and transfection methods used were previously described (10, 37–39). Mutant Bax protein with all lysines replaced with arginines (Bax Ø-Lys) was engineered by GenScript and subcloned into pcDNA 3.1(+) vector (Invitrogen).
Caspase 3/7 Assay.
Caspase 3/7 activity was measured 18 h after drug treatments using the SensoLyte AMC kit (AnaSpec) according to manufacturer's instructions, and quantified on a Synergy H1 (BioTek) plate reader.
IP, Cellular Fractionation, and Western Blot.
Cellular lysates were subjected to standard Western blotting conditions described in SI Materials and Methods. For IP, cells or neurons were treated with 10 μm MG-132 for 6 h before lysis. Protein-normalized lysates were precleared with Protein-A agarose beads (Roche Diagnostics) for 2 h at 4 °C and then immunoprecipitated using a polyclonal Bax antibody conjugated to agarose beads (sc-493AC; Santa Cruz Biotechnology) overnight at 4 °C. Beads were washed 3 × 15 min in 1× STEN buffer (50 mM Tris, pH 7.6, 150 mM NaCl, 2 mM EDTA, 0.2% Nonidet P-40). Bax was eluted 3× with an immunizing Bax peptide for the polyclonal antibody (sc-493P; Santa Cruz Biotechnology). Eluates and starting lysates were subjected to standard Western blotting conditions (antibodies and Western reagents used detailed in SI Materials and Methods). To produce cytosolic and mitochondrial fractions, cells were homogenized and mitochondria pelleted as previously described (10). In addition, the soluble supernatants were then centrifuged at 100,000 × g for 1 h to achieve a pure cytosolic fraction. All experiments were performed in triplicate, and representative images are shown.
In Vitro Ubiquitination Assay.
To the Master Mix (50 mM Tris⋅HCl pH 7.5, 2 mM MgCl2, 4 mM ATP, 1 μg/μL Flag-ubiquitin), E1 (UBE1; Boston Biochem), E2 (UbcH7; Boston Biochem), His6-Parkin (E3-150; Boston Biochem), and full-length human Bax protein, generated and purified as described (40), were added as required and incubated at 37 °C for 90 min. Bax was immunoprecipitated as above, and equal volumes of each sample were prepared for Western blot analysis. Blots were probed for ubiquitin by Flag antibody (M2, Sigma).
Immunocytochemistry.
Bax/Bak dKO MEFs with and without stable parkin expression were plated into poly-d-lysine–coated, two-well CultureSlides (BD Biosciences). Cells were transiently transfected with empty vector, Bax WT, or Bax Ø-Lys and treated with vehicle (DMSO) or 10 μM etoposide for 18 h. Cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton-X, and immunostained for Bax (sc-7480; Santa Cruz Biotechnology) and cleaved caspase 3 (9661S; Cell Signaling). Cy2- and Cy3-conjugated secondary antibodies were purchased from Jackson ImmunoResearch. Slides were coverslipped and confocal images were acquired using a Zeiss LSM 510 microscope. Images were coded and given to a blinded observer who scored the percentage of Bax-transfected cells (green) that contained cleaved caspase (red).
Statistical Analysis.
Caspase 3/7 activity data and cleaved caspase 3 quantification were compared using one-way ANOVAs, followed by Tukey's post hoc analyses.
Supplementary Material
Acknowledgments
The authors thank Drs. Anthony Letai and Helen Ardley for providing valuable reagents, Dr. Alfred Goldberg for helpful discussion, Dr. Julia Schlehe for a critical reading of the manuscript, and Katherine Amodeo for technical assistance. This work was supported by National Institutes of Health Grants AG023094 (to M.J.L.), NS065013 (to M.J.L.), and AG00222 (to A.K.B.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113248109/-/DCSupplemental.
References
- 1.Dauer W, Przedborski S. Parkinson's disease: Mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 2.Dawson TM, Dawson VL. Rare genetic mutations shed light on the pathogenesis of Parkinson disease. J Clin Invest. 2003;111:145–151. doi: 10.1172/JCI17575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitada T, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 4.Shimura H, et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- 5.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 6.Tan JM, Wong ES, Lim KL. Protein misfolding and aggregation in Parkinson's disease. Antioxid Redox Signal. 2009;11:2119–2134. doi: 10.1089/ars.2009.2490. [DOI] [PubMed] [Google Scholar]
- 7.Pilsl A, Winklhofer KF. Parkin, PINK1 and mitochondrial integrity: Emerging concepts of mitochondrial dysfunction in Parkinson's disease. Acta Neuropathol. 2012;123:173–188. doi: 10.1007/s00401-011-0902-3. [DOI] [PubMed] [Google Scholar]
- 8.Kahle PJ, Haass C. How does parkin ligate ubiquitin to Parkinson's disease? EMBO Rep. 2004;5:681–685. doi: 10.1038/sj.embor.7400188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger AK, et al. Parkin selectively alters the intrinsic threshold for mitochondrial cytochrome c release. Hum Mol Genet. 2009;18:4317–4328. doi: 10.1093/hmg/ddp384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin JH, et al. PARIS (ZNF746) repression of PGC-1α contributes to neurodegeneration in Parkinson's disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darios F, et al. Parkin prevents mitochondrial swelling and cytochrome c release in mitochondria-dependent cell death. Hum Mol Genet. 2003;12:517–526. doi: 10.1093/hmg/ddg044. [DOI] [PubMed] [Google Scholar]
- 13.Jiang H, Ren Y, Zhao J, Feng J. Parkin protects human dopaminergic neuroblastoma cells against dopamine-induced apoptosis. Hum Mol Genet. 2004;13:1745–1754. doi: 10.1093/hmg/ddh180. [DOI] [PubMed] [Google Scholar]
- 14.Wang C, et al. Stress-induced alterations in parkin solubility promote parkin aggregation and compromise parkin's protective function. Hum Mol Genet. 2005;14:3885–3897. doi: 10.1093/hmg/ddi413. [DOI] [PubMed] [Google Scholar]
- 15.Antignani A, Youle RJ. How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Curr Opin Cell Biol. 2006;18:685–689. doi: 10.1016/j.ceb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Cuervo AM, Wong ES, Martinez-Vicente M. Protein degradation, aggregation, and misfolding. Mov Disord. 2010;25(Suppl 1):S49–S54. doi: 10.1002/mds.22718. [DOI] [PubMed] [Google Scholar]
- 17.Qiu XB, Goldberg AL. Nrdp1/FLRF is a ubiquitin ligase promoting ubiquitination and degradation of the epidermal growth factor receptor family member, ErbB3. Proc Natl Acad Sci USA. 2002;99:14843–14848. doi: 10.1073/pnas.232580999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sriram SR, et al. Familial-associated mutations differentially disrupt the solubility, localization, binding and ubiquitination properties of parkin. Hum Mol Genet. 2005;14:2571–2586. doi: 10.1093/hmg/ddi292. [DOI] [PubMed] [Google Scholar]
- 19.Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP. Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J Cell Biol. 2010;189:671–679. doi: 10.1083/jcb.201001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolter KG, et al. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark IE, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 23.Pesah Y, et al. Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development. 2004;131:2183–2194. doi: 10.1242/dev.01095. [DOI] [PubMed] [Google Scholar]
- 24.Amsel AD, Rathaus M, Kronman N, Cohen HY. Regulation of the proapoptotic factor Bax by Ku70-dependent deubiquitylation. Proc Natl Acad Sci USA. 2008;105:5117–5122. doi: 10.1073/pnas.0706700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosco DA, LaVoie MJ, Petsko GA, Ringe D. Proteostasis and movement disorders: Parkinson's disease and amyotrophic lateral sclerosis. Cold Spring Harb Perspect Biol. 2011;3:a007500. doi: 10.1101/cshperspect.a007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karbowski M, Youle RJ. Regulating mitochondrial outer membrane proteins by ubiquitination and proteasomal degradation. Curr Opin Cell Biol. 2011;23:476–482. doi: 10.1016/j.ceb.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan NC, et al. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum Mol Genet. 2011;20:1726–1737. doi: 10.1093/hmg/ddr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshii SR, Kishi C, Ishihara N, Mizushima N. Parkin mediates proteasome-dependent protein degradation and rupture of the outer mitochondrial membrane. J Biol Chem. 2011;286:19630–19640. doi: 10.1074/jbc.M110.209338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palacino JJ, et al. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- 30.Periquet M, Corti O, Jacquier S, Brice A. Proteomic analysis of parkin knockout mice: Alterations in energy metabolism, protein handling and synaptic function. J Neurochem. 2005;95:1259–1276. doi: 10.1111/j.1471-4159.2005.03442.x. [DOI] [PubMed] [Google Scholar]
- 31.Lim KL, Dawson VL, Dawson TM. Parkin-mediated lysine 63-linked polyubiquitination: a link to protein inclusions formation in Parkinson's and other conformational diseases? Neurobiol Aging. 2006;27:524–529. doi: 10.1016/j.neurobiolaging.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 32.Benard G, et al. IBRDC2, an IBR-type E3 ubiquitin ligase, is a regulatory factor for Bax and apoptosis activation. EMBO J. 2010;29:1458–1471. doi: 10.1038/emboj.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cabello CR, Thune JJ, Pakkenberg H, Pakkenberg B. Ageing of substantia nigra in humans: Cell loss may be compensated by hypertrophy. Neuropathol Appl Neurobiol. 2002;28:283–291. doi: 10.1046/j.1365-2990.2002.00393.x. [DOI] [PubMed] [Google Scholar]
- 34.Ma SY, Röytt M, Collan Y, Rinne JO. Unbiased morphometrical measurements show loss of pigmented nigral neurones with ageing. Neuropathol Appl Neurobiol. 1999;25:394–399. doi: 10.1046/j.1365-2990.1999.00202.x. [DOI] [PubMed] [Google Scholar]
- 35.Zucca FA, et al. The neuromelanin of human substantia nigra: Physiological and pathogenic aspects. Pigment Cell Res. 2004;17:610–617. doi: 10.1111/j.1600-0749.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- 36.Pawlyk AC, et al. Novel monoclonal antibodies demonstrate biochemical variation of brain parkin with age. J Biol Chem. 2003;278:48120–48128. doi: 10.1074/jbc.M306889200. [DOI] [PubMed] [Google Scholar]
- 37.Banker G, Goslin K. Culturing Nerve Cells. Cambridge, MA: The MIT Press; 1996. [Google Scholar]
- 38.Crawford GD, Jr, et al. A novel N18TG2 x mesencephalon cell hybrid expresses properties that suggest a dopaminergic cell line of substantia nigra origin. J Neurosci. 1992;12:3392–3398. doi: 10.1523/JNEUROSCI.12-09-03392.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG, Selkoe DJ. Dopamine covalently modifies and functionally inactivates parkin. Nat Med. 2005;11:1214–1221. doi: 10.1038/nm1314. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki M, Youle RJ, Tjandra N. Structure of Bax: Coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.