Abstract
Objective
To examine the utility of using proteinuria in pre-operative risk stratification for acute kidney injury (AKI). AKI is a common and important complication for patients undergoing cardiac surgery. Proteinuria, which reflects structural damage to the glomeruli or renal tubules, may aid the prediction of AKI.
Methods
The ratio of urine albumin to creatinine (UACR) and dipstick proteinuria concentration were prospectively measured in 1159 patients undergoing cardiac surgery. The cohort was organized into four clinical risk categories based on the pre-operative UACR: UACR ≤ 10 mg/g (≤ 1.1 mg/mmol), 11–29 mg/g (1.2–3.3 mg/mmol), 30–299 mg/g (3.4–33.8 mg/mmol), and ≥ 300 mg/g (≥ 33.9 mg/mmol). The primary outcome was post-operative AKI, defined by the AKIN stage I criterion (serum creatinine rise by ≥50% or ≥ 0.3 mg/dL (26.5 μmol/L)).
Results
An increase in the incidence of AKI was noted across the UACR categories. Adding UACR to the clinical model to predict AKI improved the AUC from 0.64 to 0.67 (p < 0.001) and the continuous net reclassification improvement (NRI) was 30% (p < 0.001). UACR was also independently associated with risk of in-hospital dialysis, and ICU and hospital length of stay. Surgery status and pre-operative GFR were effect modifiers; the association was stronger amongst those undergoing elective surgery and those with eGFR ≥ 45 mL/min per 1.73 m2.
Conclusions
Pre-operative proteinuria provides graded stratification risk for AKI and is an independent predictor of other outcomes in patients undergoing cardiac surgery.
INTRODUCTION
Acute Kidney Injury (AKI) is a common and significant complication in patients undergoing cardiac surgery. Post operatively, it is associated with increased hospital length of stay and in-hospital mortality and those who survive AKI have an increased risk of developing chronic kidney disease.1,2 Thus, pre-operative prediction of AKI is important in clinical decision making.
One of the most important determinants for the development of AKI in cardiac surgery is pre-existing kidney function, usually assessed by estimates of glomerular filtration rate.3,4 However, another dimension that offers a clue to the integrity of the kidney is proteinuria as assessed by urine albumin to creatinine ratio (UACR) or dipstick proteinuria.5 As a reflection of structural damage to the glomeruli or kidney tubules, proteinuria is increasingly recognized as an important measure of kidney disease6 and risk factor for the development of AKI.7,8,9,10 Proteinuria adds prognostic value to glomerular filtration rate for understanding risks of AKI,9 cardiovascular disease, and death.11 Urine protein measurements are readily available, inexpensive, and can even be assessed at the bedside using a dipstick.
Several prediction models have been published to predict dialysis (incidence 1–2%) after cardiac surgery but none of these models perform well for prediction of less severe forms of AKI.12,13 These risk-assessment tools are used to balance the risks and benefits of cardiac surgery and for monitoring the quality of care and outcomes of cardiac surgery by surgeons and hospitals. Because of the intense scrutiny on cardiac surgery outcomes, even mild improvements to help risk adjust for adverse outcomes could have great clinical importance. This may allow clinicians to alter perisurgical approaches to minimize AKI or to consider preventative therapies that prove effective in clinical trials.
Thus our objective was to examine the utility of urinary albumin concentrations and urine dipstick measures of proteinuria to stratify for the risk of AKI in patients undergoing cardiac surgery.
METHODS
Design and Setting
Participants reported here are a subset of the Translational Research Investigating Biomarker Endpoints in Acute Kidney Injury (TRIBE-AKI; Clinicaltrials.gov # NCT00774137) cohort. We prospectively enrolled 1219 patients who were at risk for developing post-operative AKI. The ratio of urine albumin to creatinine (UACR) was measured on available pre-operative urine samples (n=1159) and dipstick proteinuria was measured on 1123 samples. 60 patients did not have the ratios of urine albumin to creatinine, and their characteristics were similar to the rest of the cohort. The cohort included adults undergoing cardiac surgery (coronary artery bypass grafting [CABG], surgery for valve disease or both) at six academic medical centers in North America between July 2007 and December 2009. All patients were at high risk for AKI, defined by the presence of one or more of the following criteria: pre-existing renal impairment (baseline serum creatinine > 2 mg/dL [177 μmol/L]), ejection fraction <35% or grade 3 or 4 left ventricular dysfunction, age > 65 years, diabetes mellitus, concomitant CABG and valve surgery, or repeat revascularization surgery. Exclusion criteria included prior kidney transplantation, pre-operative advanced chronic kidney disease or end-stage renal disease, or if nephrotoxic drugs were administered pre-operatively.
All participants provided written informed subject consent. Each institution’s research ethics board approved the study.
Sample collection, Biomarker Measurement and Outcomes
10 cc of fresh urine sample was collected at the pre-operative visit or on the morning of the surgery. We centrifuged the samples at 1000g for 10 minutes to remove cellular debris. The supernatant was stored at −80°C in 1 ml aliquots. All urine albumin assays were measured by immunoturbidimetry on a Siemens Dimension Plus with HM clinical analyzer, per manufacturer’s instructions. We measured urine creatinine by the modified Jaffe reaction. Based on the pre-operative urine albumin to creatinine ratio (UACR), the cohort was organized into four clinical risk categories: UACR ≤ 10 mg/g (≤ 1.1 mg/mmol), UACR 11–29 mg/g (1.2 – 3.3 mg/mmol), UACR 30–299 mg/g (3.4 – 33.8 mg/mmol) and UACR ≥ 300 mg/g (≥ 33.9 mg/mmol) for the current study.7 Urine dipstick was graded as negative, trace, 30–99 mg/dl (0.03–0.099 g/L) and ≥ 100 mg/dl (≥ 0.1 g/L) and was measured in the fresh urine using Siemens Clinitek Status ID number SN48923.
The primary outcome was post-operative AKI based on Acute Kidney Injury Network stage I (serum creatinine rise by ≥ 50% or ≥ 0.3 mg/dL (26.5 μmol/L) from the pre-operative value or requirement of dialysis). The secondary outcome was AKI based on Acute Kidney Injury Network stage II (serum creatinine rise by ≥ 100% or requirement of dialysis).
Statistical analysis
We collected demographics, co-morbidities, and procedural variables using definitions of the Society of Thoracic Surgeons (STS) (http://www.ctsnet.org/file/rptDataSpecifications252_1_ForVendorsPGS.pdf). Relative risks of AKI for the clinical risk categories, adjusted for variables that were available pre-operatively including demographics, co-morbidities, and procedural variables were calculated by logistic regression with site as a random effect (proc GLIMMIX procedure in SAS 9.2 software, SAS Institute, Cary, NC, USA). The estimated odds ratios were converted and presented as relative risks.14 The multivariate clinical model comprised of age (per year), sex, race, pre-op estimated glomerular filtration rate (eGFR) calculated by chronic kidney disease epidemiology collaboration (CKD-EPI) equation,15 elective surgery, type of surgery, cardiac catheterization in past 48 hours, and previous history of diabetes, hypertension, chronic congestive heart failure, myocardial infarction, and type of surgery. These variables are the STS variables that have been shown to predict AKI in the cardiac surgery setting.16 To evaluate the added effect of UACR on risk discrimination for AKI, we constructed receiver operating characteristic (ROC) curves and calculated the area under the curve (AUC) for the UACR alone, the multivariate clinical model alone, and then with the addition of UACR to the clinical model using R 2.11.0 (R Foundation for Statistical Computing, Vienna, Austria). We tested for a statistically significant increase in the area under the curve using the method developed by De Long et al.17 We determined the continuous net reclassification improvement (NRI) as suggested by Pencina et al.18 We evaluated for interaction with the likelihood ratio test on the following subgroups: age (<65, 65–75, 75–85, >85), diabetes, elective vs. urgent surgery, and pre-operative stages of estimated GFR.
Results
Of 1159 patients with pre-operative urine albumin creatinine ratio (UACR) measurements, 405 (35%) had UACR ≤ 10 mg/g, 355 (31%) had UACR 11–29 mg/g, 329 (28%) had UACR 30–299 mg/g, and 70 (6%) had UACR ≥ 300 mg/g. Characteristics associated with higher levels of albuminuria included older age, diabetes, heart failure, EF< 40%, higher pre-operative serum creatinine and lower pre-operative estimated GFR (Table 1). The use of angiotensin converting enzyme inhibitors, angiotensin-II receptor blockers, statins and β-blockers was not associated with the albuminuria concentrations.
Table 1.
Pre-operative Characteristics by Albuminuria in Adults undergoing Cardiac Surgery
| UACR < 10 mg/g (n=405, 35%) | UACR 11–29 mg/g (n=355, 31%) | UACR 30–299 mg/g (n=329, 28%) | UACR ≥ 300 mg/g (n=70, 6%) | P-value for trend | |
|---|---|---|---|---|---|
| Age (mean, SD) | 70.3 (10.4) | 71.8 (9.7) | 73.0 (9.7) | 70.7 (10.6) | 0.003 |
| < 65 years (n, %) | 97 (24%) | 73 (21%) | 61 (19%) | 19 (27%) | |
| 65–75 years | 164 (40%) | 135 (38%) | 118 (36%) | 24 (34%) | |
| 75–85 years | 134 (33%) | 133 (37%) | 120 (36%) | 23 (33%) | |
| >85 years | 10 (2.5%) | 14(3.9%) | 30 (9.1%) | 4 (5.7%) | |
| Male (n, %) | 288 (71%) | 234 (66%) | 225 (68%) | 40 (57%) | 0.09 |
| White Race (n, %) | 384 (95%) | 331 (93%) | 309 (94%) | 60 (86%) | 0.09 |
| Diabetes (n, %) | 141 (35%) | 137 (39%) | 155 (47%) | 46 (66%) | <.001 |
| Hypertension (n, %) | 309 (76%) | 280 (79%) | 266 (81%) | 59 (84%) | 0.06 |
| Myocardial infarction (n, %) | 114 (29%) | 83 (24%) | 70 (22%) | 25 (36%) | 0.33 |
| Heart Failure (n, %) | 96 (24%) | 77 (22%) | 98 (30%) | 25 (36%) | 0.01 |
| Ejection fraction ≤ 40% (n, %) | 64 (18%) | 49 (15%) | 84 (28%) | 24 (37%) | <.001 |
| Operative characteristics (n, %) | |||||
| Prior Cardiac Surgery | 55 (14%) | 43 (12%) | 46 (14%) | 5 (7%) | 0.58 |
| Elective Surgery | 306 (76%) | 295 (83%) | 265 (81%) | 54 (77%) | 0.16 |
| Cardiac catheterization in last 48 hours | 18 (4%) | 18 (5%) | 23 (7%) | 3 (4%) | 0.24 |
| Surgery (n, %) | 0.01 | ||||
| CABG | 228 (56%) | 148 (42%) | 147 (45%) | 35 (50%) | |
| Valve | 87 (21%) | 117 (33%) | 114 (35%) | 16 (23%) | |
| CABG and valve | 90 (22%) | 90 (25%) | 68 (21%) | 19 (27%) | |
| Pre-op medications | |||||
| ACE inhibitors | 164 (47%) | 162 (48%) | 134 (45%) | 27 (45%) | 0.56 |
| Angiotensin II receptor blockers | 75 (21%) | 64 (19%) | 74 (25%) | 10 (17%) | 0.74 |
| Aspirin | 288 (83%) | 231 (68%) | 216 (72%) | 40 (67%) | 0.01 |
| Beta blockers | 258 (74%) | 239 (71%) | 224 (75%) | 52 (87%) | 0.18 |
| Statins | 267 (77%) | 238 (70%) | 217 (73%) | 48 (80%) | 0.68 |
| Renal function (mean, SD) | |||||
| Pre-op Serum Creatinine (mg/dL)§ | 1.0 (0.2) | 1.0 (0.3) | 1.2 (0.4) | 1.3 (0.5) | <.001 |
| Pre-op eGFR(mL/min per 1.73m2) | 70 (17) | 71 (19) | 63 (21) | 57 (22) | <.001 |
| <30 mL/min (n, %) | 4 (1%) | 3 (1%) | 19 (6%) | 10 (16%) | |
| ≥30 and <45 | 26 (7%) | 29 (10%) | 50 (17%) | 14 (22%) | |
| ≥45 and <60 | 76 (21%) | 66 (22%) | 87 (29%) | 13 (20%) | |
| ≥60 | 252 (70%) | 203 (67%) | 141 (47%) | 27 (42%) | |
| STS Bedside Score* | 19.2 (4.6) | 19.9 (5.0) | 21.8 (6.3) | 24.1 (6.8) | <.001 |
Abbreviations: UACR- urine albumin to creatinine ratio, CABG- coronary artery bypass grafting, ACE- angiotensin converting enzyme, eGFR- estimated glomerular filtration rate, SD-standard deviation
Renal and Non-renal outcomes by UACR categories
During the post-operative period, 409 (35.2%) patients developed stage I acute kidney injury or worse. The incidence of AKI increased according to the pre-operative UACR ratio: UACR ≤ 10 mg/g (26% incidence), UACR 11–29 mg/g (35%), UACR 30–299 mg/g (42%), UACR ≥ 300 mg/g (57%) (p-value for trend < 0.001). The adjusted relative risk for stage I AKI increased according to the gradient of the UACR ratio, with an approximate doubling in AKI risk for the UACR ≥ 300 mg/g group compared to the UACR ≤ 10 mg/g group (Table 2). 58 (5.0%) patients developed stage II AKI or worse. The incidence of stage II or worse AKI also increased according to the gradient of the UACR ratio, however after multivariate adjustment the independent association did not meet strict statistical significance (Table 2). 17 (1.5%) patients required dialysis of whom 6 died. An additional 13 patients died without needing dialysis..UACR categories were independently associated with dialysis, length of ICU stay, length of hospital stay, but not in-hospital death.(Table 2).
Table 2.
Association of pre-operative albuminuria and dipstick proteinuria with outcomes
| Stage I AKI or worse | Stage II AKI | Other Outcomes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| UACR/Dipstick categories (n) | AKI cases (n,%) | Unadjusted RR (95% CI)* | Adjusted RR (95% CI)§ | AKI cases (n,%) | Unadjusted RR (95% CI) | Adjusted RR (95% CI)§ | In-Hospital Dialysis (n,%) | Length of stay in ICU, mean (SD) median [IQR] | Length of stay in hospital, mean (SD) median [IQR] |
| Albuminuria | |||||||||
| UACR < 10 mg/g (n=405) | 104 (26%) | 1† | 1† | 13 (3.2%) | 1† | 1† | 4 (1.0%) | 3.0 (7.5) 2 [1, 2] |
7.7 (8.7) 6 [5, 8] |
| UACR 10–29 mg/g (n=355) | 126 (35%) | 1.36 (1.09, 1.65) | 1.35 (1.07, 1.66) | 18 (5.1%) | 1.50 (0.74, 2.98) | 1.44 (0.70, 2.91) | 3 (0.9%) | 3.3 (10.6) 2 [1, 3] |
9.1 (13.9) 6 [5, 9] |
| UACR 30–299 mg/g (n=329) | 139 (42%) | 1.72 (1.42, 2.03) | 1.64 (1.33, 1.97) | 21 (6.4%) | 2.11 (1.07, 4.03) | 1.94 (0.95, 3.86) | 5 (1.5%) | 3.1 (4.4) 2 [1, 4] |
8.2 (6.5) 7 [5, 9] |
| UACR ≥ 300 mg/g (n=70) | 40 (57%) | 2.36 (1.85, 2.82) | 2.21 (1.66, 2.73) | 6 (8.6%) | 3.01 (1.16, 7.11) | 2.59 (0.93,6.58) | 5 (7.1%) | 7.1 (14.7) 2 [1, 6] |
12.4 (14.9) 7 [6, 11] |
| Unadjusted p for trend | <.0001 | 0.0154 | 0.0085 | <.0001 | <.0001 | ||||
| Adjusted p for trend | <.0001 | 0.0699 | 0.0009 | 0.0152 | 0.0187 | ||||
| Dipstick Proteinuria | |||||||||
| Negative (n=720) | 218 (30%) | 1† | 1† | 30 (4.2%) | 1† | 1† | 7 (1.0%) | 2.9 (5.6) 2 [1, 3] |
8.0 (8.3) 6 [5, 8] |
| Trace (n=198) | 77 (39%) | 1.28 (1.03, 1.55) | 1.23 (0.98, 1.52) | 15 (7.6%) | 1.93 (1.05, 3.45) | 1.75 (0.93, 3.19) | 3 (1.5%) | 4.2 (14.8) 2 [1, 3] |
9.5 (16.9) 6 [5, 8] |
| 30–99 mg/dL (n=118) | 56 (47%) | 1.59 (1.26, 1.91) | 1.56 (1.22, 1.90) | 6 (5.1%) | 1.22 (0.51, 2.79) | 1.06 (0.43, 2.51) | 2 (1.7%) | 3.5 (7.8) 2 [1, 3] |
9.2 (9.5) 7 [5, 9] |
| ≥100 mg/dL (n=87) | 41 (47%) | 1.62 (1.26, 2.00) | 1.42 (1.05, 1.81) | 7 (8.1%) | 2.09 (0.92, 4.44) | 1.86 (0.79, 4.09) | 5 (5.8%) | 5.3 (11.7) 2 [1, 4] |
10.1 (11.7) 7 [5, 10] |
| Unadjusted p for trend | <.0001 | 0.0872 | 0.0033 | 0.0004 | 0.0008 | ||||
| Adjusted p for trend | 0.0060 | 0.53 | 0.0118 | 0.24 | 0.30 | ||||
Adjusted for site as a random effected.
Adjusted for age (per year), sex, white, pre-op eGFR (per mL/min per 1.73 m2), diabetes, elective, hypertension, chronic heart failure, cardiac catheterization in past 48 hours, myocardial infarction, and type of surgery. Site is included as a random effect.
Referent
For stage I AKI with UACR, Hosmer-Lemeshow χ2=10.9, p-value 0.21
Abbreviations: AKI- acute kidney injury, RR- relative risk, UACR- urine albumin to creatinine ratio, CI- confidence interval, SD- standard deviation
In comparison to other covariates in the final model, all degrees of albuminuria, including microalbuminuria (UACR 10–29 mg/g), was comparable to or a stronger predictor variable for the outcome of stage I AKI than diabetes, hypertension, chronic heart failure, history of myocardial infarction, and combined CABG & valve surgery (Table 3).
Table 3.
Full multivariate models for Stage I and II AKI
| Multivariable-adjusted RR (95% CI) | Stage I AKI | Stage II AKI |
|---|---|---|
| UACR | ||
| <10 mg/g | 1 | 1 |
| 10–29 | 1.35 (1.07, 1.66) | 1.44 (0.7, 2.91) |
| 30–299 | 1.64 (1.33, 1.97) | 1.94 (0.95, 3.86) |
| ≥300 | 2.21 (1.66, 2.73) | 2.59 (0.93, 6.58) |
| Age (per year) | 1.003 (0.993, 1.013) | 0.993 (0.964, 1.024) |
| Female | 0.75 (0.60, 0.92) | 0.91 (0.52, 1.6) |
| White | 1.1 (0.76, 1.49) | 0.98 (0.36, 2.48) |
| eGFR (per ml/min per 1.73 m2) | 0.993 (0.988, 0.998) | 0.995 (0.981, 1.009) |
| Diabetes | 1.16 (0.96, 1.37) | 1.10 (0.63, 1.92) |
| Elective | 0.60 (0.46, 0.76) | 0.55 (0.29, 1.05) |
| Hypertension | 1.20 (0.94, 1.48) | 1.70 (0.75, 3.70) |
| Chronic heart failure | 1.28 (1.04, 1.53) | 1.59 (0.86, 2.88) |
| Cardiac catheterization in past 48 hours | 1.00 (0.99, 1.01) | 1.00 (1.00, 1.00) |
| Myocardial Infarction | 0.95 (0.76, 1.16) | 1.14 (0.61, 2.1) |
| Type of Surgery | ||
| Valve | 1 | 1 |
| CABG | 0.86 (0.66, 1.09) | 0.60 (0.28, 1.27) |
| CABG & Valve | 1.11 (0.88, 1.34) | 1.52 (0.78, 2.87) |
Abbreviations: AKI – acute kidney injury, UACR- urine albumin to creatinine ratio, CABG- coronary artery bypass grafting, eGFR- estimated glomerular filtration rate, SD-standard deviation.
Renal and Non-renal outcomes by Dipstick Proteinuria categories
The risk for stage I AKI increased across the dipstick proteinuria categories, though the relationship was not graded (p-value for trend <0.006), and the adjusted relative risks was slightly less robust than witnessed with the UACR categories (Table 2). Dipstick proteinuria was not associated with the risk of stage II AKI or with non-renal outcomes after adjustment for covariates.
Risk Discrimination for UACR and Dipstick Proteinuria categories
Addition of UACR to the clinical model increased the area under the curve from 0.64 to 0.67 (p < 0.001) and dipstick proteinuria increased the area under the curve (AUC) for stage I AKI to 0.66 (p= 0.05, Table 3). The addition of UACR or dipstick proteinuria did not increase the area under the curve significantly for the prediction of stage II AKI (Table 4).
Table 4.
Risk Discrimination and Continuous Net Reclassification Index for stage I and II AKI by UACR and Urine dipstick measurements
| Stage I AKI | Stage II AKI | |
|---|---|---|
| Area Under the ROC Curve (SE) | ||
| Albuminuria | 0.604 (0.017) | 0.591 (0.036) |
| Clinical Model | 0.669 (0.017) | 0.723 (0.036) |
| Clinical Model + Albuminuria | 0.695 (0.016) | 0.731 (0.037) |
| p-value* | 0.0055 | 0.50 |
| Dipstick | 0.573 (0.016) | 0.573 (0.036) |
| Clinical Model | 0.673 (0.017) | 0.728 (0.036) |
| Clinical Model + Dipstick | 0.688 (0.017) | 0.732 (0.037) |
| p-value* | 0.0272 | 0.70 |
| Continuous Net Reclassification Index (SE) | ||
| Albuminuria | 0.289 (0.062) | 0.290 (0.135) |
| p-value | <.0001 | 0.0312 |
| Dipstick | 0.248 (0.063) | 0.285 (0.135) |
| p-value | <.0001 | 0.0347 |
Clinical model includes age (per year), sex, white, pre-op eGFR (per mL/min per 1.73 m2), diabetes, elective surgery, hypertension, chronic heart failure, cardiac catheterization in past 48 hours, myocardial infarction, and type of surgery.
p-value comparing the addition of albuminuria to the clinical model
p-value comparing the addition of dipstick to the clinical model
Abbreviations: ROC- receiver operating curve, AKI- acute kidney injury, UACR- urine albumin to creatinine ratio, SE- standard error
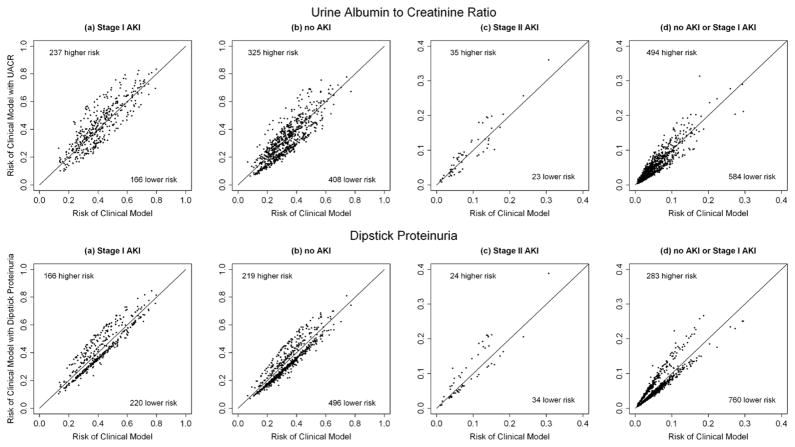
UACR and dipstick proteinuria were able to improve risk classification for AKI. The continuous net reclassification index (NRI) was 30% (p<0.001) and 31% (p=0.01) for stage I (Figure 1) and stage II AKI, respectively, with addition of UACR to the clinical model and 24% (p=0.001) for stage I AKI and 28% (p=0.03) for stage II AKI with addition of dipstick proteinuria to the clinical model (Table 4).
Figure 1. Predicted risk of clinical model with and without urine albumin creatinine ratio.
The above plots shows the predicted risk for (a) AKI and (b) no AKI for Stage I AKI and (c) Stage II AKI and (d) no AKI or Stage I AKI for Stage II AKI according to the clinical model (x-axis) and according the model with pre-operative urine albumin creatinine ratios (UACR) or dipstick proteinuria added (y-axis). The diagonal line indicates that for points above this line, the predicted risk of stage I AKI is higher in the new model and for points below this line, the predicted risk is lower.
Sub-group and sensitivity analysis
We examined the association of UACR and AKI in the following subgroups: age, diabetes, surgery status, and estimated GFR categories (eGFR ≥ 60, 45–59, 30–44, and <30 mL/min per 1.73 m2). Surgery status and baseline estimated GFR were effect modifiers (Table 5). Those with non-elective surgery and UACR ≥ 300 mg/g (≥ 33.9 mg/mol) had a lower RR than those with elective surgery and same albuminuria group. Higher levels of UACR were associated with a stepwise increase in the risk for post operative AKI in those with estimated GFR 45–59 and ≥ 60 mL/min per 1.73 m2, but were not associated with increased risk in estimated GFR 30–44, and <30 mL/min per 1.73 m2 (Table 5). A similar trend was observed also with the dipstick proteinuria categories (data not shown).
Table 5.
Relative Risks (95%CI) of UACR for AKI by pre-specified subgroups
| Variable, (n) | UACR < 10 mg/g | UACR 11–29 mg/g | UACR 30–299 mg/g | UACR > 300 mg/g | P for trend |
|---|---|---|---|---|---|
| n AKI/total (%) RR (95% CI) |
n AKI/total (%) RR (95% CI) |
n AKI/total (%) RR (95% CI) |
n AKI/total (%) RR (95% CI) |
||
| Age | |||||
| <65 (250) | 21/97 (22%) 1 |
30/73 (41%) 1.8 (1.1, 2.6) |
26/61 (43%) 2.0 (1.2, 2.8) |
12/19 (63%) 3.0 (1.8, 3.9) |
0.008 |
| 65–75 (440) | 39/164 (24%) 1 |
44/134 (33%) 1.4 (0.9, 1.9) |
50/118 (42%) 1.9 (1.3, 2.4) |
16/24 (67%) 3.0 (2.0, 3.6) |
<.001 |
| 75–85 (410) | 42/133 (32%) 1 |
46/134 (34%) 1.1(0.7, 1.5) |
50/120 (42%) 1.4(0.9, 1.8) |
12/23 (52%) 1.7 (1.0, 2.3) |
0.14 |
| >85 (59) | 2/11 (18%) 1 |
6/14 (43%) 2.3 (0.5, 4.6) |
13/30 (43%) 2.4 (0.6, 4.5) |
0/0 (0%) | 0.22 |
| Diabetes | |||||
| Yes (219) | 41/141 (29%) 1 |
47/137 (34%) 1.1 (0.7, 1.5) |
76/155 (49%) 1.70 (1.3, 2.1) |
26/46 (57%) 2.0 (1.4, 2.5) |
<.001 |
| No (461) | 63/264 (24%) 1 |
79/218 (36%) 1.5 (1.1, 1.9) |
63/174 (36%) 1.63 (1.2, 2.0) |
14/24 (58%) 2.5 (1.6, 3.3) |
0.001 |
| Surgery Status* | |||||
| Elective (920) | 64/306 (21%) 1 |
92/295 (31%) 1.4 (1.0, 1.78) |
105/265 (40%) 1.3 (0.9, 1.7) |
33/54 (61%) 1.2 (0.6, 1.8) |
<.001 |
| Non-elective (239) | 40/99 (40%) 1 |
34/60 (57%) 1.4 (1.1, 1.8) |
34/64 (53%) 2.0 (1.6, 2.4) |
7/16 (44%) 3.0 (2.3, 3.6) |
0.21 |
| eGFR‡ (mL/min per 1.73m2) | |||||
| <30 (36) | 2/4 (50%) 1 |
3/3 (100%) | 8/19 (42%) 0.8 (0.1, 1.8) |
7/10 (70%) 1.4 (0.3, 1.9) |
0.22 |
| 30–44 (119) | 10/26 (38%) 1 |
14/29 (48%) 1.3 (0.6, 1.9) |
27/50 (54%) 1.4 (0.8, 2.0) |
6/14 (43%) 1.1 (0.4, 1.9) |
0.61 |
| 45–59 (242) | 20/76 (26%) 1 |
23/66 (35%) 1.3 (0.8, 2.0) |
42/87 (48%) 2.0 (1.3, 2.6) |
9/13 (69%) 2.8 (1.6, 3.5) |
0.003 |
| ≥60 (762) | 72/299 (24%) 1 |
86/257 (33%) 1.3 (1.0, 1.7) |
62/173 (36%) 1.6 (1.2, 2.0) |
18/33 (55%) 2.5 (1.7, 3.1) |
0.005 |
Referent
p for interaction < 0.001 for elective vs. non-elective surgery
p for interaction = 0.01 for eGFR ≥45 vs. eGFR < 45 mL/min per 1.73 m2
Site is included as random effect.
Abbreviations: AKI- acute kidney injury, UACR- urine albumin to creatinine ratio, eGFR- estimated glomerular filtration rate
DISCUSSION
AKI is a major postoperative complication of cardiac surgery. Even a mild rise in creatinine as small as 0.3 mg/dl (26.5 μmol/L) (AKI Stage I) in this setting is associated with increased morbidity and mortality.19 Our study demonstrates that pre-operative albuminuria (UACR) is independently associated with development of AKI even after consideration of several important pre-operative characteristics. Furthermore, dipstick proteinuria is also an independent predictor for the development of stage I AKI. Finally, pre-operative UACR is also independently associated with in-hospital dialysis, ICU length of stay, and in-hospital length of stay. Both UACR and dipstick proteinuria have better prognostic ability for AKI in patients with estimated GFR ≥45 mL/min per 1.73 m2 than patients with estimated GFR ≤ 45 mL/min per 1.73 m2 and those undergoing elective surgery.
Our study builds upon the results of Huang et al,10 demonstrating that pre-operative proteinuria is an independent risk factor for the development of cardiac surgery associated AKI. In their study involving patients undergoing cardiac surgery, urine dipstick defined as mild (trace to 1+), had adjusted odds ratio (OR) of 1.66 for the development of AKI (95% CI 1.09 to 2.52), and or heavy proteinuria (2+ to 4+) had an adjusted OR of 2.30 (95% CI 1.35 to 3.90). Our study extends Huang’s observations by incorporating UACR for categorization and analyses. Our results are similar to the non-surgical setting where Grams et al7 demonstrated UACR is associated with AKI in a graded fashion in a community-based population. We confirmed that low grade albuminuria (UACR < 30 mg/g), which generally is not considered pathological, was independently associated with the risk for AKI.
Several clinical risk-scoring systems have been developed and validated to predict the risk of AKI in cardiac surgery. The majority consider severe AKI as the endpoint (requiring acute renal replacement therapy).12,13 However, a few studies developed models for predicting milder forms of AKI.21,22 Regardless, all the clinical risk scoring systems, except for one,21 solely utilized pre-operative serum creatinine or GFR to define chronic kidney disease. None of the studies examined the added value of albuminuria or proteinuria to predict AKI in cardiac surgery. In this multi-center cohort study of patients undergoing cardiac surgery, we found that both pre-operative UACR and dipstick proteinuria add to pre-operative risk stratification for AKI.
These risk-assessment tools are used both to allow individual patients and surgeons to balance the risks and benefits of cardiac surgery and to monitor the quality of care and outcomes of cardiac surgery. Cardiac surgery outcomes are widely monitored, thus even mild improvements to help risk adjust for adverse outcomes could have great clinical and research importance by choosing alternative procedures (e.g., percutaneous coronary revascularization or valve repair) and selection of a high-risk cohort for enrollment into randomized controlled trials. Therefore, if pre-operative proteinuria can be validated further as a relevant marker for improving risk assessment for AKI or other adverse outcomes after cardiac surgery, then it could be of value to individual patients, clinicians, hospitals and trialists.
It has been postulated that proteinuria represents endothelial dysfunction or may injure the kidney itself. There is a body of evidence which describes proteinuria as toxic to the tubules. This can result in significant tubulointerstitial injury and progression of renal disease independent of the cause of proteinuria in chronic kidney disease. Filtered albumin when taken up by renal tubular cells via receptor mediated endocytosis can trigger expression of a series of pro-inflammatory molecules like monocyte chemotactic protein-1 (MCP-1), osteopontin, regulated upon activation normal T cell expressed and secreted (RANTES), endothelin-1.10 Also, low molecular weight proteinuria can exacerbate acute ischemic injury in experimental animals.22 It is possible that patients with albuminuria have some degree of altered functional renal reserve due to the above mentioned factors and are more prone to ischemic injury during cardiac surgery.23
We found effect modification by two clinical variables: surgery status and pre-operative estimated GFR. Specifically, the relationship between the degree of pre-operative proteinuria and risk for AKI was stronger in those undergoing elective surgery and those who had higher pre-operative estimated GFR but was weak or abolished in those undergoing urgent surgery and those with estimated GFR < 45 ml/min/m2. These findings are not surprising. In patients who are already at much higher risk for AKI (urgent surgery and profoundly decreased baseline estimated GFR), it is unlikely that additional parameters will provide more information for the risk of outcomes. However, in patients with lower pre-operative risk, a measure of kidney injury, such as proteinuria, may provide additional benefit for risk stratification. For example, in those with GFR > 60 and no albuminuria, the rate of AKI was 24%. In contrast, the rate of AKI in those with GFR 30–44 was 38%. Thus, since the background or baseline risk of AKI is already higher in those with low GFR, it is more difficult to demonstrate higher risk in association with another predictor variable, in this case albuminuria. These findings are very similar to the findings by Tonelli et al.11 where the value of albuminuria to risk-stratify for the outcomes of end stage renal disease (ESRD) and all-cause mortality was greatest in those with higher baseline estimated GFR.
The strengths of this study are that it is a multicenter cohort study involving six large academic centers in North America, and representative of contemporaneous surgical practice. Furthermore, unlike previous studies8,9,10 proteinuria was measured both by UACR and dipstick at the bedside, thus we were able to compare the two methodologies of assessment of proteinuria with the outcomes. However, there are some limitations to our study. The majority of the patients in our cohort experienced only mild AKI (Acute Kidney Injury Network stage I). The number of patients with stage II AKI (or worse) was much lower, which may have limited our ability to observe a stable independent relationship between albuminuria and more severe AKI. However, the distribution of severity of AKI reflects the current epidemiology of AKI in cardiac surgery in the modern era in North America. In addition, despite the multi-center design of our study, two-thirds of participants were male and over 90% were white. The ability for the models to correctly classify patients as AKI or non-AKI using pre-operative variables was only modest, with an AUC of 0.7. Thus, additional factors such as intra-and post-operative events also influence the ultimate risk for AKI. Finally, the amount of proteinuria by dipstick can be influenced by urine concentration.
In conclusion, our results indicate that pre-operative proteinuria, both by UACR and urine dipstick, is an independent predictor for the risk of developing stage I AKI in cardiac surgery. UACR is also an independent predictor of other important outcomes, including dialysis and length of stay. However, with consideration of the cost differential between the two tests (approximately 40 cents per patient for dipstick and $130 per patient for UACR), dipstick proteinuria may be a more cost-effective method to screen patients prior to surgery, as it still improved risk classification by 24%. This implies that the number needed to screen via UACR to correctly classify one patient prior to surgery is 14, at a total cost of $1820. Future studies will need to determine whether the additional precision at the expense of increased costs is worth pursuing and then translates into improved clinical outcomes. We propose that these, widely available, and potentially bedside tests can be used as an aid in assessing risk for poor outcomes in patients undergoing cardiac surgery.
Acknowledgments
Funding/Support: The research reported in this article was supported by the American Heart Association Clinical Development award, the grant R01HL-085757 from the National Heart, Lung, and Blood Institute. The study was also supported by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR).
We would also like to thank the nursing and support staff of the pre admission clinic, anesthesia units, and the cardiac care units at all the participating sites.
Members of AKI-TRIBE consortium
Yale-New Haven: Dr. Michael Dewar, Dr. Umer Darr, Dr. Sabet Hashim, Dr. Richard Kim, Dr. John Elefteriades, Dr. Arnar Geirsson, Dr. Susan Garwood, Dr. Prakash Nadkarni, Dr. Simon Li..
Danbury: Dr. Cary Passik.
London: Dr. Michael Chu, Dr. Martin Goldbach, Dr. Lin Ruo Guo, Dr. Bob Kiaii, Dr. Neil McKenzie, Dr. Mary Lee Myers, Dr. Richard Novick, Dr. Mac Quantz.
Chicago: Dr. Jay Koyner, Dr. Patrick Murray, Dr. Shahab A. Akhter, Dr. Jai Raman, Dr. Valluvan Jeevanandam, MD
Cincinnati: Dr. Catherine D. Krawczeski, MD, Michael Bennett, PhD, Qing Ma, MD..
Footnotes
Financial Disclosures:
Dr. Devarajan is a consultant to Abbott Diagnostics and Biosite, Inc. No conflicts for other authors.
References
- 1.Loef BG, Epema AH, Smilde TD, Henning RH, Ebels T, Navis G, et al. Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol. 2005;16:195–200. doi: 10.1681/ASN.2003100875. [DOI] [PubMed] [Google Scholar]
- 2.Ishani A, Nelson D, Clothier B, Schult T, Nugent S, Greer N, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;14:226–33. doi: 10.1001/archinternmed.2010.514. [DOI] [PubMed] [Google Scholar]
- 3.Higgins TL, Estafanous FG, Loop FD, Beck GJ, Blum JM, Paranandi L. Stratification of morbidity and mortality outcome by preoperative risk factors in coronary artery bypass patients. A clinical severity score. JAMA. 1992;267:2344–8. [PubMed] [Google Scholar]
- 4.Lombardi R, Ferreiro A. Risk factors profile for acute kidney injury after cardiac surgery is different according to the level of baseline renal function. Ren Fail. 2008;30:155–60. doi: 10.1080/08860220701808129. [DOI] [PubMed] [Google Scholar]
- 5.Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–77. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–59. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 7.Grams ME, Astor BC, Bash LD, Matsushita K, Wang Y, Coresh J. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J Am Soc Nephrol. 2010;21:1757–64. doi: 10.1681/ASN.2010010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu CY, Ordonez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74:101–7. doi: 10.1038/ki.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James MT, Hemmelgarn BR, Wiebe N, Pannu N, Manns BJ, Klarenbach SW, et al. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet. 2010;376:2096–103. doi: 10.1016/S0140-6736(10)61271-8. [DOI] [PubMed] [Google Scholar]
- 10.Huang TM, Wu VC, Young GH, Lin YF, Shiao CC, Wu PC, et al. Preoperative proteinuria predicts adverse renal outcomes after coronary artery bypass grafting. J Am Soc Nephrol. 2011;22:156–63. doi: 10.1681/ASN.2010050553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tonelli M, Muntner P, Lloyd A, Manns BJ, James MT, Klarenbach S, et al. Using proteinuria and estimated glomerular filtration rate to classify risk in patients with chronic kidney disease: a cohort study. Ann Intern Med. 2011;154:12–21. doi: 10.7326/0003-4819-154-1-201101040-00003. [DOI] [PubMed] [Google Scholar]
- 12.Chertow GM, Lazarus JM, Christiansen CL, Cook EF, Hammermeister KE, Grover F, et al. Preoperative renal risk stratification. Circulation. 1997;95:878–84. doi: 10.1161/01.cir.95.4.878. [DOI] [PubMed] [Google Scholar]
- 13.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–8. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta RH, Grab JD, O’Brien SM, Bridges CR, Gammie JS, Haan CK, et al. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114:2208–16. doi: 10.1161/CIRCULATIONAHA.106.635573. quiz. [DOI] [PubMed] [Google Scholar]
- 17.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 18.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 20.Palomba H, de Castro I, Neto AL, Lage S, Yu L. Acute kidney injury prediction following elective cardiac surgery: AKICS Score. Kidney Int. 2007;72:624–31. doi: 10.1038/sj.ki.5002419. [DOI] [PubMed] [Google Scholar]
- 21.Brown JR, Cochran RP, Leavitt BJ, Dacey LJ, Ross CS, MacKenzie TA, et al. Multivariable prediction of renal insufficiency developing after cardiac surgery. Circulation. 2007;116:I139–43. doi: 10.1161/CIRCULATIONAHA.106.677070. [DOI] [PubMed] [Google Scholar]
- 22.Zager RA, Teubner EJ, Adler S. Low molecular weight proteinuria exacerbates experimental ischemic renal injury. Lab Invest. 1987;56:180–8. [PubMed] [Google Scholar]
- 23.Glassock RJ. Is the presence of microalbuminuria a relevant marker of kidney disease? Curr Hypertens Rep. 2010;12:364–8. doi: 10.1007/s11906-010-0133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]