Abstract
There have been conflicting findings as to whether the P3 brain potential to targets in oddball tasks is reduced in depressed patients. The P3 to novel distracter stimuli in a three-stimulus oddball task has a more frontocentral topography than P3 to targets and is associated with different cognitive operations and neural generators. The novelty P3 potential was predicted to be reduced in depressed patients. EEG was recorded from 30 scalp electrodes (nose reference) in 20 unmedicated depressed patients and 20 matched healthy controls during a novelty oddball task with three stimuli: infrequent target tones (12%), frequent standard tones (76%) and nontarget novel stimuli, e.g., animal or environment sounds (12%). Novel stimuli evoked a P3 potential with shorter peak latency and more frontocentral topography than the parietal-maximum P3b to target stimuli. The novelty P3 was markedly reduced in depressed patients compared to controls. Although there was a trend for patients to also have smaller parietal P3b to targets, this group difference was not statistically significant. Nor was there a group difference in the earlier N1 or N2 potentials. The novelty P3 reduction in depressed patients is indicative of a deficit in orienting of attention and evaluation of novel environmental sounds.
Keywords: Depression, ERP, P3, Novelty, Attention
1. Introduction
Individuals having a depressive disorder commonly experience difficulties in concentration or attention and other cognitive functions, most notably memory and executive function (Austin et al., 2001; Porter et al., 2003). The P3 or P300 event-related brain potential (ERP) provides physiologic measures associated with attentional engagement and memory operations during cognitive task performance (Polich, 2007). The study of P3 in depressed patients could therefore provide information concerning the neurophysiologic mechanisms underlying their cognitive deficits. There have, however, been conflicting reports as to whether or not depressed patients have reduced P3 amplitude. An early review found that about half of the studies showed reduced P3 amplitude in depressed patients when compared to normal controls (Roth et al., 1986). We reviewed the findings of more recent studies that compared P3 amplitudes for depressed patients and healthy controls in auditory target detection (oddball) tasks (Bruder et al., submitted for publication). Ten studies found significantly smaller P3 amplitude in depressed patients as compared to healthy controls (Blackwood et al., 1987; Muir et al., 1991; Gangadhar et al., 1993; Ancy et al., 1996; Wagner et al., 1997; Yanai et al., 1997; Anderer et al., 2002; Röschke and Wagner, 2003; Urretavizcaya et al., 2003; Kawasaki et al., 2004), whereas five studies did not (Sara et al.,1994; Bruder et al.,1998; Vandoolaeghe et al.,1998; Kaustio et al., 2002; Kaiser et al., 2003). The difference in P3 amplitude between patients and controls had small to large effect sizes (Cohen's d), which ranged widely across studies from 0.11 to 2.25. The difference in findings across studies could be related to differences in patients' clinical features, with P3 reductions being more evident in depressed patients having a melancholic depression (Gangadhar et al., 1993; Ancy et al., 1996; Urretavizcaya et al., 2003), psychotic depression (Kaustio et al., 2002; Karaaslan et al., 2003), or suicidal features (Hansenne et al., 1996).
Another issue is that the P3 potential is not a unitary phenomenon, but consists of multiple subcomponents associated with different cognitive operations and neural generators (Kayser and Tenke, 2006a; Polich, 2007). P3 is typically measured in a two-stimulus oddball task, in which the subject responds to an infrequent target stimulus in a background of a frequent standard stimulus. The most commonly studied subcomponent observed in this task is the classical P3b potential to target stimuli, which has a parietal maximum scalp distribution and a peak latency ranging from 300–500 ms. The P3b component is often preceded by a component with a more frontocentral topography, i.e., P3a. Although this frontal aspect of P3 can be observed to target stimuli during an oddball task, it is most prominent to nontarget distracter stimuli that are interspersed along with the target and standard stimuli in a 3-stimulus oddball task (Polich and Criado, 2006). Novel distracter stimuli (e.g., environmental sounds) elicit a short latency “novelty P3” with a frontocentral distribution, which is indistinguishable from the P3a potential (Spencer et al., 1999; Simons et al., 2001). The P3a or novelty P3 is thought to reflect frontal attention mechanisms, whereas P3b reflects temporal–parietal activity associated with context updating and subsequent memory storage (Polich, 2007).
Studies examining P3 subcomponents in depressed patients could therefore provide new information concerning the specific nature of their cognitive deficit and the underlying neurophysiologic mechanisms. In a study recording ERPs during two-stimulus tonal and phonetic oddball tasks (Bruder et al., 2002), we used principal components analysis (PCA) to identify and measure overlapping P3 subcomponents in patients having a depressive disorder alone (n = 58), an anxiety disorder alone (n = 22), comorbidity of these disorders (n = 18), and healthy controls (n = 49). An early P3 subcomponent (peak latency 315 ms) was larger in patients having an anxiety disorder alone when compared to depressed patients or healthy controls. Depressed patients having a comorbid anxiety disorder tended to have a smaller early P3 than healthy controls, but those having a depressive disorder alone did not. The timing and frontocentral topography of this early P3 subcomponent resembled that seen for P3a. A later positive subcomponent (peak latency 400 ms) with a parietal maximum did not differ between patients having a depressive disorder alone and controls, but was larger in depressed patients having a comorbid anxiety disorder when compared to the other groups.
A limitation of the above study is that P3 amplitudes were measured to target stimuli in two-stimulus oddball tasks, which is not ideal for measuring the frontocentral P3a. We therefore undertook a study in which brain ERPs in depressed patients and controls were measured during a novelty oddball task (Friedman et al., 1993). This was designed to evaluate whether depressed patients differ from controls in the novelty P3 associated with orienting of attention or the target P3b associated with resource allocation and memory operations. Given evidence for the role of prefrontal and anterior cingulate cortex in depression (Drevets et al., 1997; Bremner et al., 2004; Siegle et al., 2004) and in the generation of P3a or novelty P3 (Knight and Scabiani, 1998; Dien et al., 2003), we predicted that the frontocentral novelty P3 would be reduced in depressed patients when compared to healthy controls, whereas there would be less difference between these groups in the parietal P3b to target stimuli.
2. Methods
2.1. Subjects
The patient group consisted of 20 depressed outpatients who were attending a university-affiliated research clinic, and the matched-control group consisted of 20 healthy adults who were recruited from the New York metropolitan area. The patients and controls were screened to exclude those with any of the following: serious suicide risk, substance abuse disorders (including alcohol abuse) within the last 6 months, psychotic disorders, antisocial personality disorder, seizure disorder, organic mental disorder, history of head trauma, or other neurological disorder. A standard audiogram was used to exclude those having a hearing loss greater than 30 dB in either ear at 500, 1000 or 2000 Hz or if they had an ear difference greater than 10 dB. All participants gave written informed consent before participating in the study and were paid $15 per hour.
Diagnostic assessment of patients was by Structured Interview for Clinical Diagnosis, patient version (SCID-P; First et al., 1994), conducted by research psychiatrists during a pretreatment session. Patients met DSM-IV criteria for major depressive disorder (n = 15) or dysthymia (n = 5). Most had unipolar depressions without melancholic or psychotic features. Three patients met criteria for Bipolar II disorder and only one for major depression with melancholic features. One patient also met criteria for social phobia and one for obsessive–compulsive disorder. Secondary analyses excluding these two patients were conducted to determine whether inclusion of patients having a comorbid anxiety disorder affected the P3 findings. Controls were interviewed using the Structured Clinical Interview for DSM-IV, nonpatient edition (SCID-NP; First et al., 1996) to exclude those with current or past DSM-IV Axis I psychopathology.
Table 1 gives the demographic characteristics of the depressed patients and healthy controls. About an equal number of patients and controls were males or females and there was no significant difference between groups in age or education level. All patients and controls were right handed and there was no difference between groups in handedness laterality quotient (LQ) on the Edinburgh Inventory (Oldfield, 1971). When compared to controls, the depressed patients had higher depression scores on the Beck Depression Inventory (BDI; Beck et al., 1961) and higher trait anxiety scores on the State-Trait Anxiety Index (STAI; Spielberger et al., 1983). There was no overlap of the BDI scores for patients (range = 15–43) and controls (range = 0–7).
Table 1.
Characteristics of patients and controls.
| Depressed patients (n = 20) | Healthy controls (n = 20) | |
|---|---|---|
| Gender (male/female) | 11/9 | 9/11 |
| Age (years) | 33.4 (7.7) | 31.1 (6.2) |
| Education (years) | 16.1 (2.0)a | 16.4 (1.7) |
| Handedness (LQ)b | 81.2 (20.8) | 78.2 (19.5) |
| Depression (BDI)c | 24.8 (8.2) | 1.4 (2.1) |
| Trait anxiety (STAI)d | 79.6 (7.7) | 43.7 (9.6)e |
n = 18.
Laterality Quotient (LQ) on Edinburgh Inventory.
Beck Depression Inventory (BDI); patients have significantly higher depression scores compared to controls (t = 12.45, df = 38, P<0.001).
State Trait Anxiety Inventory (STAI); patients have higher trait anxiety scores than controls (t = 12.79, df = 36, P<0.001).
n = 18.
2.2. Procedure
The novelty oddball task was administered during a pretreatment session before patients received medication. Patients were unmedicated a minimum of 7 days before testing, although most patients were drug-free for a considerably longer period or were not previously treated with an antidepressant. No patient was tested within 6 weeks of receiving fluoxetine or 1 week of receiving other antidepressants and no patient had received a monoamine oxidase inhibitor.
In the novelty oddball task (Friedman et al., 1993; Fabiani and Friedman, 1995), ERPs were measured to infrequent target tones, frequent standard tones, and to nontarget novel sounds (e.g., animal sounds, environment sounds, musical instruments). Each subject first received 4 blocks of 50 trials in a standard auditory oddball task. Each block contained a series of two tones in a random order (ISI = 1,000 ms), with one tone (500 or 350 Hz) being the frequent stimulus (P = 0.88) and the other being the infrequent target stimulus (P = 0.12). Subjects responded with a button press to the target tones. The session continued with 8 additional blocks of the novelty oddball task, in which novel sounds (P = 0.12) were intermixed with the infrequent target tones (P = 0.12) and the frequent tones (P = 0.76). Subjects were not informed that the novel sounds would be presented, and if they asked questions about their presence, they were reminded to respond only to the target tones. Target tones were 300 ms in duration and the 48 unique novel sounds ranged in duration from 100 to 400 ms (mean = 301.4 ms, S.D. = 71.8). All stimuli were presented binaurally at 75 dB SPL over TDH-49 headphones. Target (500 or 350 Hz) and response hand (right or left) for a given block were counterbalanced within subjects in each group. Further details concerning the novelty task are given elsewhere (Friedman et al., 1993).
Scalp EEG was recorded from 13 lateral, homologous pairs of electrode sites (FP1/2, F3/4, F7/8, FC5/6, FT9/10, C3/4, T7/8, CP5/6, TP9/10, P3/4, P7/8, P9/10, O1/2) and from four midline electrode sites (Fz, Cz, Pz, and Oz) using extended 10–20-system placements with an electrode cap (Electro Cap International, Inc.) and a nose reference. Electrodes at supra- and infra-orbital sites surrounding the right eye recorded blinks and vertical eye movements (bipolar), while electrodes at right and left outer canthi recorded horizontal eye movements (bipolar). All electrodes were tin, with impedances below 5 kΩ. EEG was recorded using a Grass Neurodata system at a gain of 10 k (5 k and 2.5 k for horizontal and vertical eye channels, respectively), with a bandpass of 0.1–30 Hz using a NeuroScan recording system (200 samples/s). Recordings were screened for electrolyte bridges between electrodes (Tenke and Kayser, 2001) and activity at bridged electrodes was replaced by spherical spline interpolation from unaffected sites. Only one control subject showed bridging at any of the sites reported here (only O1 to Oz), and exclusion of this subject's data did not affect our findings. The filtered, continuous EEG was then blink corrected using a spatial, singular value decomposition filter generated from identified blinks and artifact-free EEG periods (NeuroScan, 2003). Additional artifact detection and correction was performed using a reference-free approach (Kayser and Tenke, 2006b). Trials containing artifacts in more than 8 channels were rejected; otherwise, contaminated channels were interpolated from the data of the artifact-free channels by means of spherical splines (Perrin et al., 1989). Artifact detection and electrode replacement was verified by visual inspection. Artifact-free data were averaged for correct responses to targets, novels and nontargets. The number of trials contributing to averages did not differ between groups for targets (controls: mean = 42, S.D. = 4; patients: mean = 41, S.D. = 6; t = 0.86, df = 38, ns), novels (controls: mean = 39, S.D. = 5; patients: mean = 37, S.D. = 7, t = 1.03, df = 38, ns), or nontargets (controls: mean = 272, S.D. = 17; patients: mean = 263, S.D. = 29; t = 1.22, df = 38, ns).
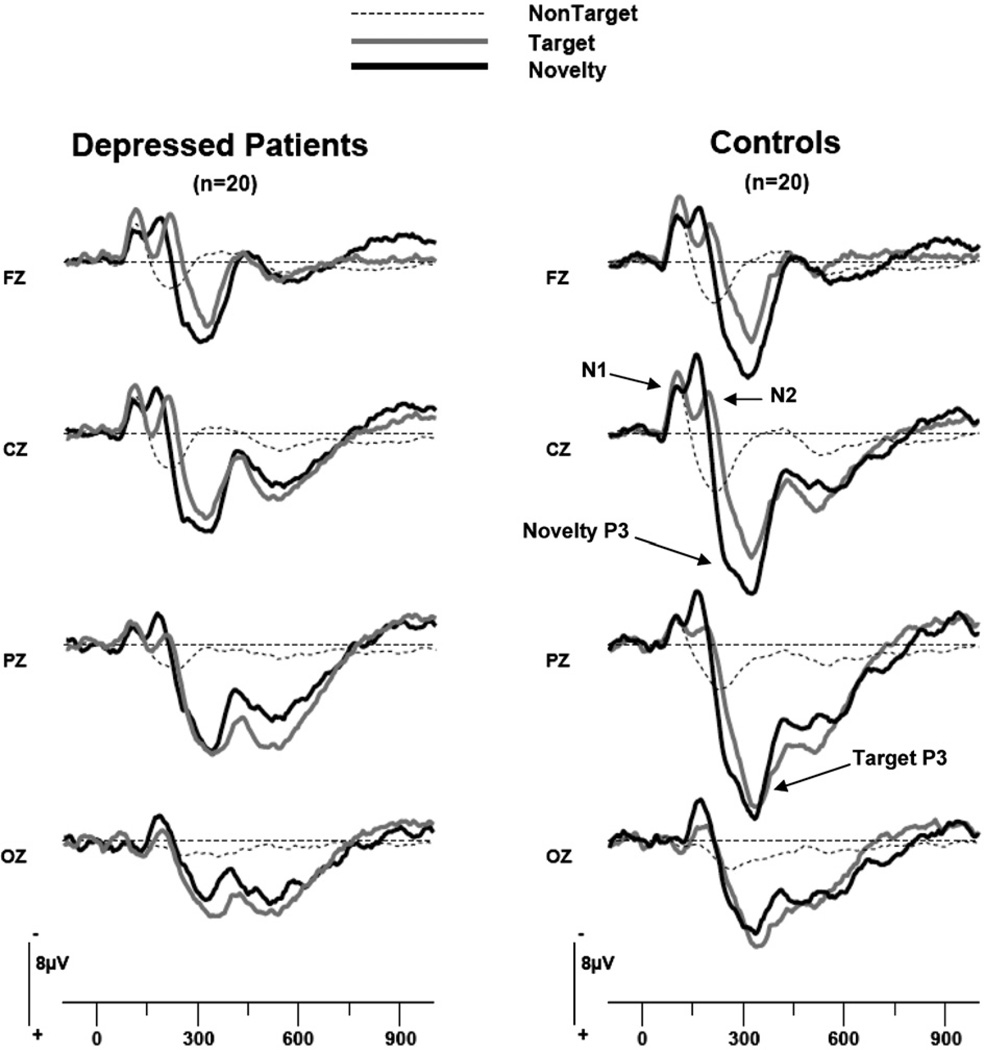
Grand average ERP waveforms for each stimulus condition (Fig. 1) and average waveforms for each patient and control subject were carefully inspected to select time windows that bracketed the peaks for the ERP components and optimized their measurement across subjects: 70–145 ms N1, 150–240 ms for N2, and 220–470 ms for P3. These windows were used to compute mean integrated amplitudes for each component at each recording site. The mean latency of P3 peaks to novel and target stimuli were also computed for patients and controls at the midline parietal site (Pz) where P3 amplitude is maximal. Although there was no significant difference in the P3 latency for patients and controls, P3 latency was shorter for novels (mean = 312 ms, S.D. = 36) than for targets (mean = 324 ms, S.D. = 34; F = 5.36, df = 1,38, P = 0.026). The waveforms in Fig. 1 illustrate, however, that there was considerable overlap of the P3 components for novel and target stimuli, and therefore the same broad window (220–470 ms) was used to incorporate both the novelty P3 and target P3 for each patient and control.
Fig. 1.
Grand average ERP waveforms for depressed patients and healthy controls to nontarget, target and novel stimuli.
2.3. Statistical analyses
The primary statistical analyses evaluated group differences in P3 amplitude at midline sites (Fz, Cz, Pz, and Oz) to novel, target, and nontarget stimuli. Preliminary analyses did not reveal evidence that group differences interacted with gender and therefore gender was not considered in subsequent analyses. Amplitudes in the P3 window (220–470 ms) were submitted to repeated-measures ANOVA with Group (depressed, control) as a between-subjects factor and two within-subjects factors: Stimulus (novel, target, nontarget) and Site (frontal, central, parietal, occipital). Given the focus of this study on the novelty P3 and target P3b, analyses of simple effects examined group differences in P3 amplitude separately for each stimulus condition. Analyses also examined group differences in the earlier N1 or N2 windows. These ANOVA used the same Group, Stimulus, and Site factors as in the above P3 analyses. A secondary analysis examining laterality differences in P3 amplitude included an additional within-subject factor of Hemisphere using medial–lateral electrode sites (F3/4; C3/4; P3/4; O1/2). An ANOVA of P3 latency at Pz included Group (depressed, control) as a between-subjects factor and stimulus (novel, target) as a within-subjects factor. Greenhouse-Geisser epsilon (ε) correction was used to compensate for violations of sphericity when appropriate. The sources of significant interactions were systematically examined through simple effects and contrasts between groups. Response accuracy and latency for target stimuli were also analyzed using an ANOVA with the same grouping factor.
3. Results
3.1. Behavioral performance
Both patients and controls performed well and there was no significant difference in their performance. Patients had 98.4% (S.D. = 1.4) and controls 98.1% (S.D. = 1.3) correct responses to targets (t = 0.99, df = 38, P = 0.33). They made few false alarms to nontargets or novel stimuli (patients = 1.4%, S.D. = 1.3; controls = 1.0%, S.D. = 1.2; t = 0.99, df = 38, P = 0.33). The groups also showed comparable mean reaction time to targets (patients = 458 ms, S.D. = 79; controls = 420 ms, S.D. = 82; Group: t = 1.76, df = 38, P = 0.09).
3.2. Novelty and target P3
The grand average ERP waveforms at midline sites for patients and controls to nontarget, target and novel stimuli are shown in Fig. 1. The waveforms show the expected component structure with early N1 and N2 potentials, most clearly evident for targets at the central site (Cz). The novelty P3 is also evident at this site and the target P3 is largest at the parietal site (Pz). The greater P3 to novels than targets at the Fz and Cz sites reflects the more frontocentral topography of the novelty P3.
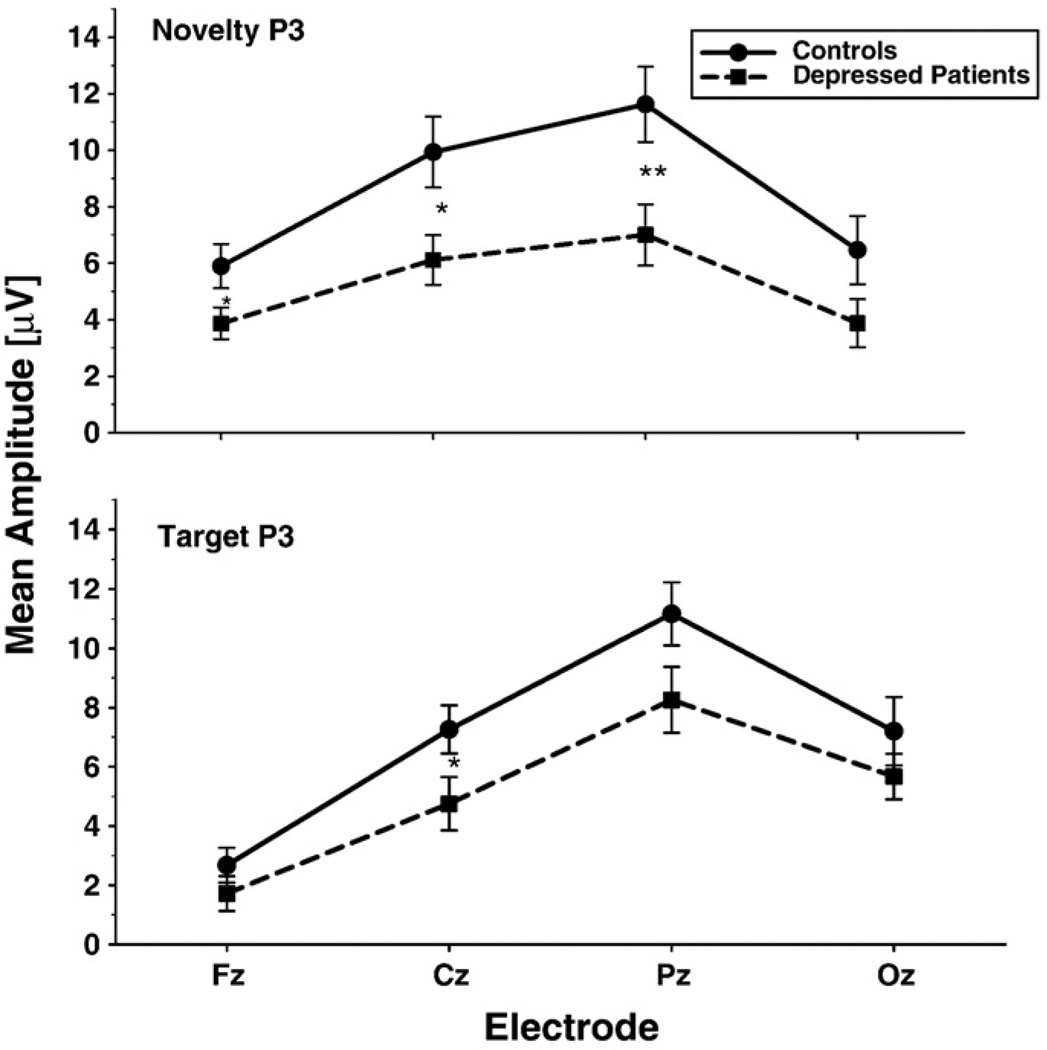
As can be seen in Fig. 1, the amplitude of P3 to both novels and targets was overall smaller in depressed patients than in healthy controls. ANOVA of amplitude in the P3 window (220–470 ms) confirmed the existence of a significant Group difference, F = 6.06, df = 1, 38, P = 0.018. There was also a trend for a Group by Stimulus interaction (F = 3.01 df = 2,76, P = 0.06, ε = 0.91). Analysis of simple effects indicated that the group difference in P3 amplitude was significant for novel stimuli (F = 6.23, df = 1,38, P = 0.017; effect size = 0.80), but not for target (F = 3.44, df = 1,38, P = 0.072; effect size = 0.59) or nontarget stimuli (F = 2.96, df = 1,38, P = 0.094; effect size = 0.56). Fig. 2 shows the mean amplitude of the novelty P3 and target P3 for depressed patients and controls at midline sites. At the parietal site, where the target P3 was maximum, patients had significantly smaller novelty P3 than controls (t = 2.68, df = 38, P = 0.01) with an effect size of 0.85. There was also a nonsignificant trend for smaller target P3 in patients at the parietal site (t = 1.89, df = 38, P = 0.067) with an effect size of 0.60. The above results remained the same when the two patients having a comorbid anxiety disorder were excluded from the analyses. Thus, the overall ANOVA of amplitude in the P3 window yielded a main effect of Group (F = 6.36, df = 1,36, P = 0.016) and a significant Group by Stimulus interaction (F = 3.32, df = 2,72, P = 0.047).
Fig. 2.
Mean amplitude of novelty P3 and target P3b in depressed patients and healthy controls at frontal (Fz), central (Cz), parietal (Pz), and occipital (Oz) midline sites (error bars = standard errors). Significant simple group effects at each site are indicated by: *P<0.05, **P = 0.01.
An ANOVA of P3 amplitude at medial–lateral sites over each hemisphere showed the same Group difference as seen for the midline sites (F = 5.37, df = 1,38, P = 0.026), but there was no interaction involving Group and Hemisphere, which indicates that the smaller P3 in patients was not dependent on hemisphere. There was a significant main effect of Hemisphere (F = 5.71, df = 1,38, P = 0.022) and a Hemisphere by Condition by Site interaction (F = 9.36, df = 6,228, P<0.001). Analyses of simple effects indicated that this interaction reflected larger P3 amplitude over right than left hemisphere at only central and parietal sites for targets and novels (Hemisphere by Site interaction: for targets, F = 15.56, df = 3,114, P<0.001, ε = 0.76; and novels, F = 4.08, df = 3,114, P = 0.015, ε = 0.80).
3.3. N1 and N2
Fig. 1 shows that patients and controls had comparable N1 amplitude. In the ANOVA of amplitudes in the N1 window, there was no significant group difference and no interactions involving group. The N2 potential is clearly evident in Fig. 1 for target and novel stimuli but not nontarget stimuli, which was reflected in a significant Stimulus effect (F = 21.79, df = 2,76, P<0.001, ε = 0.93). There was, however, no significant group difference in the N2 window and no Group by Stimulus interaction.
3.4. Correlational analyses
Correlations were performed to examine whether the novelty P3 and target P3 in patients were related to the severity of depression or anxiety symptoms. There was no significant correlation between novelty P3 amplitude for patients at the midline parietal site (Pz) and depression scores on the BDI (r = 0.08, ns) or trait anxiety scores on the STAI (r = 0.07, ns). Similarly, there was no significant correlation between target P3 amplitude for patients at parietal site and scores on the BDI (r = 0.14, ns) or STAI (r = 0.04, ns).
4. Discussion
Novel distracter stimuli in a 3-stimulus oddball task evoked a novelty P3 potential with a more frontocentral topography than the parietal-maximum P3b potential to target stimuli (Friedman et al., 2001; Polich, 2007; Spencer et al., 1999). While depressed patients showed overall reduced P3 amplitude when compared to healthy controls, the size of this reduction was larger for novel than target stimuli. The novelty P3 was significantly reduced in depressed patients, whereas the parietal P3b to target stimuli was only marginally reduced in these patients. The effect size at the midline parietal site was relatively large for novelty P3 (Cohen's d = 0.85), but smaller for target P3b (0.60). The marginal size of the group difference in P3b to targets may account for why some studies have found reduced P3 amplitude in depressed patients, while others have not. Prior studies failed to differentiate P3 subcomponents and most used a two-stimulus oddball task, which yields a weaker frontal P3a than the novelty oddball task (Polich and Criado, 2006). We have previously argued that simple oddball tasks are not challenging enough to elicit robust P3b reductions in depressed patients having subtle cognitive deficits. Larger differences in P3b between depressed patients and controls may be found by increasing the cognitive demands of the task (e.g., Bruder et al., 1995; Pierson et al., 1996).
In contrast, prior studies have found evidence that novelty P3 or P3a is increased in anxiety disorders. Patients having an anxiety disorder (primarily social phobia or panic disorders; n = 22) showed an abnormally large early P3 potential with a frontocentral topography resembling P3a (Bruder et al., 2002), and patients having a post-traumatic stress disorder (n = 24) showed larger P3 to novel sounds at frontal sites when compared to controls (Kimble et al., 2000). The opposite P3a or novelty P3 abnormality in depressive and anxiety disorders suggests a possible relation to specific symptom features. This is supported by the findings of Partiot et al. (1993), who measured ERPs during a simple go/no-go reaction-time task and found that a subgroup of depressed patients with retarded and blunted affect had reduced frontal P3a amplitude when compared to a subgroup with anxious–agitated symptoms. We did not, however, find evidence of a relation between amplitude of novelty or target P3 and severity of depression or anxiety ratings in patients. This suggests that the diagnostic category or subtype of depression is more closely related to P3 reductions than symptom severity. There is evidence that melancholic (Gangadhar et al.,1993; Ancy et al.,1996; Urretavizcaya et al., 2003) and psychotic (Kaustio et al., 2002; Karaaslan et al., 2003) subtypes of depression have the largest reductions of P3 amplitude. It is also important to note that reduction of novelty P3 is not specific to depression, but has also been found in schizophrenia patients (Grillon et al., 1990; Merrin and Floyd, 1994).
The N1 and N2 components did not differ between depressed patients and controls, which indicates that their reduced novelty P3 is unlikely due to an earlier deficit in auditory processing or detection of deviant novel stimuli. The novelty P3 reduction may indicate a deficit in automatic orienting of attention and evaluation of novel environmental sounds. Both ERP and fMRI studies suggest that frontal mechanisms are involved in orienting of attention to novel stimuli (for review, see Polich and Criado, 2006). Frontal cortex, and particularly the anterior cingulate, is of key importance for attentional processing, and has been found to be dysfunctional in depressed patients during a variety of cognitive tasks (Bremner et al., 2004; Drevets et al., 1997; Siegle et al., 2004). The P3a potential to novel stimuli is markedly reduced by prefrontal lesions (Knight et al., 1998). Intracranial recordings have found evidence for generators of novelty P3 in multiple areas, including frontal and posterior association cortex, hippocampus and anterior cingulate cortex (Halgren et al., 1995). ERP studies using source localization techniques have localized generators of novelty P3 to anterior cingulate, whereas the P3b to target stimuli has sources in the region of the temporal–parietal junction (Dien et al., 2003; Mecklinger and Ullsperger, 1995). Focal lesions in the posterior hippocampal region also result in reduction of novelty P3a particularly over prefrontal regions, whereas posterior P3b amplitude to targets is unaffected by hippocampal damage (Knight, 1996). This led Knight et al. (1998) to conclude that a distributed frontal-hippocampal system is involved in novelty processing. The P3 novelty reduction in depressed patients may therefore involve dysfunction of a distributed network, including prefrontal, anterior cingulate, and hippocampal regions.
Nieuwenhuis and Aston-Jones (2005) reviewed studies suggesting that P3 reflects activity in the locus coeruleus–norepinephrine system. Evidence for the role of the noradrenergic neurotransmitter system in modulating P3 was provided by the finding of opposing effects of α2 noradrenergic agonists and antagonists on the frontal P3a (Turetsky and Fein, 2002). The α2 antagonist yohimbine, which has anxiogenic effects, increased P3a amplitude in healthy adults, whereas the α2 agonist clonidine decreased P3a amplitude. The P3b component was not, however, affected by either noradrenergic agent. They suggest that norepinephrine enhances the physiological processes underlying the evaluation of novelty and selective attention to potentially important stimuli. The P3a or novelty decrement in depressed patients may therefore be a physiologic marker of a norepinephrine deficiency, which is thought to characterize at least a subgroup of depressed patients (Schildkraut, 1974). However, Polich (2007) reviewed evidence supporting the hypothesis that P3a is related to frontal attention mechanisms mediated by dopaminergic activity, whereas P3b is related to temporoparietal activity mediated by the norepinephrine system. Although further research on the neurotransmitter systems mediating P3 is needed, the widespread neural networks that appear to underlie P3a and P3b suggest that both noradrenergic and dopaminergic neurotransmitter systems are likely to be of importance. Studies could, for instance, investigate whether antidepressants that block reuptake of norepinephrine, e.g., bupropion, normalize P3a or novelty P3 in depressed patients. The possible value of novelty P3 for predicting clinical responsiveness to these antidepressants that act on the noradrenergic system could also be investigated in this context.
Some limitations of this study deserve mention. First, the sample of depressed patients in the current study consisted of patients who mainly had a unipolar, non-melancholic depression with little comorbidity. It is therefore unclear to what extent the findings generalize to other diagnostic subtypes of depression or to patients with comorbid anxiety disorders. Second, the novelty P3 component overlaps the P3b component, which leaves open the possible contribution of P3b to group differences in the mean integrated amplitude of the novelty P3. The use of multivariate techniques, such as principal components analysis (PCA), could aide in identifying and measuring these separate P3 subcomponents. An independent replication and extension of this study using a larger electrode array (67 channels) applied PCA to reference-free Laplacian transformations of ERPs during the novelty oddball task (Tenke et al., 2009). This analysis identified an early mid-central source (245-ms peak latency) that was unique to novel stimuli and was markedly reduced in depressed patients when compared to healthy controls. Again, group differences were less evident for later sources corresponding to P3b. This supports the hypothesis that the novelty P3 reduction in depression is indicative of a deficit in early shifting of attention to novel distracter stimuli, involving a source localizable to the midline frontocentral region. Findings of a recent study by Ruchsow et al. (2008) suggest that impaired control processes involved in inhibiting responses to novel stimuli may also contribute to the P3 reduction at midline central sites. They measured ERPs of 21 patients having a major depression and 21 healthy controls during a Go/Nogo flanker task. The P3 to Go trials has a parietal distribution, whereas the Nogo-P3, like the novelty P3, has a more frontocentral distribution. Depressed patients showed reduced Nogo-P3, but P3b to Go trials did not differ from controls. Further study should be given to the possibility that a common deficit in cognitive control processes may underlie reductions of novelty P3 and Nogo-P3 in depression.
Acknowledgement
This research was supported in part by a National Institute of Mental Health grant MH36295.
References
- Ancy J, Gangadhar BN, Janakiramaiah N. “Normal” P300 amplitude predicts rapid response to ECT in melancholia. Journal of Affective Disorders. 1996;41:211–215. doi: 10.1016/s0165-0327(96)00090-0. [DOI] [PubMed] [Google Scholar]
- Anderer P, Saletu B, Semlitsch HV, Pascual-Marqui RD. Structural and energetic processes related to P300: LORETA findings in depression and effects of antidepressant drugs. Methods and Findings in Experimental and Clinical Pharmacology. 2002;24:85–91. [PubMed] [Google Scholar]
- Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression. British Journal of Psychiatry. 2001;178:200–206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Blackwood DHR, Whalley LJ, Christie JE, Blackburn IM, St Clair DM, McInnes A. Changes in auditory P3 event-related potential in schizophrenia and depression. British Journal of Psychiatry. 1987;150:154–160. doi: 10.1192/bjp.150.2.154. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Vaccarino V, Charney DS. Deficits in hippocampal and anterior cingulate functioning during verbal declarative memory encoding in midlife major depression. American Journal of Psychiatry. 2004;161:637–645. doi: 10.1176/appi.ajp.161.4.637. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Stewart JW, Towey JP, Leite P, Voglmaier M, Quitkin FM. Brain event-related potentials to complex tones in depressed patients: relations to perceptual asymmetry and clinical features. Psychophysiology. 1995;32:373–381. doi: 10.1111/j.1469-8986.1995.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Towey JP, Leite P, Fong R, Stewart JE, McGrath PJ, Quitkin FM. Brain ERPs of depressed patients to complex tones in an oddball task: relation of reduced P3 asymmetry to physical anhedonia. Psychophysiology. 1998;35:54–63. [PubMed] [Google Scholar]
- Bruder GE, Kayser J, Tenke CE, Leite P, Schneier FR, Stewart JW, Quitkin FM. Cognitive ERPs in depressive and anxiety disorders during tonal and phonetic oddball tasks. Clinical Electroencephalography. 2002;33:119–124. doi: 10.1177/155005940203300308. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Kayser J, Tenke CE. Event- related brain potentials in depression: clinical, cognitive and neurophysiologic implications. In: Luck SJ, Kappenman ES, editors. Event-Related Potential Components: The Ups and Downs of Brainwave Recordings. Oxford University Press; submitted for publication. [Google Scholar]
- Dien J, Spencer KM, Donchin E. Localization of the event-related potential novelty response as defined by principal components analysis. Cognitive Brain Research. 2003;17:637–650. doi: 10.1016/s0926-6410(03)00188-5. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Friedman D. Changes in brain activity patterns in aging: the novelty oddball. Psychophysiology. 1995;32:579–594. doi: 10.1111/j.1469-8986.1995.tb01234.x. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. The Structural Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (SCID-I/P), Version 2.0) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1994. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders—Non Patient Edition (SCID-NP) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain's evaluation of novelty. Neuroscience and Biobehavioral Reviews. 2001;25:355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Friedman D, Simpson G, Hamberger M. Age-related changes in scalp topography to novel and target stimuli. Psychophysiology. 1993;30:383–396. doi: 10.1111/j.1469-8986.1993.tb02060.x. [DOI] [PubMed] [Google Scholar]
- Gangadhar BN, Ancy J, Janakiramaiah N, Umapathy C. P300 amplitude in non-bipolar, melancholic depression. Journal of Affective Disordors. 1993;28:57–60. doi: 10.1016/0165-0327(93)90077-w. [DOI] [PubMed] [Google Scholar]
- Grillon C, Courchesne E, Rezvan A, Geyer MA, Braff DL. Increased distractibility in schizophrenic patients. Electrophysiologic and behavioral evidence. Archives of General Psychiatry. 1990;47:171–179. doi: 10.1001/archpsyc.1990.01810140071010. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Marinkovic K, Devaux B, Vignal JP, Biraben A. Intracerebral potentials to rare target and distractor auditory and visual stimuli. II. Medial, lateral and posterior temporal lobe. Electroencephalography and Clinical Neurophysiology. 1995;94:229–250. doi: 10.1016/0013-4694(95)98475-n. [DOI] [PubMed] [Google Scholar]
- Hansenne M, Pitchot W, Moreno AG, Zaldua IU, Ansseau M. Suicidal behavior in depressive disorder: an event-related potential study. Biological Psychiatry. 1996;40:116–122. doi: 10.1016/0006-3223(95)00372-x. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Unger J, Kiefer M, Markela J, Mundt C, Weisbrod M. Executive control deficit in depression: event-related potentials in a Go/Nogo task. Psychiatry Research. 2003;122:169–184. doi: 10.1016/s0925-4927(03)00004-0. [DOI] [PubMed] [Google Scholar]
- Karaaslan F, Gonul AS, Oguz A, Erdinc E, Esel E. P300 changes in major depressive disorders with and without psychotic features. Journal of Affective Disorders. 2003;73:283–287. doi: 10.1016/s0165-0327(01)00477-3. [DOI] [PubMed] [Google Scholar]
- Kaustio O, Partanen J, Valkonen-Korhonen M, Viinamäki H, Lehtonen J. Affective and psychotic symptoms relate to different types of P300 alteration in depressive disorder. Journal of Affective Disorders. 2002;71:43–50. doi: 10.1016/s0165-0327(01)00410-4. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clinical Neurophysiology. 2006a;117:348–368. doi: 10.1016/j.clinph.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Electrical distance as a reference-free measure for identifying artifacts in multichannel electroencephalogram (EEG) recordings. Psychophysiology. 2006b;43:S51. [Google Scholar]
- Kawasaki T, Tanaka S, Wang J, Hokama H, Hiramatsu K. Abnormalities of P300 cortical current density in unmedicated depressed patients revealed by LORETA analysis of event-related potentials. Psychiatry and Clinical Neurosciences. 2004;58:68–75. doi: 10.1111/j.1440-1819.2004.01195.x. [DOI] [PubMed] [Google Scholar]
- Kimble M, Kaloupek D, Kaufman M, Deldin P. Stimulus novelty differentially affects attentional allocation in PTSD. Biological Psychiatry. 2000;47:880–890. doi: 10.1016/s0006-3223(99)00258-9. [DOI] [PubMed] [Google Scholar]
- Knight R. Contribution of human hippocampal region to novelty detection. Nature. 1996;383:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabiani D. Anatomic bases of event-related potentials and their relationship to novelty detection in humans. Journal of Clinical. Neurophysiology. 1998;15:3–13. doi: 10.1097/00004691-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Mecklinger A, Ullsperger P. The P300 to novel and target events: a spatiotemporal dipole model analysis. Neuroreport. 1995;7:241–245. [PubMed] [Google Scholar]
- Merrin EL, Floyd TC. P300 responses to novel auditory stimuli in hospitalized schizophrenic patients. Biological Psychiatry. 1994;36:527–542. doi: 10.1016/0006-3223(94)90617-3. [DOI] [PubMed] [Google Scholar]
- Muir WJ, St. Clair DM, Blackwood DHR. Long-latency auditory event-related potentials in schizophrenia and in bipolar and unipolar affective disorder. Psychological Medicine. 1991;21:867–879. doi: 10.1017/s003329170002986x. [DOI] [PubMed] [Google Scholar]
- NeuroScan, Inc. SCAN 4.3 – Vol. II. EDIT 4.3 – Offline Analysis of Acquired Data (Document Number 2203, Revision D) El Paso, TX: Compumedics Neuroscan; 2003. [Google Scholar]
- Nieuwenhuis S, Aston-Jones G. Decision making, the P3, and the locus coeruleus–norepinephrine system. Psychological Bulletin. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Partiot A, Pierson A, Le Houezec J, Dodin V, Renault B, Jouvent R. Loss of automatic processes and blunted-affect in depression: a P3 study. European Psychiatry. 1993;8:309–318. [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping [Corrigenda EEG 02274, Electroencephalography and Clinical Neurophysiology 1990, 76, 565] Electroencephalography and Clinical Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Pierson A, Ragot R, Van Hooff J, Partiot A, Renault B, Jouvent R. Heterogeneity of information-processing alterations according to dimensions of depression: an event-related potentials study. Biological Psychiatry. 1996;40:98–115. doi: 10.1016/0006-3223(95)00329-0. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. International Journal of Psychophysiology. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Porter RJ, Gallagher P, Thompson JM, Young AH. Neurocognitive impairment in drug-free patients with major depressive disorder. British Journal of Psychiatry. 2003;182:214–220. doi: 10.1192/bjp.182.3.214. [DOI] [PubMed] [Google Scholar]
- Röschke J, Wagner P. A confirmatory study on the mechanisms behind reduced P300 waves in depression. Neuropsychopharmcology. 2003;28:S9–S12. doi: 10.1038/sj.npp.1300139. [DOI] [PubMed] [Google Scholar]
- Roth WT, Duncan CC, Pfefferbaum A, Timsit-Berthier M. Applications of cognitive ERPs in psychiatric patients. In: McCallum WC, Zappoli R, Denoth F, editors. Cerebral Psychophysiology: Studies in Event-Related Potentials.. EEG Suppl. vol. 38. Amsterdam: Elsevier; 1986. pp. 419–438. [PubMed] [Google Scholar]
- Ruchsow M, Groen G, Kiefer M, Beschoner P, Hermle L, Ebert D, Falkenstein M. Electrophysiological evidence for reduced inhibitory control in depressed patients in partial remission: a Go/Nogo study. International Journal of Psychophysiology. 2008;68:209–218. doi: 10.1016/j.ijpsycho.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Sara G, Gordon E, Kraiuhin C, Coyle S, Howson A, Meares R. The P300 ERP component: an index of cognitive dysfunction in depression? Journal of Affective Disordors. 1994;31:29–38. doi: 10.1016/0165-0327(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Schildkraut JJ. Biochemical criteria for classifying depressive disorders and predicting responses to pharmacotherapy: preliminary findings from studies of norepinephrine metabolism. Contributions to biochemistry. Pharmakopsychiatrie Neuro Psychopharmakologie. 1974;7:98–107. doi: 10.1055/s-0028-1094408. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME. Pupillary assessment and computational modeling of the Stroop task in depression. International Journal of Psychophysiology. 2004;52:63–76. doi: 10.1016/j.ijpsycho.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Simons R, Graham F, Miles M, Chen X. On the relationship of P3a and the novelty-P3. Biological Psychology. 2001;56:207–218. doi: 10.1016/s0301-0511(01)00078-3. [DOI] [PubMed] [Google Scholar]
- Spencer K, Dien J, Donchin E. A componential analysis of the ERP elicited by novel events using a dense electrode array. Psychophysiology. 1999;36:409–414. doi: 10.1017/s0048577299981180. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Tenke CE, Kayser J. A convenient method for detecting electrolyte bridges in multichannel electroencephalogram and event-related potential recordings. Clinical Neurophysiology. 2001;112:545–550. doi: 10.1016/s1388-2457(00)00553-8. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J, Stewart JW, Bruder GE. Novelty P3 reductions in depression: characterization using principal components analysis (PCA) of current source density (CSD) waveforms. Psychophysiology. 2009 Sep 15; doi: 10.1111/j.1469-8986.2009.00880.x. e-publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Fein G. Alpha2-noradrenergic effects on ERP and behavior indices of auditory information processing. Psychophysiology. 2002;39:147–157. doi: 10.1017/S0048577202991298. [DOI] [PubMed] [Google Scholar]
- Urretavizcaya M, Moreno I, Benlloch L, Cardoner N, Serrallonga J, Menchon JM, Vallejo J. Auditory event-related potentials in 50 melancholic patients: increased N100, N200 and P300 latencies and diminished P300 amplitude. Journal of Affective Disordors. 2003;74:293–297. doi: 10.1016/s0165-0327(02)00016-2. [DOI] [PubMed] [Google Scholar]
- van Vandoolaeghe E, Hunsel F, Nuyten D, Maes M. Auditory event related potentials in major depression: prolonged P300 latency and increased P200 amplitude. Journal of Affective Disorders. 1998;48:105–113. doi: 10.1016/s0165-0327(97)00165-1. [DOI] [PubMed] [Google Scholar]
- Wagner P, Röschke J, Fell J, Frank C. Differential pathophysiological mechanisms of reduced P300 amplitude in schizophrenia and depression: a single trial analysis. Schizophrenia Research. 1997;25:221–229. doi: 10.1016/s0920-9964(97)00027-3. [DOI] [PubMed] [Google Scholar]
- Yanai I, Fujikawa T, Osada M, Yamawaki S, Touhouda Y. Changes in auditory P300 in patients with major depression and silent cerebral infarction. Journal of Affective Disorders. 1997;46:263–271. doi: 10.1016/s0165-0327(97)00100-6. [DOI] [PubMed] [Google Scholar]