Abstract
Background
Gabapentin is most commonly prescribed for chronic pain, but acute perioperative effects including preemptive analgesia and hemodynamic stabilization have also been reported. Adrenal chromaffin cells are a widely used model to investigate neurosecretion and adrenal catecholamines play important physiological roles and contribute to the acute stress response. However, the effects of gabapentin on adrenal catecholamine release have never been tested.
Methods
Primary cultures of bovine adrenal chromaffin cells were treated with gabapentin or vehicle for 18–24 h. We quantified catecholamine secretion from dishes of cells using high performance liquid chromatography, and resolved exocytosis of individual secretory vesicles from single cells using carbon fiber amperometry. Voltage-gated calcium channel currents (ICa) were recorded using patch-clamp electrophysiology, and intracellular [Ca2+] using fluorescent imaging.
Results
Gabapentin produced statistically significant reductions in catecholamine secretion evoked by cholinergic agonists (24 ± 3 % n = 12) or KCl (16 ± 4 % n = 8) (mean ± SEM) but did not inhibit Ca2+ entry or ICa. Amperometry (n = 51 cells) revealed that gabapentin inhibited the number of vesicles released upon stimulation, with no change in quantal size or kinetics of these unitary events.
Conclusions
We show Ca2+ entry was not inhibited by gabapentin, but was less effective at triggering vesicle fusion. Our work also demonstrates that chromaffin cells are a useful model to further investigate the cellular mechanism(s) by which gabapentin controls neurosecretion. Moreover, it identifies altered adrenal catecholamine release as a potential contributor to some of the beneficial perioperative effects of gabapentin.
INTRODUCTION
Gabapentin is most commonly prescribed for chronic pain states but there is increasing interest in potentially beneficial effects of perioperative dosing. For example, several randomized control trials indicate that gabapentin has acute preemptive analgesic effects on intraoperative and postoperative pain1. Preoperative gabapentin also blunted the hemodynamic response to direct laryngoscopy and tracheal intubation 2,3. Although the underlying mechanism(s) remain unclear, previous clinical studies have shown these procedures stimulate a sympathoadrenal stress response evidenced by increases in circulating catecholamine levels 4–7
While it is clear that gabapentin has a constellation of analgesic, antihyperalgesic, and perioperative hemodynamic effects, the cellular mechanism(s) that underlie these actions are not well understood. Although it was originally developed as a mimetic of the inhibitory neurotransmitter γ-aminobutyric acid, the consensus now holds that gabapentin exerts little effect on γ-aminobutyric acid signaling, but rather binds with high affinity to the α2δ subunit of voltage-gated calcium channels (Ca2+-channels) 8. Calcium influx through Ca2+ channels triggers exocytosis of secretory vesicles, leading to the hypothesis that gabapentin reduces transmitter release by reducing Ca2+ entry. Indeed, some studies reported acute inhibition of Ca2+ channel currents (ICa) by gabapentin or pregabalin 9–12, although others found no effect 13–15. Perhaps of more relevance to the perioperative setting, longer-term (17–48 h) incubation with gabapentin / pregabalin reduces the amplitude of ICa by disrupting trafficking which results in fewer channels at the plasma membrane 13,14,16,17. Gabapentin might also inhibit neurotransmitter release by other mechanisms. Consistent with this, spontaneous synaptic release events or exocytosis evoked by hypertonic sucrose were inhibited by gabapentin / pregabalin, even though these events are thought to occur independent from Ca2+ entry 18,19. Thus, regulation of neurotransmitter and hormone release by gabapentin is likely complex and might involve effects on the transmitter release machinery in addition to altered Ca2+ entry and/or altered excitability in neuronal circuits.
The rationale for our study, the first to investigate the effects of gabapentin on adrenal chromaffin cells, was twofold. First, chromaffin cells are widely used to investigate neurosecretion 20–22, so provide a good model to evaluate the cellular mechanisms of gabapentin. Second, chromaffin cells release a complex cocktail of catecholamines, endogenous opioids 23 and other transmitters that play central roles in the acute stress response and possibly in stress-related enhancement of mechanical hyperalgesia 24. Therefore, we postulated that perioperative hemodynamic stabilization, and perhaps other stress-related effects of gabapentin, might be partly mediated through reduced catecholamine release.
MATERIALS AND METHODS
Cell culture
Adult bovine adrenal glands were obtained from a local slaughterhouse and chromaffin cells were prepared by digestion with collagenase followed by density gradient centrifugation as described previously25. The cells were plated onto coverslips coated with collagen (at a density of 0.3–0.4 x 106 cells/mL for [Ca2+]i measurements or ~0.2 x 106 cell /mL for patch clamp recordings). For secretion studies cells were plated in 24-well tissue culture plates at a density of ~ 0.3 x 106 cells per well. Fibroblasts were effectively suppressed with cytosine arabinoside (10 μM) (Sigma-Aldrich; St Louis MO), leaving relatively pure chromaffin cell cultures. The culture medium consisted of Dulbecco’s modified Eagle medium \ F12 (1:1) supplemented with fetal bovine serum (10%), glutamine (2 mM), penicillin/streptomycin (100 unit mL−1/100 μg mL−1), cytosine arabinoside (10 μM) and 5-fluorodeoxyuridine (10 μM). Unless noted otherwise, all tissue culture reagents were from Invitrogen (Carlsbad, CA) apart from fetal bovine serum which was from Hyclone (Logan, UT). All experiments were performed 2–5 days following cell isolation.
[Ca2+]i Measurements
Free cytosolic Ca2+ concentration ([Ca2+]i) was measured in cells loaded with the fluorescent Ca2+ indicator Fura-2 (Molecular Probes, Eugene OR) using an InCyt IM2 fluorescence imaging system (Intracellular Imaging Inc., Cincinnati, OH) as described previously 25. Cells were washed twice with HEPES-buffered Hanks Balanced Salt Solution (Invitrogen) and incubated for 30–45 min with 3 μM Fura-2 AM at 37°C. Cells were then washed in Fura-free solution for 30–60 min before recording. For recording, the coverslip with the cells attached was transferred to a recording chamber and mounted on the stage of a Nikon TE2000 fluorescence microscope (Nikon Instruments Inc., Melville, NY). The recording chamber had a volume of ~300–400 μL and was continually perfused with fresh solution from gravity-fed reservoirs at a flow rate of ~4 ml/min. The extracellular solution comprised (in mM): 136 NaCl, 2 KCl, 1 MgCl, 10 Glucose, 10 HEPES, 2 CaCl, pH 7.3 osmolarity ~ 305 mOsm. Cells were alternately excited at wavelengths of 340 nm and 380 nm and emission at 510 nm detected using a pixelfly digital camera. The ratio of fluorescence at 340 nm/380 nm excitation was collected every 2 s throughout the experiment and converted to [Ca2+]i using an in vitro calibration curve generated by adding 15.8 μM Fura-2 pentapotassium salt to solutions containing 1 mM MgCl2 and known concentrations of Ca2+ (0 1350 nM). Data analysis was performed using OriginPro software (Originlab Corporation, Northampton, MA).
Catecholamine release experiments
Cells in 24-well plates were washed twice with extracellular solution and incubated in this solution for 30 mins at ~37 °C. For gabapentin (Tocris Cookson, Ellisville, MO, or Ascent Scientific, Princeton, NJ) treated cells, the drug was also present in all solutions throughout the experiment. The solution was then removed and replaced with fresh solution to determine basal release or solution containing 100 μM carbachol (Sigma-Aldrich) to stimulate secretion. Alternatively, cells were stimulated using the selective nicotinic receptor agonist 1,1-dimethyl-4-phenylpiperazinium iodide (DMPP) (30 μM) (Sigma-Aldrich) or a solution with elevated (30 mM) KCl. After the 5-min stimulation period at ~37 °C the cells were placed on ice and the solution collected and added to an equal volume of ice-cold 0.4 M perchloric acid. Perchloric acid was also added to the tissue culture wells to lyse the cells and extract the non-released catecholamines. The catecholamine content of the samples was determined by a specific high performance liquid chromatography (HPLC) assay utilizing an Antec Decade (oxidation potential: 0.7 V) electrochemical detector in the Neurochemistry Core of the Center for Molecular Neuroscience at Vanderbilt University. Samples (20 μL) were injected using a Waters 717+ autosampler onto a Kinetex 2.6u C18 100A 100x4.6mm HPLC column (Phenomenex, Torrance, CA) and biogenic amines eluted with a mobile phase consisting of 89.5% 0.1 M TCA, 10−2 M sodium acetate, 10−4 M EDTA and 10.5 % methanol (pH 3.8). The amount of catecholamine released into the bathing medium during the 5-min stimulation period was expressed as a percentage of the total catecholamine content for that dish of cells. Each plate consisted of control and gabapentin treated conditions performed in duplicate. Duplicates for each condition were averaged and compared. Personnel performing the catecholamine detection were blinded to which samples were from control or gabapentin treated cells. Each dataset were obtained from several independent experiments performed on different plates of cells (n is indicated in text). Each dataset was also performed on several different preparations of cells (i.e., cells isolated from different animals). To calculate an EC50 for inhibition of catecholamine secretion, data from experiments using different concentrations of gabapentin were plotted and fit with a Boltzmann function of the form: Y = Ymax / (1 + 10^(Log EC50 -X)); where Y = percent inhibition of secretion and X is the concentration of gabapentin. The Hill slope was assumed to be 1 and the curve was fit with the least squares method in Prism5 software (GraphPad Software Inc., San Diego, CA).
Patch-clamp electrophysiology
Electrodes were pulled from borosilicate glass capillary tubes (World Precision Instruments, Sarasota, FL), coated with dental wax (Electron Microscopy Sciences, Hatfield, PA) and fire polished to a final resistance of ~2 MΩ when filled with a CsCl-based internal solution. Cells were voltage-clamped in the conventional whole-cell configuration using an Axopatch 200B amplifier, Digidata 1400A interface, and PClamp10 (Clampex) acquisition software (Molecular Devices, Sunnyvale, CA). Analog data were filtered at 2 kHz and digitized at 20 μs/point (50 kHz). Series resistance was partially compensated using the Axopatch circuitry and data for ICa were subjected to linear capacitance and leak subtraction using standard pulse / number protocols. Cell membrane capacitance was determined for each cell using the membrane test protocol in Clampex software. ICa were activated by brief 20 ms step depolarizations to a predetermined peak (10–30 mV) from a holding potential of −80 mV. To assess inactivation, 500 ms voltage step commends were used as detailed in the results section. Data were analyzed using PClamp10 (Clampfit), OriginPro software and GraphPad Prism. The patch pipette solution consisted of (in mM): CsCl 110, MgCl2 4, HEPES 20, EGTA 10, GTP 0.35, adenosine triphosphate 4, creatine phosphate 14, pH 7.3, osmolarity approximately 310–315 mOsm. For experiments investigating Ca2+-dependent inactivation the concentration of EGTA was reduced to 0.5mM. The extracellular solution was (in mM): 136 NaCl, 2 KCl, 1 MgCl.6H20, 10 Glucose, 10 HEPES, 5 CaCl, pH 7.3 osmolarity ~ 310 mOsm. For gabapentin treated cells, the drug was included in all extracellular solutions throughout the experiment.
Carbon fiber amperometry
Cells were placed into the recording bath and continuously washed with the same extracellular buffer used for calcium imaging and HPLC secretion experiments (see above). The carbon fiber amperometry electrode (Dagan Corporation, Minneapolis MN) was backfilled with 3 M KCl and positioned so that it just touched the surface of the cell. A potential of +700 mV was applied to the carbon fiber using a VA-10X amplifier (NPI Electronic GmbH, Tamm, Germany). Electrodes were changed frequently and control cells and gabapentin treated cells recorded alternately to minimize potential variability due to cell preparation, time in culture etc. Data were acquired using a 16-bit BNC-2090 analogue-to-digital converter (National Instruments, Austin, TX) and WinEDR acquisition software written by Dr. John Dempster, Ph.D. (Senior Lecturer, Strathclyde Institute for Pharmacy & Biomedical Sciences, University of Strathclyde, Glasgow, Scotland). Amperometric currents were filtered at 2 kHz and continuously sampled at 5 kHz. To evoke exocytosis, cells were stimulated by bath perfusion with 30 mM KCl. Amperometric spikes were detected and analyzed using a series of macros written by Dr. Eugene Mosharov, Ph.D. (see26) in IgorPro software (Wavemetrics Inc., Oswego OR). Events were detected using an amplitude threshold of 5 pA (which was 4–5 times the root mean squared (rms) noise of the baseline current), and were subsequently confirmed by visual inspection. All detected events were counted to assess overall secretory activity. However, to accurately analyze the individual spike parameters (charge, amplitude, half width, slope) we excluded spikes that overlapped by > 10% and spikes with rise times > 10 ms that likely occurred distant from the carbon fiber and thus cannot be reliably collected. A median value for each spike parameter was calculated for each cell, and these values pooled and compared between control and gabapentin treated cells. Data collection and initial spike detection was performed in a blinded manner (i.e., the experimenter was unaware of whether cells were from control or gabapentin treated groups).
Drugs and reagents
The AM-ester of Fura-2 was prepared as 1 mM stock in dimethyl sulfoxide and aliquots frozen for up to 1–2 weeks. The pentapotassium salt was prepared as an aqueous stock and stored at 4 °C. Gabapentin was prepared as a stock solution (100 – 300 mM) in sterile water and aliquots frozen. Carbachol and DMPP stocks were prepared in sterile water and kept refrigerated.
Statistical analyses
Unless otherwise noted all data are presented as mean ± SEM. All statistical analyses were performed using OriginPro software or GraphPad Prism software. Calcium imaging data were compared using one-way ANOVA with a Bonferroni multiple comparisons test. Catecholamine secretion detected using HPLC was compared between control and gabapentin treated cells using two-tailed, paired or independent Student’s t-test as appropriate. Comparison of control and gabapentin treated cells at 0.1 and 1 mM were performed using a repeated measures ANOVA with Dunnett’s multiple comparisons test. Peak ICa density was compared using using two-tailed, paired or independent Student’s t-test as appropriate. To compare the inactivation time constants of ICa, current amplitude was normalized to peak and the decay from this peak was fit with a double exponential function using Prism5 software. The calculated fast and slow time constants generated by the fit were determined for each cell, and pooled values compared between control and gabapentin treated cells using a two-tailed independent t-test. For amperometry recordings the number of events followed a non-Gaussian distribution (determined using a Shapiro-Wilk normality test or D’Agostino & Pearson omnibus normality) so these data were analyzed using the nonparametric Mann-Whitney U-test. When analyzing individual spike characteristics (charge, amplitude, half width, and slope), if the parameters from all spikes in all cells are simply pooled then those cells with a high number of events will have greater weight than those cells with a low number of events. To avoid this we calculated median spike values for each cell which were then pooled and compared between control and gabapentin treated groups using two-tailed t-tests (for full discussion see 26). In all cases data were considered to be significantly different if p < 0.05.
RESULTS
Gabapentin treatment reduces catecholamine release evoked by cholinergic agonists
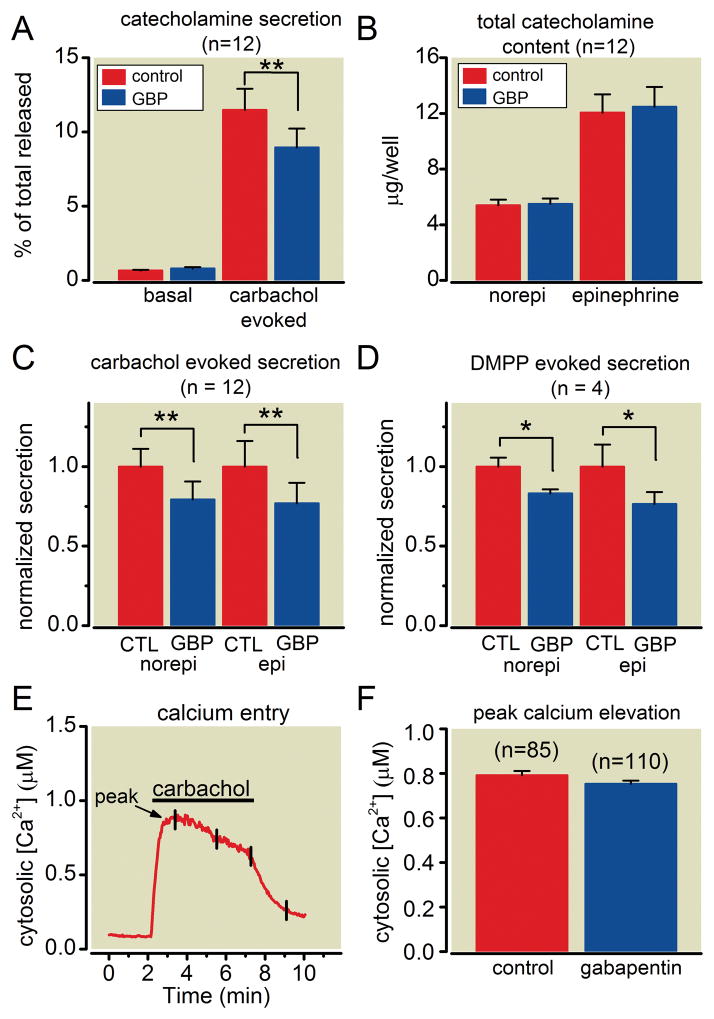
In situ, chromaffin cells are innervated by cholinergic splanchnic nerve fibers. Activation of nicotinic acetylcholine receptors (nAChRs) on the chromaffin cells causes membrane depolarization, activation of voltage-gated Ca2+ channels, and influx of Ca2+ that triggers exocytosis of large dense core secretory granules. To mimic this cholinergic stimulation we used carbachol, an acetylcholine analogue that is resistant to cholinesterase activity. We wanted the timeline of gabapentin exposure in these cellular studies to broadly correlate with reported intra-and early postoperative clinical effects of preoperative gabapentin dosing. Thus, cells were incubated for 18 – 24 h in culture medium supplemented with 1 mM gabapentin, or an equivalent amount of vehicle (sterile water) for time matched controls. Cells seeded in 24-well tissue culture plates (~300,000 cells per well) were stimulated for 5 min with 100 μM carbachol and catecholamines were quantified using HPLC. As shown in figure 1A, basal secretion was not altered by gabapentin (p = 0.15). Carbachol evoked robust catecholamine secretion in control cells (11.5 ± 1.4 % of total content, n = 12, mean ± SEM) and there was a statistically significant reduction in gabapentin treated cells (8.9 ± 1.3 % of total content; n = 12; p = 0.00023). This decrease in carbachol-evoked release did not reflect altered catecholamine synthesis or storage because the total cellular content of epinephrine (p = 0.16) and norepinephrine (p = 0.43) was not altered in gabapentin treated cells (fig. 1B).
Figure 1. Gabapentin reduces catecholamine secretion but not calcium entry evoked by cholinergic stimulation.
(A) Chromaffin cells were seeded on 24-well plates and treated with 1mM gabapentin (GBP) or vehicle (control) for 18–24 h. The amount of catecholamines released under basal conditions (in the absence of secretagogue) or during a 5-min stimulation with carbachol (100 μM) was determined using high performance liquid chromatography (HPLC) and expressed as a percentage of total cellular content (mean ± SEM). Gabapentin significantly reduced carbachol-evoked secretion (** p = 0.00023) but not basal release (p = 0.15). (B) Gabapentin treatment (GBP) did not alter the total cellular content of norepinephrine (p = 0.43) or epinephrine (p = 0.16) compared to control cells (mean ± SEM). (C) Secretion evoked by carbachol in gabapentin treated cells (GBP) was normalized to controls (CTL). Gabapentin reduced both epinephrine (epi) (p = 0.0007) and norepinephrine (norepi) (p = 0.0001) secretion to a similar extent (mean ± SEM). (D) Same layout as in panel C except using a 5-min stimulation with 1,1-dimethyl-4-phenylpiperazinium iodide (DMPP) (30 μM), a selective nicotinic receptor agonist. Gabapentin significantly reduced both epinephrine (epi) (p = 0.042) and norepinephrine (norepi) (p = 0.026) secretion. (E) Representative experiment showing the [Ca2+]i increase evoked by a 5-min application of 100μM carbachol in FURA-2 loaded chromaffin cells (n = 8 cells, mean ± SEM - for clarity error bars are only shown for a few data points). (F) Pooled data from multiple experiments like that shown in panel E. The peak increase in [Ca2+]i evoked by carbachol was not significantly altered in gabapentin treated cells compared to control cells (mean ± SEM; p = 0.10).
There is evidence for two populations of chromaffin cells that either express or lack phenylethanolamine N-methyltransferase, the enzyme that converts norepinephrine to epinephrine. Moreover, differential control of epinephrine and norepinephrine secretion has been reported 27,28. However, this was not the case for gabapentin; epinephrine secretion was inhibited by 24 ± 3 % (p = 0.0007; n = 12, mean ± SEM) and norepinephrine secretion was inhibited by 23 ± 4 % (p = 0.0001; n =12, mean ± SEM) (fig. 1C). Carbachol activates both nicotinic and muscarinic receptors on chromaffin cells, but we found similar results when secretion was stimulated with a 5-min application of the selective nicotinic receptor agonist DMPP (30 μM) (fig. 1D). Gabapentin inhibited epinephrine secretion by 22 ± 4 % (p = 0.042; n = 4, mean ± SEM) and norepinephrine secretion by 16 ± 3 % (p = 0.026; n = 4, mean ± SEM), values that were not statistically different from those obtained using carbachol (p = 0.85 for norepinephrine and p = 0.98 for epinephrine).
To determine if carbachol evoked Ca2+ entry was altered by gabapentin we used fluorescent imaging of individual chromaffin cells loaded with Fura-2. Cells were continuously perfused with fresh extracellular recording solution and exposed to a 5-min application of carbachol (100 μM) to parallel the secretion studies outlined above. Carbachol elicited a sustained increase in intracellular [Ca2+] that reversed upon washout (fig. 1E). The magnitude of the Ca2+ elevation was not statistically different in cells treated with gabapentin compared to control cells (fig. 1F).
Gabapentin treatment reduces catecholamine release evoked by KCl, even though Ca2+ entry was increased
To bypass involvement of cholinergic receptors, we stimulated the cells with 30 mM KCl which directly depolarizes the membrane potential leading to Ca2+ influx through voltage-gated Ca2+ channels. Previous studies have shown that the secretory response to 30 mM KCl mimics that seen with acute stress 29. There was a statistically significant reduction in KCl-evoked secretion from gabapentin treated cells compared to controls (fig. 2A) (p = 0.019; n = 8). There was a statistically significant inhibition of both epinephrine (p = 0.002; n = 8) and norepinephrine (p = 0.006; n = 8) secretion by gabapentin (fig. 2B). KCl elicited a robust increase in intracellular [Ca2+] that decayed by ~ 40 % during the 5-min application (fig. 2C). The peak Ca2+ elevation (fig. 2D) and the Ca2+ elevation at the end of the 5-min stimulation (fig. 2D) were greater in gabapentin cells than in controls, and in both cases this difference was statistically significant (p < 0.0001). Thus, gabapentin reduced catecholamine release even though Ca2+ entry was modestly increased.
Figure 2. Gabapentin reduces KCl-evoked catecholamine secretion even though calcium entry was increased.
(A) Chromaffin cells were seeded on 24-well plates and treated with 1mM gabapentin (GBP) or vehicle (control) for 18–24 h. The amount of catecholamines released under basal conditions (in the absence of secretagogue) or during a 5-min stimulation with 30 mM KCl was determined using high performance liquid chromatography and expressed as a percentage of total cellular content (mean ± SEM). Gabapentin treatment significantly reduced KCl-evoked secretion (* p < 0.019; n = 8) but not basal release (p = 0.42; n = 8). (B) Secretion evoked by KCl in gabapentin treated cells (GBP) was normalized to controls from the same plate (mean ± SEM). There was a statistically significant inhibition of both epinephrine (p = 0.002; n = 8) and norepinephrine (p = 0.006; n = 8) secretion by gabapentin. (C) Representative experiment showing the [Ca2+]i increase evoked by a 5-min application of 30mM KCl in FURA-2 loaded chromaffin cells (n = 18 cells, mean ± SEM - for clarity error bars are only shown for a few data points). (D) Pooled data from multiple experiments like that shown in panel C. The peak increase in [Ca2+]i (peak) and the sustained increase at the end of the KCl application (end) were significantly greater in gabapentin treated cells (n = 97) compared to controls (mean ± SEM; n = 128) (** p < 0.0001).
The inhibition of catecholamine release by gabapentin is concentration dependent
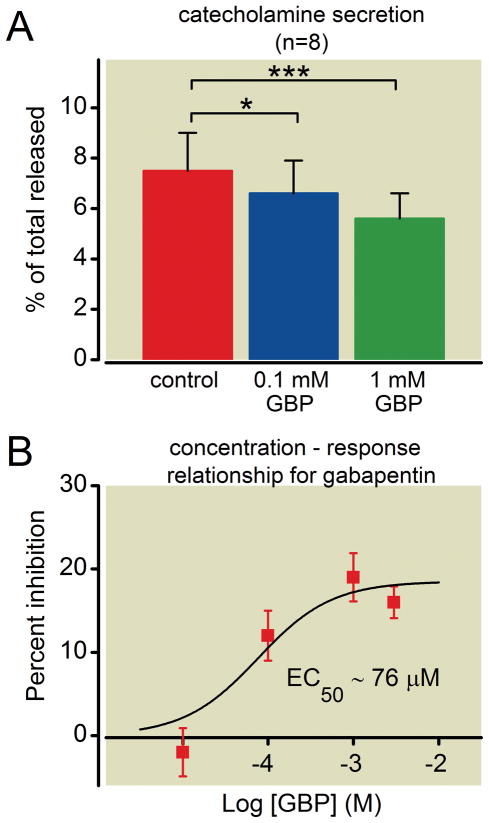
Clinically, gabapentin is given in a range of concentration and dosing paradigms. Plasma concentrations approach tens of micromolar and highly concentrative transport of gabapentin by the system L-amino acid transporter can occur in tissues or cells. Neutral amino acids including L-leucine and L-isoleucine competitively inhibit this transport and gabapentin binding to the α2δ subunit of Ca2+-channels 30. Each of these amino acids are present in tissue culture medium at ~ 0.4 mM, (substantially higher than in plasma) so to overcome this competitive antagonism, we initially used 1mM gabapentin, consistent with previous studies 14. However, in other experiments we directly compared the ability of 0.1 mM and 1 mM gabapentin to inhibit catecholamine secretion evoked by KCl or carbachol. Both concentrations of gabapentin reduced catecholamine release compared to controls by a statistically significant amount (p = 0.0002, repeated measures ANOVA; p < 0.05 for 0.1 mM and p < 0.001 for 1mM using Dunnett’s post test) (fig. 3A). Furthermore, the inhibition produced by 1 mM gabapentin (22 ± 3 %; n = 8, mean ± SEM) was statistically greater than that by 0.1 mM gabapentin (12 ± 3 %; n = 8, mean ± SEM) (p = 0.039). In figure 3B all data from the HPLC analyses of catecholamine secretion are combined to generate a concentration response curve which predicts an EC50 of 76 μM, close to relevant plasma concentrations in patients.
Figure 3. The inhibition of catecholamine secretion by gabapentin is concentration-dependent.
(A) Chromaffin cells were seeded on 24-well plates and treated with vehicle (control) or gabapentin (0.1mM GBP or 1mM GBP) for 18–24 h. Evoked catecholamine secretion was determined using high performance liquid chromatography (HPLC) and expressed as a percentage of total cellular content (mean ± SEM). Both concentrations of gabapentin significantly reduced secretion compared to controls (n = 8; * p < 0.05; *** p < 0.001). (B) Data from all HPLC experiments were pooled and percent inhibition of evoked catecholamine secretion (mean ± SEM) plotted against Log 10 of gabapentin concentration (10 μM, n = 4; 100 μM, n = 8; 1mM, n = 20; 3 mM, n = 4). A concentration response curve was generated by fitting the data to a Boltzmann function with a Hill slope = 1, and yielded an estimated EC50 of 76 μM.
Gabapentin treatment does not alter the density or kinetics of voltage-gated Ca2+ channel currents
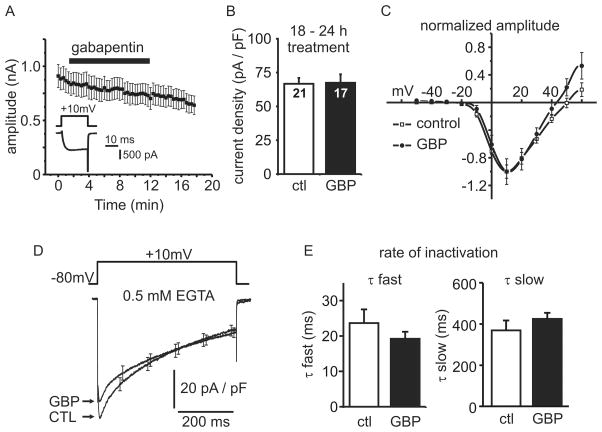
To enable more direct and precise insight into the effects of gabapentin on voltage-gated Ca2+ channel currents (ICa) we used whole-cell patch clamp electrophysiology. We have previously shown that chromaffin cells express predominantly N-type, and P/Q-type calcium channels with a smaller (10–15 %) contribution from L-type channels 31,32. Acute application of gabapentin or pregabalin has been reported to inhibit ICa in some studies 9–12 but not in others 13–15, and has never been tested in chromaffin cells. Therefore, we first investigated the effects of acute gabapentin application on ICa. Chromaffin cells were voltage-clamped at −80 mV and ICa was elicited by a 20 ms step depolarization to +10 mV once every 20 s. Bath perfusion of gabapentin (1 mM) for 10-minutes had no obvious effect on ICa amplitude (fig. 4A). The gradual rundown in current amplitude during the course of the experiment (12 ± 4 % decline; n = 6, mean ± SEM) was not significantly different (p = 0.68) than in control cells (15 ± 4 %; n = 7; mean ± SEM; not shown).
Figure 4. Gabapentin does not alter the density or kinetics of voltage-gated Ca2+ channel currents.
(A) Whole cell patch-clamp electrophysiology was used to record ICa elicited by 20 ms step depolarizations (see inset), and the peak amplitude (mean ± SEM) plotted against time. Acute application of 1mM gabapentin (indicated by black bar) had no effect on ICa amplitude. (B) Cells were treated with vehicle (ctl) or 1mM gabapentin (GBP) for 18–24 h prior to recording. Peak ICa was normalized to cell size (membrane capacitance) to yield current density. There was no change in current density (mean ± SEM) in gabapentin treated cells (n = 17) compared to control cells (n = 21) (p = 0.92). (C) The current-voltage relationship for ICa was not shifted by treatment with 1mM gabapentin for 18 – 24 h. ICa amplitude (mean ± SEM) was normalized to the peak inward current in each cell to facilitate comparison of the voltage-dependence. (D) Inactivation of ICa during a 500 ms step depolarization in control cells and cells treated for 18– 24 h with 1mM gabapentin. Traces represent the data from 8 control cells and 10 gabapentin treated cells (mean ± SEM). For clarity, error bars are shown for only a few data points. (E) Inactivation of ICa during a 500 ms step was fit with a double exponential decay and the time constants from each cell were pooled. There was no difference in either the fast (τ fast) (p = 0.28) or slow (τ slow) (p = 0.31) inactivation time constants between control (n = 8) and gabapentin treated cells (n = 10) (mean ± SEM).
Next, to parallel the secretion studies outlined above (fig. 1–3), we compared ICa in control cells and cells treated for 18–24 h with 1 mM gabapentin. The peak current density, calculated by normalizing peak current amplitude to membrane capacitance (cell size), was not significantly different in gabapentin treated cells compared to matched controls (fig. 4B; p = 0.92). There was also no discernible shift in the current-voltage relationship of ICa (fig. 4C). Voltage-gated Ca2+ channels are subject to both voltage-dependent inactivation and Ca2+-dependent inactivation 33–37 that control the amount and timing of Ca2+ entry during sustained or repetitive stimulation. Pregabalin disrupts inactivation of ICa in Calyx of Held neurons 9, so we investigated if gabapentin altered inactivation of ICa in chromaffin cells. For these experiments we lowered the concentration of EGTA in the patch-pipette solution from 10 mM to 0.5 mM because Ca2+-dependent inactivation of N- and P/Q-type calcium channels is blocked by higher concentrations of EGTA or other Ca2+ chelators 37,38. Cells were stimulated with a 500 ms step depolarization from −80 mV to +10 mV (fig. 4D) to elicit an inward ICa. The peak density of ICa was slightly smaller in cells treated with gabapentin (1 mM, 18–24 h) than in controls, but this difference was not statistically significant (54 ± 4.6 pA/pF, n = 10 compared to 64 ± 4.4 pA/pF, n = 8, mean ± SEM, p = 0.11). The extent of inactivation at the end of the 500 ms step (% decay from peak amplitude) was not significantly altered in gabapentin treated cells (66 ± 3 %, n = 10, mean ± SEM) compared to control cells (74 ± 4 %, n = 8, mean ± SEM; p = 0.13). The inactivation of ICa was well fit with a double exponential decay and we found no statistically significant difference in the fast or slow time constants derived from these fits (fig. 4E). We repeated the same experiment but with 10 mM EGTA in the patch pipette solution which blocks Ca2+-dependent inactivation but leaves voltage-dependent inactivation intact. Gabapentin had no effect on this voltage-dependent inactivation (32 ± 2 % n = 7 in controls compared to 33 ± 2 % n = 6 in gabapentin treated cells; p = 0.82, mean ± SEM).
We also tested whether G protein mediated inhibition of ICa was altered by gabapentin. We and others have shown previously that adenosine triphosphate coreleased with catecholamines mediates feedback inhibition of ICa and thus transmitter release in chromaffin cells 21. This inhibition is due to activation of P2Y autoreceptors and downstream G proteins which directly inhibit the channels 21. Gabapentin treatment did not alter this important regulatory pathway; application of adenosine triphosphate (100 μM) inhibited ICa in control cells by 48 ± 7 % (n = 6, mean ± SEM) and in gabapentin treated cells by 49 ± 3 % (n = 9, mean ± SEM) (p = 0.98 comparing control to gabapentin treated cells). Thus, we found no statistically significant difference in the density, kinetics, or regulation of ICa in gabapentin treated cells compared to controls.
Gabapentin reduces the number but not the quantal size or kinetics of secretory vesicle fusion events
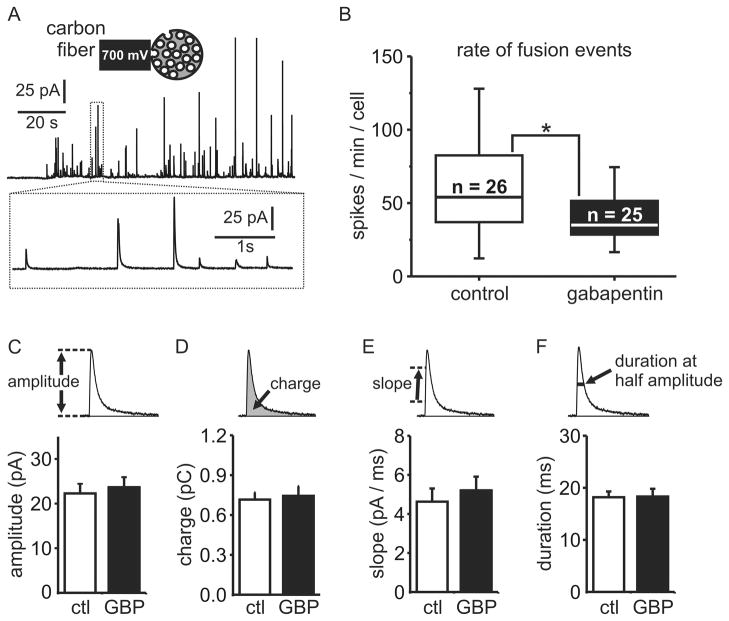
The overall reduction in catecholamine release that we report could be due to fewer vesicles fusing with the plasma membrane and/or altered content or release of catecholamine from each vesicle. Hydralazine and some beta-blockers have been reported to accumulate in secretory vesicles of chromaffin cells and thereby displace catecholamines leading to reduced vesicular content and altered release kinetics 39,40. Therefore, to assess these possibilities and gain more precise mechanistic insight we used carbon fiber amperometry. Briefly, a carbon fiber electrode was positioned immediately adjacent to an individual chromaffin cell (fig. 5A). A potential of +700 mV was applied to the fiber such that catecholamines released from the adjacent cell were rapidly oxidized generating two electrons for each molecule of catecholamine. Because of the sensitivity and precise spatial and temporal resolution, this approach can readily resolve catecholamine release from individual vesicles fusing with the plasma membrane. Each fusion event leads to a transient current “spike” (fig. 5A). These spikes can be counted as a measure of the number of vesicles that undergo exocytosis. We compared secretion evoked by 30 mM KCl and found a statistically significant reduction (~35 %) in the rate of fusion events in gabapentin treated cells compared to control cells (fig. 5B).
Figure 5. Gabapentin reduces the number of secretory vesicle fusion events but not the quantal size or kinetics of catecholamine release from each vesicle.
Carbon fiber amperometry was used to quantify the number, quantal size, and kinetics of individual vesicular fusion events. (A) A representative amperometry recording from a control cell stimulated with 30 mM KCl. Each upward deflection (spike) on the current trace is produced by oxidation of the catecholamines released from a single vesicular fusion event. The inset shows a cartoon representation of the recoding configuration (above) and an expanded view of a few spikes is shown below. (B) The number of amperometric spikes was determined for each cell over a 2-min period. The box graph shows the 25th percentile, median, and 75th percentile distribution of vesicular fusion rate (spikes per minute) for control cells (n = 26 ) and cells treated for 18–24 hours with 1mM gabapentin (n = 25). The whiskers represent the smallest and largest non-outliers in each population of cells. Gabapentin produced a statistically significantly reduction in the rate of fusion events compared to matched controls (* p = 0.046, Mann-Whitney test). (C–F) Other parameters of the amperometric spikes (amplitude, charge, slope, and duration) were analyzed and a median value for each cell calculated. Pooled values (mean ± SEM) for each parameter are shown and were compared. No statistically significant differences were found between gabapentin treated cells (GBP) and control cells (ctl).
We also analyzed the individual amperometric spikes in control and gabapentin treated cells. The charge (integral) of each spike is directly proportional to the number of catecholamine molecules released from a single vesicle. The amplitude of the spikes reflects the peak concentration of catecholamine at the electrode tip whereas the “half-width” and slope reflect the duration and speed of catecholamine release respectively. These characteristics were determined for each spike and a median value calculated for each cell. These median spike parameters were then pooled and we found no statistically significant differences between control and gabapentin treated cells (fig. 5C–F) (p > 0.63 for all comparisons). Thus our data reveal that gabapentin reduced the number of vesicles that underwent exocytosis, but did not alter the amount or kinetics of catecholamine released from those vesicles that did fuse with the plasma membrane.
DISCUSSION
To our knowledge this is the first study to investigate the effects of gabapentin on stimulus-secretion coupling in adrenal chromaffin cells. Our rationale for doing so was motivated by two primary goals. First, chromaffin cells are widely used as a model to investigate regulation of Ca2+-channels and neurosecretion 20–22. Thus, we reasoned this would be a good system in which to evaluate the cellular mechanisms of gabapentin. Second, catecholamines, endogenous opioids 23, and other hormones released from chromaffin cells play central roles in the sympathoadrenal stress response, and might contribute to stress-related enhancement of mechanical hyperalgesia 24. Therefore, we postulated that altered adrenal catecholamine release might contribute to hemodynamic stabilization and perhaps other beneficial perioperative effects of gabapentin.
Gabapentin reduces catecholamine release from adrenal chromaffin cells
Gabapentin has been reported to block elevated serum levels of norepinephrine produced by spinal nerve ligation 41, or conversely to activate the descending noradrenergic system and increase norepinephrine release in the spinal cord, likely through modulation of glutamatergic signaling in the locus coeruleus 42–45. Thus, the effects of gabapentin on catecholamine levels in vivo likely involve central and peripheral sites of action, including indirect effects through altered network excitability and direct targeting of neurosecretory cells and mechanisms. However, the effects of gabapentin on adrenal chromaffin cells, a primary source for circulating catecholamines, was unknown prior to this study. To mirror the relevant time-frame for intra-and early postoperative effects of preoperative gabapentin dosing, we treated cells with gabapentin for 18–24 h and compared catecholamine release to matched controls. We show that gabapentin did not alter the catecholamine content of the chromaffin cells, but produced a statistically significant reduction in secretion evoked by cholinergic agonists or by direct membrane depolarization (30 mM KCl) (fig. 1–3). The modest extent of this inhibition (~ 20–25 %) is in line with previous reports investigating neurotransmitter release in brain slices 44,46, and expected for a drug that is generally well tolerated. Thus, we demonstrate for the first time that gabapentin has direct actions on chromaffin cells that might contribute to modulation of the sympathoadrenal stress response. It will be of interest in future studies to determine if gabapentin also has indirect effects by altering sympathetic drive to the adrenal medulla.
The inhibition of catecholamine release was not mediated by altered calcium entry
Currently, the consensus view is that gabapentin and pregabalin target the α2δ1 or α2δ2 subunit of voltage-gated Ca2+ channels to mediate many of their physiological effects (for review see 8). Calcium influx through voltage-gated Ca2+ channels triggers neurotransmitter and hormone release leading to the hypothesis that gabapentin reduced transmitter release by inhibiting Ca2+ entry. Indeed, some studies reported acute inhibition of ICa by gabapentin or pregabalin 9–12, although others found no effect 13–15. Here we report that acute (10-min) gabapentin application had no effect on ICa in chromaffin cells (fig. 4A).
Of more relevance to our investigations of catecholamine secretion, previous studies showed that longer-term (17–48 h) incubation with gabapentin / pregabalin reduced the amplitude of ICa due to a decrease in the number of channels at the plasma membrane 13,14,16. This was dependent on the α2δ subunit, but appeared to involve an intracellular action of gabapentin to disrupt trafficking / recycling of the channels from endosomal compartments to the plasma membrane 17. Using Fura-2 imaging, we found no effect of gabapentin (18–24 h) on the intracellular [Ca2+] elevation produced by cholinergic agonists (fig. 1), while the [Ca2+] elevation evoked by 30 mM KCl was slightly increased (fig. 2). We used patch-clamp electrophysiology and found no statistically significant differences in ICa density or kinetics in gabapentin treated cells (18–24 h incubation) compared to controls (fig. 4). We also found no statistically significant alteration in regulation of ICa by endogenous P2Y purinergic autoreceptors, an important mechanism that mediates feedback inhibition of catecholamine secretion 21. These data suggest the enhanced calcium entry seen with KCl stimulation was not due to direct effects of gabapentin on ICa. Pregabalin was previously reported to enhance Ca2+ entry in a subset of sensory neurons perhaps through modulation of K+ channels 47, but we did not investigate this possibility in the current study.
It remains unclear why gabapentin had no effect on ICa in chromaffin cells, but it is possible that longer incubations are required to observe the trafficking effect. Alternatively, specific Ca2+ channel subunit combinations or splice variants might be required 13, and not highly expressed in chromaffin cells. Regardless of the reason, our data provide direct evidence that gabapentin can inhibit catecholamine secretion independently from effects on Ca2+ entry, perhaps through disruption of the exocytotic machinery or vesicular trafficking events.
Gabapentin reduced the number but not the quantal size of secretory vesicle release events
In general, an overall reduction in transmitter release could reflect fusion of fewer secretory vesicles with the plasma membrane and/or alterations in the amount or kinetics of transmitter release from each vesicle. One advantage to using chromaffin cells is the ability to directly detect and quantify catecholamine release from individual vesicles using carbon fiber amperometry. Previous work demonstrated that second messenger pathways can alter the content and/or emptying of secretory vesicles upon stimulation48–53. Furthermore, hydralazine and β blockers can accumulate in secretory vesicles and thereby reduce the amount and kinetics of catecholamine release from each vesicle39,40. Using carbon fiber amperometry we show that gabapentin reduced the number of vesicles that undergo exocytosis, but did not change the amount or kinetics of catecholamine released from these unitary events (fig. 5).
Taken together, our data suggest that Ca2+ entry is unaltered by gabapentin but is less effective at triggering vesicle fusion. Consistent with this, gabapentin / pregabalin inhibited spontaneous synaptic release events or exocytosis evoked by hypertonic sucrose, even though these events are thought to occur independent from Ca2+ entry 18,19. It is possible that binding to the α2δ subunit might allosterically disrupt coupling of Ca2+ channels with the exocytotic machinery 18. Gabapentin and pregabalin are also substrates for the neutral L-amino acid transporter so may act intracellularly. Recently, α2δ subunits were identified on neuropeptide containing large dense core secretory vesicles isolated from dorsal spinal cord neurons 54. Perhaps analogous to the disruption of channel recycling from endosomal compartments 17, gabapentin might alter secretory vesicle trafficking, docking, or priming, and thus control the number of fusion competent vesicles at the plasma membrane.
Relating cellular mechanism to perioperative physiological effects
There is increasing interest in potentially beneficial effects of acute (up to 24 h) preoperative gabapentin 1. While much of this work has focused on analgesia, several reports have demonstrated that gabapentin blunted the hemodynamic response to direct laryngoscopy / tracheal intubation 2,3, or skull pin insertion55. Although the mechanism was not directly addressed in these studies, these procedures are known to elicit an acute stress response including increased levels of circulating catecholamines 4–7. This led us to postulate that gabapentin might act in part through reducing sympathoadrenal stress hormone release. Consistent with this hypothesis, we provide the first demonstration that gabapentin inhibits catecholamine release from isolated adrenal chromaffin cells. As already mentioned, gabapentin exerts widespread actions, perhaps including altered neuronal excitability and sympathetic drive to the adrenal medulla in addition to the direct effects on chromaffin cells reported here. Thus, further in vivo studies are required to corroborate our findings, but careful attention needs to be paid to the anesthetic regimen that might in itself alter the hemodynamic response and/or sympathoadrenal hormone release 4,56. Previous work has shown that opioids 31,48 and anesthetics including etomidate, propofol, and isoflurane can modulate calcium signaling and transmitter release in chromaffin cells 57–60. Given the perioperative exposure to these classes of drug, it is pertinent to determine if, and how, they impact regulation of catecholamine release by gabapentin. Other mechanisms such as altered neuronal excitability, vagal reflexes, or direct effects on the cardiovascular system might also contribute to the hemodynamic stabilization produced by gabapentin. Pregabalin inhibits L-type Ca2+ channels in smooth muscle cells isolated from cerebrovascular arteries 61, but gabapentin does not alter ICa in cardiac myocytes 62. Generally, gabapentin is well tolerated and thought to have little effect on cardiovascular function under resting conditions, consistent with the idea that perioperative hemodynamic stabilization involves blunting of the acute stress response.
Surgery is well known to elicit a stress response that can persist for hours or days postoperatively. It is tempting to speculate that by reducing sympathoadrenal stress hormone release gabapentin might impact a variety of postoperative physiological parameters including hemodynamics and inflammation. This might relate to some of the antihyperalgesic effects of gabapentin because chronic elevation of epinephrine and peripheral nerve catecholamine chemosensitivity have been implicated in the induction and maintenance of selected forms of mechanical hyperalgesia and neuropathic pain24,63–65. Although more studies are needed, this paper identifies altered adrenal catecholamine release as a feasible mechanism underlying some of the perioperative effects of gabapentin. Our work also demonstrates the utility of adrenal chromaffin cells as a model system to dissect the cellular mechanism(s) of this widely prescribed drug.
Summary Statement.
What we already know about this topic
Gabapentin has acute preemptive analgesic effects and blunts the hemodynamic response to laryngoscopy and tracheal intubation.
Gabapentin binds with high affinity to a subunit of voltage-gated calcium channels.
Calcium influx triggers secretory vesicle exocytosis.
What this article tells us that is new
Gabapentin reduced catecholamine release from adrenal chromaffin cells without altering their catecholamine content.
Calcium entry was unaltered by gabapentin but calcium was less effective in triggering vesicle fusion.
Acknowledgments
Sources of financial support for the work
This work was supported in part by an Innovation Grant from the Department of Anesthesiology, Vanderbilt University School of Medicine, Nashville Tennessee and by the National Institutes of Health, National Institute of Neurological Diseases and Stroke, Bethesda, Maryland [Grant R01 NS052446].
Individuals or organizations to be acknowledged
We would like to thank Ray Johnson, B.S., Manager of the Neurochemistry Core, Center for Molecular Neuroscience, Vanderbilt University School of Medicine, Nashville, Tennessee, for assistance with high performance liquid chromatography analyses of catecholamines. WinEDR software was authored and freely distributed by Dr. John Dempster, Ph.D. (Senior Lecturer, Strathclyde Institute for Pharmacy & Biomedical Sciences, University of Strathclyde, Glasgow, Scotland).
Footnotes
Meetings at which the work has been presented (name, exact date, location)
American Society of Interventional Pain Physicians; Washington, D.C., June 27, 2010.
American Society of Anesthesiologists; poster abstract; San Diego, California, October 19, 2010
American Society of Anesthesiologists; research abstract; Chicago, Illinois, October 16, 2011
Contributor Information
Robert D. Todd, Assistant Professor, Department of Anesthesiology, Vanderbilt University School of Medicine, Nashville Tennessee
Sarah M. McDavid, Research Assistant III, Department of Anesthesiology, Vanderbilt University School of Medicine, Nashville Tennessee
Rebecca L. Brindley, Post-doctoral Fellow, Department of Anesthesiology, Vanderbilt University School of Medicine, Nashville Tennessee
Mark L. Jewell, Graduate Student, Department of Pharmacology, Vanderbilt University School of Medicine, Nashville Tennessee
Kevin P.M. Currie, Assistant Professor, Department of Anesthesiology and Department of Pharmacology, Vanderbilt University School of Medicine, Nashville Tennessee
References
- 1.Kong VK, Irwin MG. Gabapentin: A multimodal perioperative drug? Br J Anaesth. 2007;99:775–86. doi: 10.1093/bja/aem316. [DOI] [PubMed] [Google Scholar]
- 2.Fassoulaki A, Melemeni A, Paraskeva A, Petropoulos G. Gabapentin attenuates the pressor response to direct laryngoscopy and tracheal intubation. Br J Anaesth. 2006;96:769–73. doi: 10.1093/bja/ael076. [DOI] [PubMed] [Google Scholar]
- 3.Memis D, Turan A, Karamanlioglu B, Seker S, Ture M. Gabapentin reduces cardiovascular responses to laryngoscopy and tracheal intubation. Eur J Anaesthesiol. 2006;23:686–90. doi: 10.1017/S0265021506000500. [DOI] [PubMed] [Google Scholar]
- 4.Derbyshire DR, Chmielewski A, Fell D, Vater M, Achola K, Smith G. Plasma catecholamine responses to tracheal intubation. Br J Anaesth. 1983;55:855–60. doi: 10.1093/bja/55.9.855. [DOI] [PubMed] [Google Scholar]
- 5.Shribman AJ, Smith G, Achola KJ. Cardiovascular and catecholamine responses to laryngoscopy with and without tracheal intubation. Br J Anaesth. 1987;59:295–9. doi: 10.1093/bja/59.3.295. [DOI] [PubMed] [Google Scholar]
- 6.Brossy MJ, James MF, Janicki PK. Haemodynamic and catecholamine changes after induction of anaesthesia with either thiopentone or propofol with suxamethonium. Br J Anaesth. 1994;72:596–8. doi: 10.1093/bja/72.5.596. [DOI] [PubMed] [Google Scholar]
- 7.Russell WJ, Morris RG, Frewin DB, Drew SE. Changes in plasma catecholamine concentrations during endotracheal intubation. Br J Anaesth. 1981;53:837–9. doi: 10.1093/bja/53.8.837. [DOI] [PubMed] [Google Scholar]
- 8.Taylor CP. Mechanisms of analgesia by gabapentin and pregabalin--calcium channel α2-δ [Cavα2-δ] ligands. Pain. 2009;142:13–6. doi: 10.1016/j.pain.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Di Guilmi MN, Urbano FJ, Inchauspe CG, Uchitel OD. Pregabalin modulation of neurotransmitter release is mediated by change in intrinsic activation/inactivation properties of ca(v)2.1 calcium channels. J Pharmacol Exp Ther. 2011;336:973–82. doi: 10.1124/jpet.110.172171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stefani A, Spadoni F, Bernardi G. Gabapentin inhibits calcium currents in isolated rat brain neurons. Neuropharmacology. 1998;37:83–91. doi: 10.1016/s0028-3908(97)00189-5. [DOI] [PubMed] [Google Scholar]
- 11.Sutton KG, Martin DJ, Pinnock RD, Lee K, Scott RH. Gabapentin inhibits high-threshold calcium channel currents in cultured rat dorsal root ganglion neurones. Br J Pharmacol. 2002;135:257–65. doi: 10.1038/sj.bjp.0704439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin DJ, McClelland D, Herd MB, Sutton KG, Hall MD, Lee K, Pinnock RD, Scott RH. Gabapentin-mediated inhibition of voltage-activated Ca2+ channel currents in cultured sensory neurones is dependent on culture conditions and channel subunit expression. Neuropharmacology. 2002;42:353–66. doi: 10.1016/s0028-3908(01)00181-2. [DOI] [PubMed] [Google Scholar]
- 13.Mich PM, Horne WA. Alternative splicing of the Ca2+ channel beta4 subunit confers specificity for gabapentin inhibition of Cav2.1 trafficking. Mol Pharmacol. 2008;74:904–12. doi: 10.1124/mol.108.045153. [DOI] [PubMed] [Google Scholar]
- 14.Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC. Pharmacological disruption of calcium channel trafficking by the α2δ ligand gabapentin. Proc Natl Acad Sci U S A. 2008;105:3628–33. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vega-Hernandez A, Felix R. Down-regulation of N-type voltage-activated Ca2+ channels by gabapentin. Cell Mol Neurobiol. 2002;22:185–90. doi: 10.1023/A:1019865822069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heblich F, Tran Van Minh A, Hendrich J, Watschinger K, Dolphin AC. Time course and specificity of the pharmacological disruption of the trafficking of voltage-gated calcium channels by gabapentin. Channels (Austin) 2008;2:4–9. doi: 10.4161/chan.2.1.6045. [DOI] [PubMed] [Google Scholar]
- 17.Tran-Van-Minh A, Dolphin AC. The α2δ ligand gabapentin inhibits the Rab11-dependent recycling of the calcium channel subunit α2δ-2. J Neurosci. 2010;30:12856–67. doi: 10.1523/JNEUROSCI.2700-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Micheva KD, Taylor CP, Smith SJ. Pregabalin reduces the release of synaptic vesicles from cultured hippocampal neurons. Mol Pharmacol. 2006;70:467–76. doi: 10.1124/mol.106.023309. [DOI] [PubMed] [Google Scholar]
- 19.Cunningham MO, Woodhall GL, Thompson SE, Dooley DJ, Jones RS. Dual effects of gabapentin and pregabalin on glutamate release at rat entorhinal synapses in vitro. Eur J Neurosci. 2004;20:1566–76. doi: 10.1111/j.1460-9568.2004.03625.x. [DOI] [PubMed] [Google Scholar]
- 20.Bader MF, Holz RW, Kumakura K, Vitale N. Exocytosis: The chromaffin cell as a model system. Ann N Y Acad Sci. 2002;971:178–83. doi: 10.1111/j.1749-6632.2002.tb04461.x. [DOI] [PubMed] [Google Scholar]
- 21.Currie KP. Inhibition of Ca2+ channels and adrenal catecholamine release by G protein coupled receptors. Cell Mol Neurobiol. 2010;30:1201–8. doi: 10.1007/s10571-010-9596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borges R, Camacho M, Gillis KD. Measuring secretion in chromaffin cells using electrophysiological and electrochemical methods. Acta Physiol (Oxf) 2008;192:173–84. doi: 10.1111/j.1748-1716.2007.01814.x. [DOI] [PubMed] [Google Scholar]
- 23.Viveros OH, Wilson SP. The adrenal chromaffin cell as a model to study the co-secretion of enkephalins and catecholamines. J Auton Nerv Syst. 1983;7:41–58. doi: 10.1016/0165-1838(83)90068-1. [DOI] [PubMed] [Google Scholar]
- 24.Khasar SG, Dina OA, Green PG, Levine JD. Sound stress-induced long-term enhancement of mechanical hyperalgesia in rats is maintained by sympathoadrenal catecholamines. J Pain. 2009;10:1073–7. doi: 10.1016/j.jpain.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dzhura EV, He W, Currie KP. Linopirdine modulates calcium signaling and stimulus-secretion coupling in adrenal chromaffin cells by targeting M-type K+ channels and nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2006;316:1165–74. doi: 10.1124/jpet.105.095570. [DOI] [PubMed] [Google Scholar]
- 26.Mosharov EV, Sulzer D. Analysis of exocytotic events recorded by amperometry. Nat Methods. 2005;2:651–8. doi: 10.1038/nmeth782. [DOI] [PubMed] [Google Scholar]
- 27.Yadid G, Youdim MB, Zinder O. Preferential release of epinephrine by glycine from adrenal chromaffin cells. Eur J Pharmacol. 1992;221:389–91. doi: 10.1016/0014-2999(92)90729-n. [DOI] [PubMed] [Google Scholar]
- 28.Cahill AL, Perlman RL. Phorbol esters cause preferential secretion of norepinephrine from bovine chromaffin cells. J Neurochem. 1992;58:768–71. doi: 10.1111/j.1471-4159.1992.tb09784.x. [DOI] [PubMed] [Google Scholar]
- 29.Fulop T, Smith C. Matching native electrical stimulation by graded chemical stimulation in isolated mouse adrenal chromaffin cells. J Neurosci Methods. 2007;166:195–202. doi: 10.1016/j.jneumeth.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the α2δ subunit of a calcium channel. J Biol Chem. 1996;271:5768–76. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- 31.Currie KPM, Fox AP. ATP serves as a negative feedback inhibitor of voltage-gated Ca2+ channel currents in cultured bovine adrenal chromaffin cells. Neuron. 1996;16:1027–36. doi: 10.1016/s0896-6273(00)80126-9. [DOI] [PubMed] [Google Scholar]
- 32.Currie KP, Fox AP. Comparison of N- and P/Q-type voltage-gated calcium channel current inhibition. J Neurosci. 1997;17:4570–9. doi: 10.1523/JNEUROSCI.17-12-04570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–55. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 34.Stotz SC, Zamponi GW. Structural determinants of fast inactivation of high voltage-activated Ca(2+) channels. Trends Neurosci. 2001;24:176–81. doi: 10.1016/s0166-2236(00)01738-0. [DOI] [PubMed] [Google Scholar]
- 35.Cox DH, Dunlap K. Inactivation of N-type calcium current in chick sensory neurons: Calcium and voltage dependence. J Gen Physiol. 1994;104:311–36. doi: 10.1085/jgp.104.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee A, Wong ST, Gallagher D, Li B, Storm DR, Scheuer T, Catterall WA. Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature. 1999;399:155–9. doi: 10.1038/20194. [DOI] [PubMed] [Google Scholar]
- 37.Liang H, DeMaria CD, Erickson MG, Mori MX, Alseikhan BA, Yue DT. Unified mechanisms of Ca2+ regulation across the Ca2+ channel family. Neuron. 2003;39:951–60. doi: 10.1016/s0896-6273(03)00560-9. [DOI] [PubMed] [Google Scholar]
- 38.McDavid S, Currie KP. G-proteins modulate cumulative inactivation of N-type (Cav2.2) calcium channels. J Neurosci. 2006;26:13373–83. doi: 10.1523/JNEUROSCI.3332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machado JD, Gomez JF, Betancor G, Camacho M, Brioso MA, Borges R. Hydralazine reduces the quantal size of secretory events by displacement of catecholamines from adrenomedullary chromaffin secretory vesicles. Circ Res. 2002;91:830–6. doi: 10.1161/01.res.0000039530.30495.6f. [DOI] [PubMed] [Google Scholar]
- 40.Montesinos MS, Camacho M, Machado JD, Viveros OH, Beltran B, Borges R. The quantal secretion of catecholamines is impaired by the accumulation of β-adrenoceptor antagonists into chromaffin cell vesicles. Br J Pharmacol. 2010;159:1548–56. doi: 10.1111/j.1476-5381.2010.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mao YF, Liu XR, Liao XZ, Lv YH, Xu H, Deng XM, Yan SK, Xiong YC, Zhang WD. Blood serum profiling of the rat spinal nerve ligation model using ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Eur J Pharmacol. 2009;615:61–5. doi: 10.1016/j.ejphar.2009.03.079. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi Y, Takasu K, Honda M, Ono H, Tanabe M. Neurochemical evidence that supraspinally administered gabapentin activates the descending noradrenergic system after peripheral nerve injury. Eur J Pharmacol. 2007;556:69–74. doi: 10.1016/j.ejphar.2006.10.059. [DOI] [PubMed] [Google Scholar]
- 43.Hayashida K, DeGoes S, Curry R, Eisenach JC. Gabapentin activates spinal noradrenergic activity in rats and humans and reduces hypersensitivity after surgery. Anesthesiology. 2007;106:557–62. doi: 10.1097/00000542-200703000-00021. [DOI] [PubMed] [Google Scholar]
- 44.Takasu K, Ono H, Tanabe M. Gabapentin produces PKA-dependent pre-synaptic inhibition of GABAergic synaptic transmission in LC neurons following partial nerve injury in mice. J Neurochem. 2008;105:933–42. doi: 10.1111/j.1471-4159.2008.05212.x. [DOI] [PubMed] [Google Scholar]
- 45.Hayashida K, Obata H, Nakajima K, Eisenach JC. Gabapentin acts within the locus coeruleus to alleviate neuropathic pain. Anesthesiology. 2008;109:1077–84. doi: 10.1097/ALN.0b013e31818dac9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fink K, Meder W, Dooley DJ, Gothert M. Inhibition of neuronal Ca(2+) influx by gabapentin and subsequent reduction of neurotransmitter release from rat neocortical slices. Br J Pharmacol. 2000;130:900–6. doi: 10.1038/sj.bjp.0703380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McClelland D, Evans RM, Barkworth L, Martin DJ, Scott RH. A study comparing the actions of gabapentin and pregabalin on the electrophysiological properties of cultured DRG neurones from neonatal rats. BMC Pharmacol. 2004;4:14. doi: 10.1186/1471-2210-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoon EJ, Hamm HE, Currie KP. G protein betagamma subunits modulate the number and nature of exocytotic fusion events in adrenal chromaffin cells independent of calcium entry. J Neurophysiol. 2008;100:2929–39. doi: 10.1152/jn.90839.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marcantoni A, Carabelli V, Vandael DH, Comunanza V, Carbone E. PDE type-4 inhibition increases L-type Ca(2+) currents, action potential firing, and quantal size of exocytosis in mouse chromaffin cells. Pflugers Arch. 2009;457:1093–110. doi: 10.1007/s00424-008-0584-4. [DOI] [PubMed] [Google Scholar]
- 50.Fulop T, Smith C. Physiological stimulation regulates the exocytic mode through calcium activation of protein kinase C in mouse chromaffin cells. Biochem J. 2006;399:111–9. doi: 10.1042/BJ20060654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Machado JD, Segura F, Brioso MA, Borges R. Nitric oxide modulates a late step of exocytosis. J Biol Chem. 2000;275:20274–9. doi: 10.1074/jbc.M000930200. [DOI] [PubMed] [Google Scholar]
- 52.Graham ME, Fisher RJ, Burgoyne RD. Measurement of exocytosis by amperometry in adrenal chromaffin cells: Effects of clostridial neurotoxins and activation of protein kinase C on fusion pore kinetics. Biochimie. 2000;82:469–79. doi: 10.1016/s0300-9084(00)00196-6. [DOI] [PubMed] [Google Scholar]
- 53.Machado JD, Morales A, Gomez JF, Borges R. cAmp modulates exocytotic kinetics and increases quantal size in chromaffin cells. Mol Pharmacol. 2001;60:514–20. [PubMed] [Google Scholar]
- 54.Zhao B, Wang HB, Lu YJ, Hu JW, Bao L, Zhang X. Transport of receptors, receptor signaling complexes and ion channels via neuropeptide-secretory vesicles. Cell Res. 2011;21:741–53. doi: 10.1038/cr.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Misra S, Koshy T, Unnikrishnan KP, Suneel PR, Chatterjee N. Gabapentin premedication decreases the hemodynamic response to skull pin insertion in patients undergoing craniotomy. J Neurosurg Anesthesiol. 2011;23:110–7. doi: 10.1097/ANA.0b013e3181da3c3b. [DOI] [PubMed] [Google Scholar]
- 56.Kovac AL. Controlling the hemodynamic response to laryngoscopy and endotracheal intubation. J Clin Anesth. 1996;8:63–79. doi: 10.1016/0952-8180(95)00147-6. [DOI] [PubMed] [Google Scholar]
- 57.Charlesworth P, Pocock G, Richards CD. Calcium channel currents in bovine adrenal chromaffin cells and their modulation by anaesthetic agents. J Physiol. 1994;481(Pt 3):543–53. doi: 10.1113/jphysiol.1994.sp020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie Z, Currie KP, Fox AP. Etomidate elevates intracellular calcium levels and promotes catecholamine secretion in bovine chromaffin cells. J Physiol. 2004;560:677–90. doi: 10.1113/jphysiol.2004.070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herring BE, McMillan K, Pike CM, Marks J, Fox AP, Xie Z. Etomidate and propofol inhibit the neurotransmitter release machinery at different sites. J Physiol. 2011;589:1103–15. doi: 10.1113/jphysiol.2010.200964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herring BE, Xie Z, Marks J, Fox AP. Isoflurane inhibits the neurotransmitter release machinery. J Neurophysiol. 2009;102:1265–73. doi: 10.1152/jn.00252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bannister JP, Adebiyi A, Zhao G, Narayanan D, Thomas CM, Feng JY, Jaggar JH. Smooth muscle cell α2δ-1 subunits are essential for vasoregulation by CaV1.2 channels. Circ Res. 2009;105:948–55. doi: 10.1161/CIRCRESAHA.109.203620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alden KJ, Garcia J. Differential effect of gabapentin on neuronal and muscle calcium currents. J Pharmacol Exp Ther. 2001;297:727–35. [PubMed] [Google Scholar]
- 63.Carroll I, Mackey S, Gaeta R. The role of adrenergic receptors and pain: The good, the bad, and the unknown. Sem Anes Periop Pain. 2007;26:17–21. [Google Scholar]
- 64.Kim SH, Na HS, Sheen K, Chung JM. Effects of sympathectomy on a rat model of peripheral neuropathy. Pain. 1993;55:85–92. doi: 10.1016/0304-3959(93)90187-T. [DOI] [PubMed] [Google Scholar]
- 65.Treede RD, Davis KD, Campbell JN, Raja SN. The plasticity of cutaneous hyperalgesia during sympathetic ganglion blockade in patients with neuropathic pain. Brain. 1992;115(Pt 2):607–21. doi: 10.1093/brain/115.2.607. [DOI] [PubMed] [Google Scholar]